Silencing TUBB3 Expression Destroys the Tegument and Flame Cells of Echinococcus multilocularis Protoscoleces
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Preparation and Culture
2.2. siRNA Preparation
2.3. siRNA Delivery to E. multilocularis PSCs
2.4. Viability of PSCs Treated with siRNA Targeting EmTUBB3
2.5. Quantitative Real-Time PCR (qRT–PCR)
2.6. Morphological Observation
2.7. Statistical Analysis
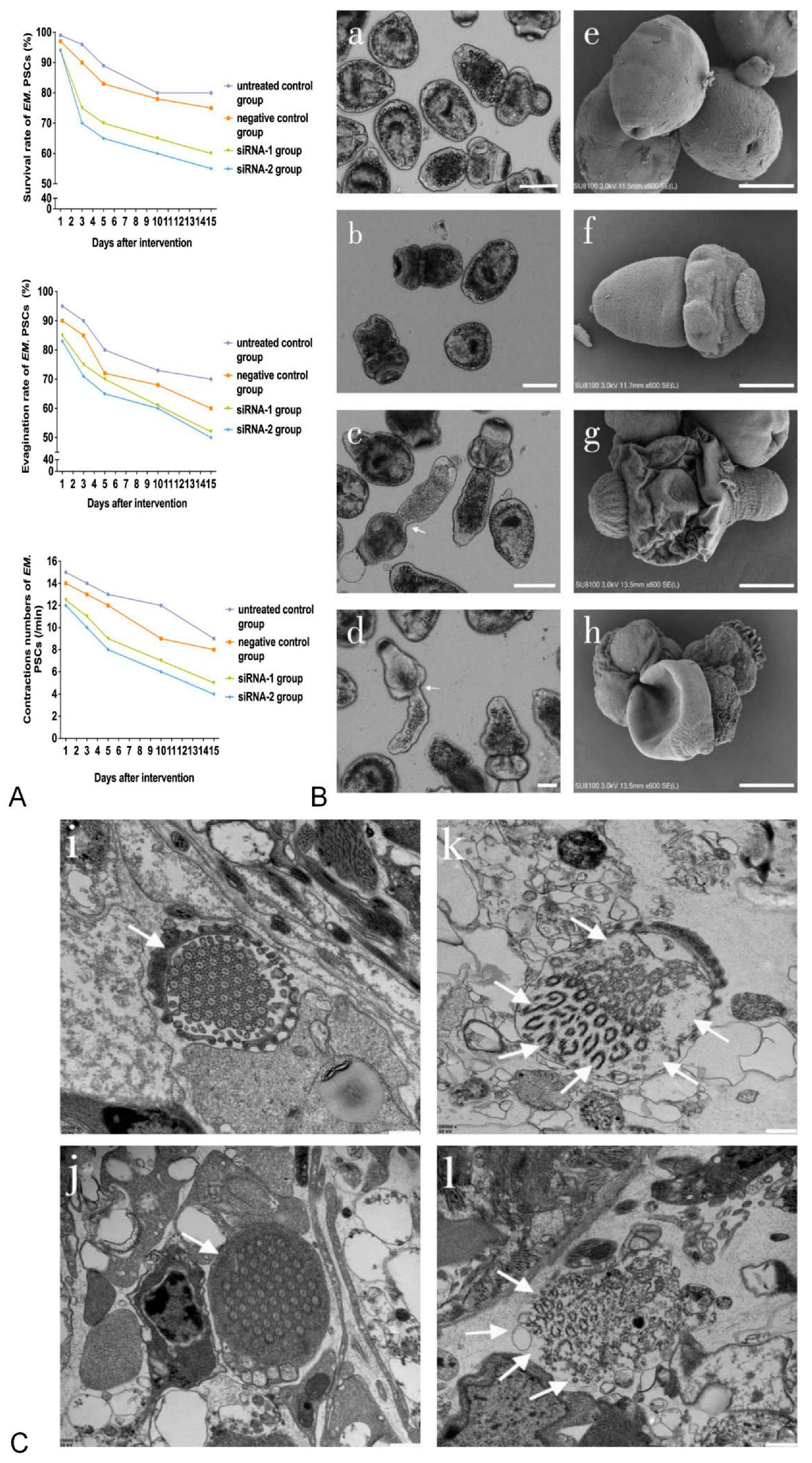
3. Results
3.1. TUBB3 Expression in E. multilocularis PSCs after Treatment with siRNA
3.2. Viability of and Morphological Changes in PSCs Treated with siRNA-1 and siRNA-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st century. Clin. Microbiol. Rev. 2019, 32, e00075–e00118. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Keller, K.; Magnotta, M.; Ragland, N. The global burden of alveolar echinococcosis. PLoS Negl. Trop. Dis. 2010, 4, e722. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, A.; Ammann, R.W.; Candinas, D.; Clavien, P.A.; Eckert, J.; Gottstein, B.; Halkic, N.; Muellhaupt, B.; Prinz, B.M.; Reichen, J.; et al. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg. Infect. Dis. 2007, 13, 878–882. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Gray, D.J.; Zhang, W.; Yang, Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012, 344, e3866. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, E.; Kern, P.; Vuitton, D.A.; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, M. An old drug as a promising new cure for the hard-to-treat echinococcosis. eBioMedicine 2020, 55, 102749. [Google Scholar] [CrossRef]
- Brehm, K.; Kronthaler, K.; Jura, H.; Frosch, M. Cloning and characterization of beta-tubulin genes from Echinococcus multilocularis. Mol. Biochem. Parasitol. 2000, 107, 297–302. [Google Scholar] [CrossRef]
- Lacey, E. Mode of action of benzimidazoles. Parasitol. Today 1990, 6, 112–115. [Google Scholar] [CrossRef]
- Soucek, K.; Kamaid, A.; Phung, A.D.; Kubala, L.; Bulinski, J.C.; Harper, R.W.; Eiserich, J.P. Normal and prostate cancer cells display distinct molecular profiles of alpha-tubulin posttranslational modifications. Prostate 2006, 66, 954–965. [Google Scholar] [CrossRef]
- Whipple, R.A.; Matrone, M.A.; Cho, E.H.; Balzer, E.M.; Vitolo, M.I.; Yoon, J.R.; Ioffe, O.B.; Tuttle, K.C.; Yang, J.; Martin, S.S. Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement. Cancer Res. 2010, 70, 8127–8137. [Google Scholar] [CrossRef]
- Xue, X.; Gao, W.; Sun, B.; Xu, Y.; Han, B.; Wang, F.; Zhang, Y.; Sun, J.; Wei, J.; Lu, Z.; et al. Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene 2013, 32, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, X.; Zhang, L.; Yan, B.; Xie, S.; Liu, R.; Liu, M.; Zhou, J. Histone deacetylase 6 and cytoplasmic linker protein 170 function together to regulate the motility of pancreatic cancer cells. Protein Cell 2014, 5, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, P.; Tang, F.; Lian, H.; Chen, X.; Zhang, Y.; He, X.; Liu, W.; Xie, C. HDAC6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma. Cancer Lett. 2016, 379, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Gradilone, S.A.; Radtke, B.N.; Bogert, P.S.; Huang, B.Q.; Gajdos, G.B.; LaRusso, N.F. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013, 73, 2259–2270. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, K.; Nakagawa, H.; Hosokawa, M.; Mochizuki, Y.; Ueda, K.; Piao, L.; Chung, S.; Hamamoto, R.; Eguchi, H.; Ohigashi, H.; et al. Involvement of the tubulin tyrosine ligase-like family member 4 polyglutamylase in PELP1 polyglutamylation and chromatin remodeling in pancreatic cancer cells. Cancer Res. 2010, 70, 4024–4033. [Google Scholar] [CrossRef]
- Rocha, C.; Papon, L.; Cacheux, W.; Marques Sousa, P.; Lascano, V.; Tort, O.; Giordano, T.; Vacher, S.; Lemmers, B.; Mariani, P.; et al. Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO J. 2014, 33, 2247–2260. [Google Scholar] [CrossRef]
- McCarroll, J.A.; Sharbeen, G.; Liu, J.; Youkhana, J.; Goldstein, D.; McCarthy, N.; Limbri, L.F.; Dischl, D.; Ceyhan, G.O.; Erkan, M.; et al. βIII-tubulin: A novel mediator of chemoresistance and metastases in pancreatic cancer. Oncotarget. 2015, 6, 2235–2249. [Google Scholar] [CrossRef]
- Koziol, U.; Brehm, K. Recent advances in Echinococcus genomics and stem cell research. Vet. Parasitol. 2015, 213, 92–102. [Google Scholar] [CrossRef]
- McGonigle, L.; Mousley, A.; Marks, N.J.; Brennan, G.P.; Dalton, J.P.; Spithill, T.W.; Day, T.A.; Maule, A.G. The silencing of cysteine proteases in Fasciola hepatica newly excysted juveniles using RNA interference reduces gut penetration. Int. J. Parasitol. 2008, 38, 149–155. [Google Scholar] [CrossRef]
- Krautz-Peterson, G.; Bhardwaj, R.; Faghiri, Z.; Tararam, C.A.; Skelly, P.J. RNA interference in schistosomes: Machinery and methodology. Parasitology 2010, 137, 485–495. [Google Scholar] [CrossRef]
- Sánchez Alvarado, A.; Newmark, P.A. Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc. Natl. Acad. Sci. USA 1999, 96, 5049–5054. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xu, M.J.; Nisbet, A.J.; Huang, C.Q.; Lin, R.Q.; Yuan, Z.G.; Song, H.Q.; Zhu, X.Q. Ascaris suum: RNAi mediated silencing of enolase gene expression in infective larvae. Exp. Parasitol. 2011, 127, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Issa, Z.; Grant, W.N.; Stasiuk, S.; Shoemaker, C.B. Development of methods for RNA interference in the sheep gastrointestinal parasite, Trichostrongylus colubriformis. Int. J. Parasitol. 2005, 35, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.S.; Fraser, A.G.; Dong, Y.; Poulin, G.; Durbin, R.; Gotta, M.; Kanapin, A.; Le Bot, N.; Moreno, S.; Sohrmann, M.; et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003, 421, 231–237. [Google Scholar] [CrossRef]
- Mizukami, C.; Spiliotis, M.; Gottstein, B.; Yagi, K.; Katakura, K.; Oku, Y. Gene silencing in Echinococcus multilocularis protoscoleces using RNA interference. Parasitol. Int. 2010, 59, 647–652. [Google Scholar] [CrossRef]
- Pierson, L.; Mousley, A.; Devine, L.; Marks, N.J.; Day, T.A.; Maule, A.G. RNA interference in a cestode reveals specific silencing of selected highly expressed gene transcripts. Int. J. Parasitol. 2010, 40, 605–615. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Zhang, C.; Guo, B.; Wei, Q.; Li, L.; Yang, N.; Peter McManus, D.; Gao, X.; Zhang, W.; et al. Echinococcus granulosus sensu stricto: Silencing of thioredoxin peroxidase impairs the differentiation of protoscoleces into metacestodes. Parasite 2018, 25, 57. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Afgar, A.; Mohammadi, M.A.; Mortezaei, S.; Sadeghi, B.; Fasihi Harandi, M. Calmodulin-specific small interfering RNA induces consistent expression suppression and morphological changes in Echinococcus granulosus. Sci. Rep. 2019, 9, 3894. [Google Scholar] [CrossRef]
- Spiliotis, M.; Mizukami, C.; Oku, Y.; Kiss, F.; Brehm, K.; Gottstein, B. Echinococcus multilocularis primary cells: Improved isolation, small-scale cultivation and RNA interference. Mol. Biochem. Parasitol. 2010, 174, 83–87. [Google Scholar] [CrossRef]
- Luft, C.; Ketteler, R. Electroporation knows no boundaries: The use of electrostimulation for siRNA delivery in cells and tissues. SLAS Discov. 2015, 20, 932–942. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Afgar, A.; Mohammadi, M.A.; Mortezaei, S.; Faridi, A.; Sadeghi, B.; Fasihi Harandi, M. Biological and morphological consequences of dsRNA-induced suppression of tetraspanin mRNA in developmental stages of Echinococcus granulosus. Parasit. Vectors 2020, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Levron, C.; Poddubnaya, L.G.; Kuchta, R.; Freeman, M.; Wang, Y.H.; Scholz, T. SEM and TEM study of the armed male terminal genitalia of the tapeworm Paraechinophallus japonicus (Cestoda: Bothriocephalidea). J. Parasitol. 2008, 94, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.M.; Song, Z.Y.; Xing, R.H.; Jin, Y.M.; Guo, Y.H.; Li, H.; Lu, K.; Shi, Y.J.; Cheng, G.F.; et al. The molecular characterization and RNAi silencing of SjZFP1 in Schistosoma japonicum. Parasitol. Res. 2015, 114, 903–911. [Google Scholar] [CrossRef]
- Ohashi, H.; Umeda, N.; Hirazawa, N.; Ozaki, Y.; Miura, C.; Miura, T. Expression of vasa (vas)-related genes in germ cells and specific interference with gene functions by double-stranded RNA in the monogenean, Neobenedenia girellae. Int. J. Parasitol. 2007, 37, 515–523. [Google Scholar] [CrossRef]
- Wang, N.; Zhan, J.; Guo, C.; Li, C.; Shen, N.; Gu, X.; Xie, Y.; Peng, X.; Yang, G. Molecular characterisation and functions of Fis1 and PDCD6 genes from Echinococcus granulosus. Int. J. Mol. Sci. 2018, 19, 2669. [Google Scholar] [CrossRef]
- Hu, D.; Song, X.; Xie, Y.; Zhong, X.; Wang, N.; Zheng, Y.; Gu, X.; Wang, T.; Peng, X.; Yang, G. Molecular insights into a tetraspanin in the hydatid tapeworm Echinococcus granulosus. Parasit. Vectors 2015, 8, 311. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Wu, J.; Wang, H.; Guo, B.; Wu, C.; Shou, X.; Yang, N.; Zhang, Z.; McManus, D.P.; et al. Cloning and characterization of an Echinococcus granulosus ecdysteroid hormone nuclear receptor HR3-like gene. Parasite 2017, 24, 36. [Google Scholar] [CrossRef]
- Toya, M.; Takeichi, M. Organization of non-centrosomal microtubules in epithelial cells. Cell Struct. Funct. 2016, 41, 127–135. [Google Scholar] [CrossRef]
- Dalton, J.P.; Skelly, P.; Halton, D.W. Role of the tegument and gut in nutrient uptake by parasitic platyhelminths. Can. J. Zool. 2004, 82, 211–232. [Google Scholar] [CrossRef]
- Ravidà, A.; Cwiklinski, K.; Aldridge, A.M.; Clarke, P.; Thompson, R.; Gerlach, J.Q.; Kilcoyne, M.; Hokke, C.H.; Dalton, J.P.; O’Neill, S.M. Fasciola hepatica surface tegument: Glycoproteins at the interface of parasite and host. Mol. Cell. Proteom. 2016, 15, 3139–3153. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.D.; McManus, D.P. (Eds.) The adult cestode: Special structural features relevant to its physiology. In The Physiology and Biochemistry of Cestodes; Cambridge University Press: New York, NY, USA, 1989; pp. 5–34. [Google Scholar]
- Rohde, K.; Watson, N.A.; Roubal, F.R. Ultrastructure of protonephridial system of Anoplodiscus cirrusspiralis (Monogenea Monopisthocotylea). Int. J. Parasitol. 1992, 22, 443–457. [Google Scholar] [CrossRef]
- Rios-Valencia, D.G.; López-Villegas, E.O.; Diaz Chiguer, D.; Marquez Navarro, A.; Díaz-Martín, R.D.; Nogueda-Torres, B.; Ambrosio, J.R. In Vitro analyses reveal the effect of synthetic cytokinin forchlorfenuron (FCF) on a septin-like protein of Taeniid cysticerci. J. Parasitol. Res. 2019, 2019, 8578936. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Islas, L.E.; Arrangoiz, E.; Vega, E.; Robert, L.; Villanueva, R.; Reynoso-Ducoing, O.; Willms, K.; Zepeda-Rodríguez, A.; Fortoul, T.I.; Ambrosio, J.R. Visualization and 3D reconstruction of flame cells of Taenia solium (cestoda). PLoS ONE 2011, 6, e14754. [Google Scholar] [CrossRef]
- Bray, D. Cell Movements from Molecules to Motility, 2nd ed.; Garland Publishing: New York, NY, USA, 2001; p. 372. [Google Scholar]
- Moreno, M.J.; Casado, N.; Urrea-París, M.A.; Rodriguez-Caabeiro, F. Evidence of tubulin in the scolex gland ducts of Gymnorhynchus gigas plerocercoid (Cestoda: Trypanorhyncha). Folia Parasitol. 2001, 48, 163–164. [Google Scholar] [CrossRef]
- Bahia, D.; Avelar, L.G.; Vigorosi, F.; Cioli, D.; Oliveira, G.C.; Mortara, R.A. The distribution of motor proteins in the muscles and flame cells of the Schistosoma mansoni miracidium and primary sporocyst. Parasitology 2006, 133, 321–329. [Google Scholar] [CrossRef]
- Palomares, F.; Palencia, G.; Ambrosio, J.R.; Ortiz, A.; Jung-Cook, H. Evaluation of the efficacy of albendazole sulphoxide and praziquantel in combination on Taenia crassiceps cysts: In vitro studies. J. Antimicrob. Chemother. 2006, 57, 482–488. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Liu, C.; Huo, L.; Tao, Y.; Zhang, H. Silencing TUBB3 Expression Destroys the Tegument and Flame Cells of Echinococcus multilocularis Protoscoleces. Animals 2022, 12, 2471. https://doi.org/10.3390/ani12182471
Shi Q, Liu C, Huo L, Tao Y, Zhang H. Silencing TUBB3 Expression Destroys the Tegument and Flame Cells of Echinococcus multilocularis Protoscoleces. Animals. 2022; 12(18):2471. https://doi.org/10.3390/ani12182471
Chicago/Turabian StyleShi, Qiqi, Congshan Liu, Lele Huo, Yi Tao, and Haobing Zhang. 2022. "Silencing TUBB3 Expression Destroys the Tegument and Flame Cells of Echinococcus multilocularis Protoscoleces" Animals 12, no. 18: 2471. https://doi.org/10.3390/ani12182471
APA StyleShi, Q., Liu, C., Huo, L., Tao, Y., & Zhang, H. (2022). Silencing TUBB3 Expression Destroys the Tegument and Flame Cells of Echinococcus multilocularis Protoscoleces. Animals, 12(18), 2471. https://doi.org/10.3390/ani12182471




