Oregano Oil Combined with Macleaya Cordata Oral Solution Improves the Growth Performance and Immune Response of Broilers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Oral Solution Preparation
2.3. Animal Experiments and Sample Collection
2.3.1. Birds, Housing, and Experimental Groups
2.3.2. Sample Collection
2.4. Data Recording and Experimental Testing
2.5. Statistical Analysis
3. Results
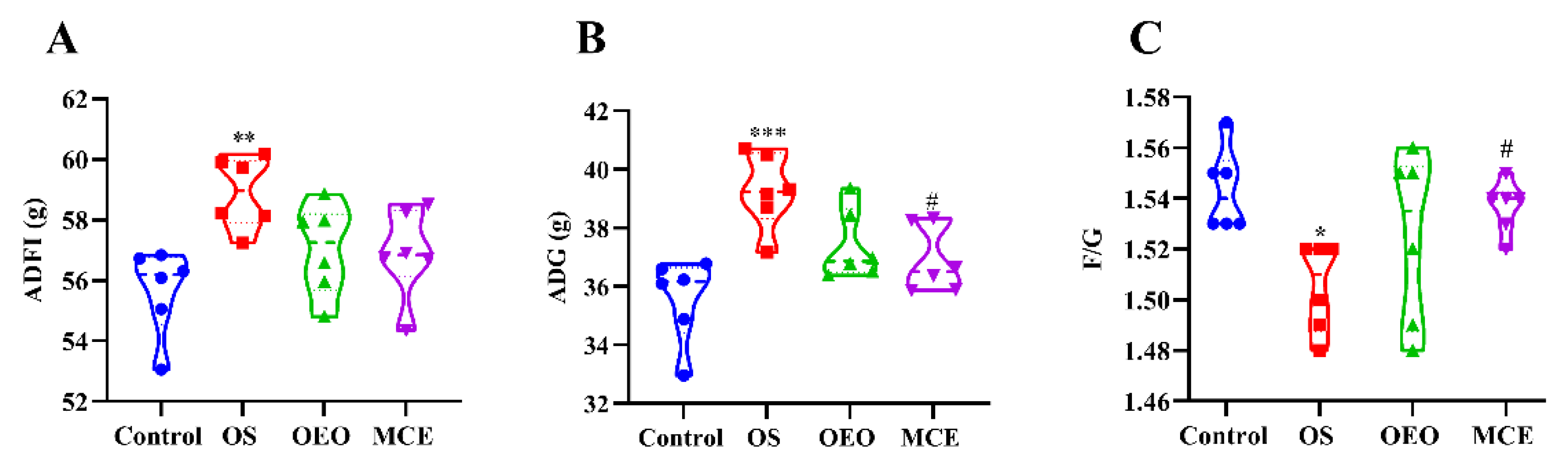
3.1. Evaluation of In Vivo Growth Promotion
3.2. Serum Biochemical Indices
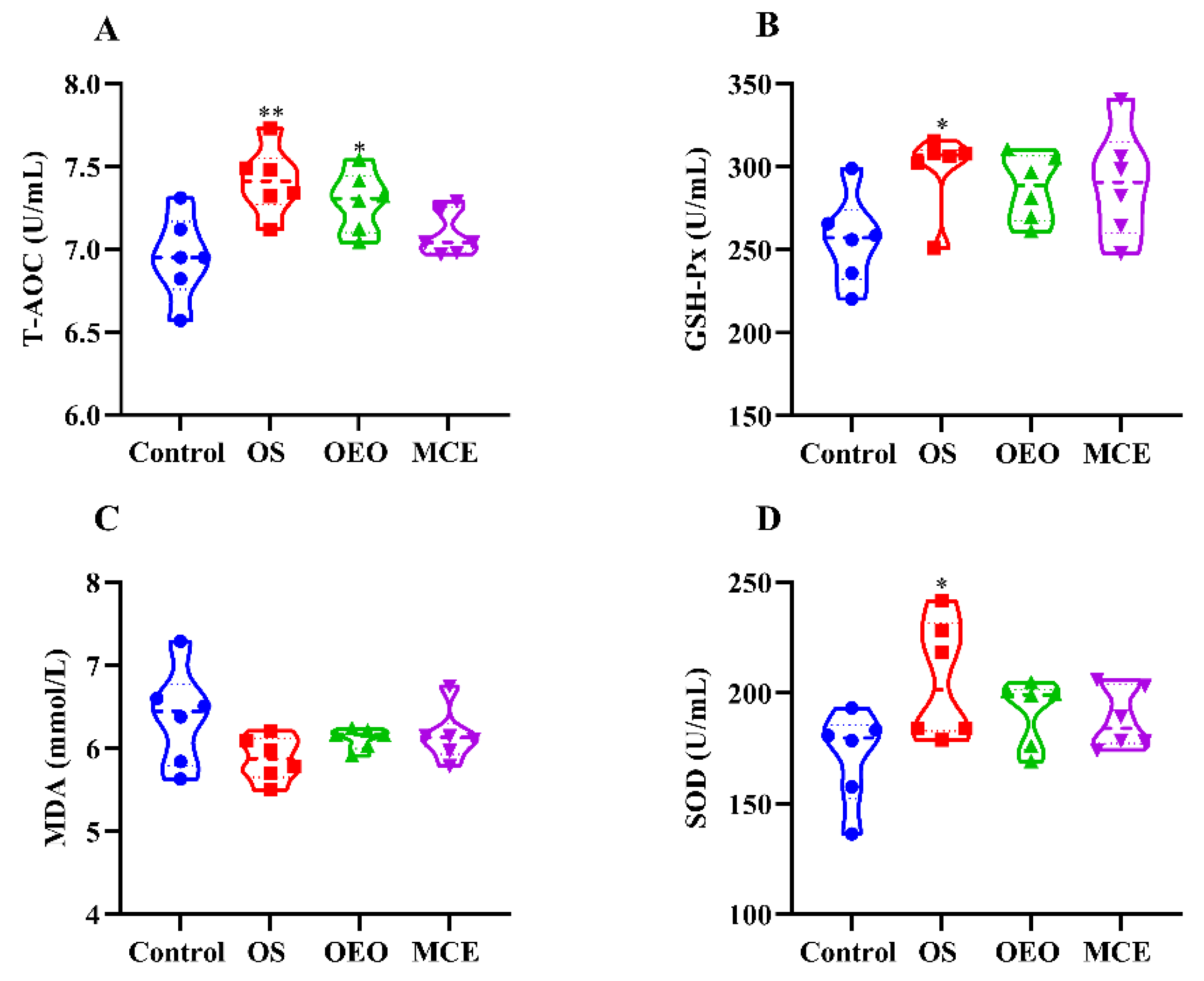
3.3. Serum Antioxidant Indices
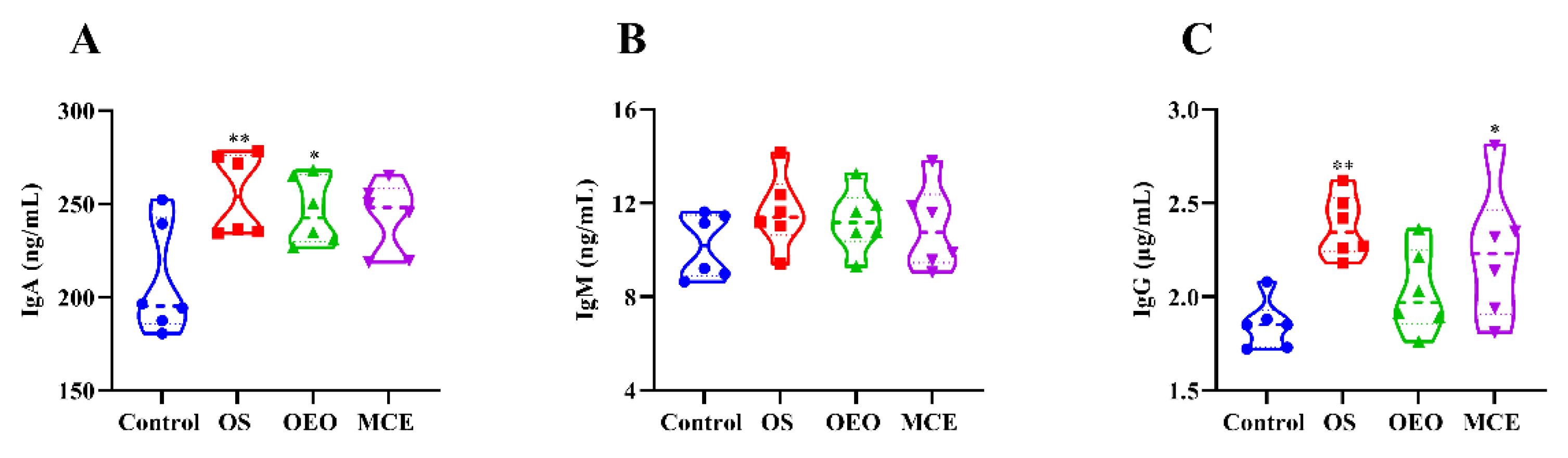
3.4. Serum Immune Indices
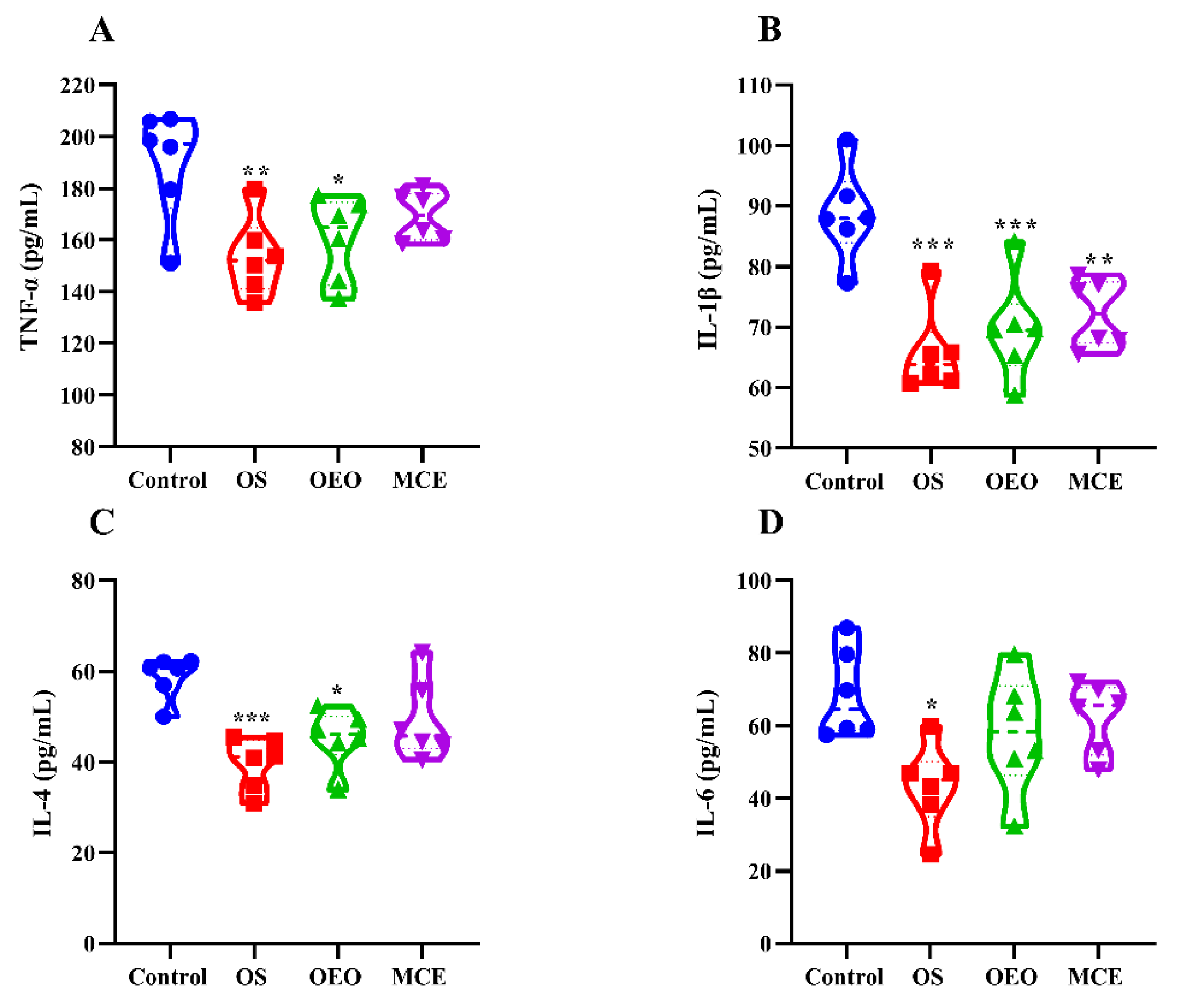
3.5. Serum Inflammatory Cytokines
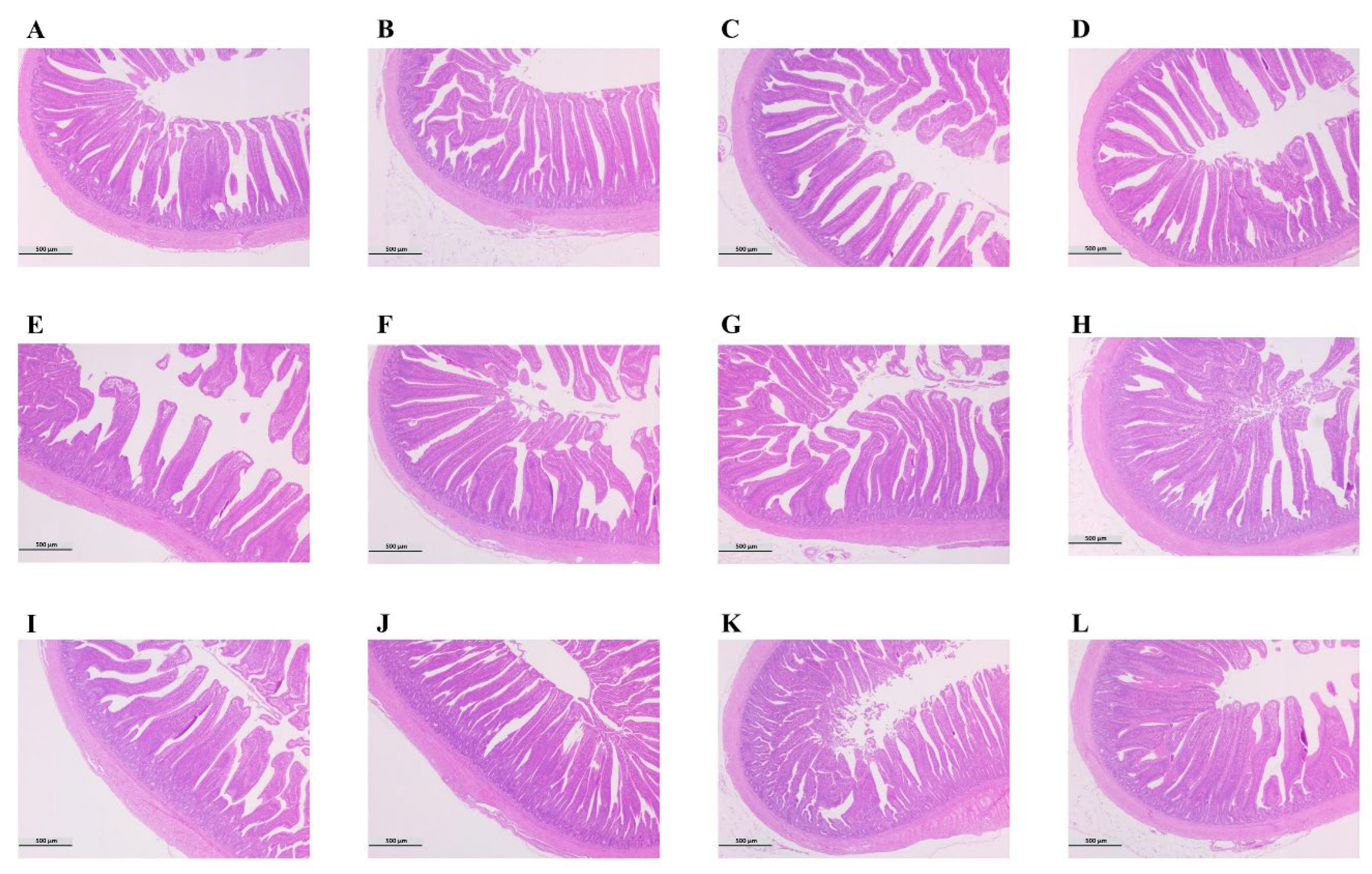
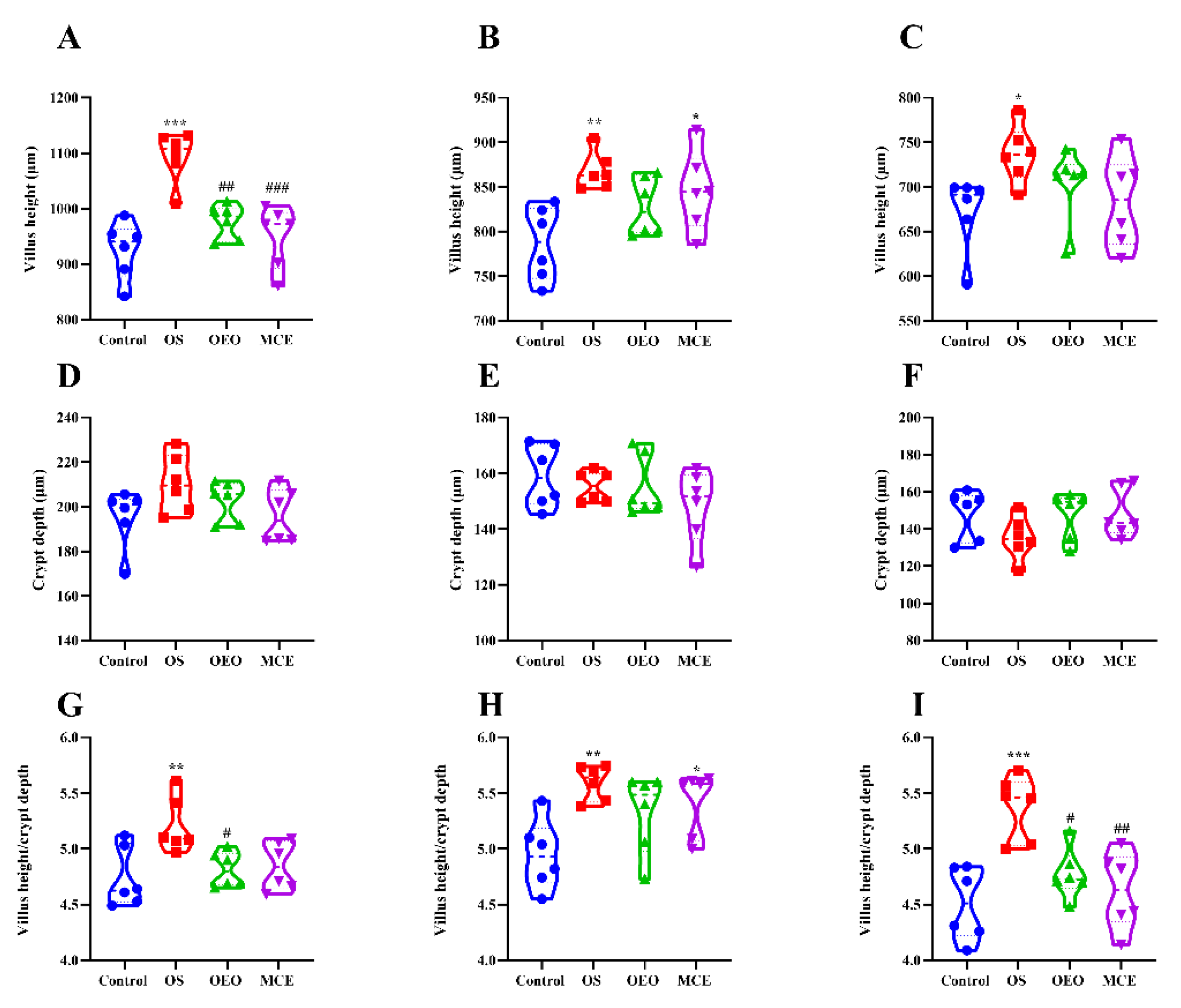
3.6. Changes in the Intestinal Morphology of Broilers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Wang, X.F.; Xing, T.; Li, J.L.; Zhu, X.D.; Zhang, L.; Gao, F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2021, 100, 100909. [Google Scholar] [CrossRef] [PubMed]
- He, C.L.; Fu, B.D.; Shen, H.Q.; Jiang, X.L.; Zhang, C.S.; Wu, S.C.; Zhu, W.; Wei, X.B. Xiang-qi-tang increases avian pathogenic Escherichia coli-induced survival rate and regulates serum levels of tumor necrosis factor alpha, interleukin-1 and soluble endothelial protein C receptor in chicken. Biol. Pharm. Bull. 2011, 34, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hunkapiller, A.A.; Layton, A.C.; Chang, Y.J.; Robbins, K.R. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog. Dis. 2013, 10, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, L.; Wang, Q.; Zeng, H.; Xu, J.; Chen, Z. Antibiotics in aquaculture ponds from Guilin, South of China: Occurrence, distribution, and health risk assessment. Environ. Res. 2022, 204, 112084. [Google Scholar] [CrossRef] [PubMed]
- Suresh, G.; Das, R.K.; Kaur Brar, S.; Rouissi, T.; Avalos Ramirez, A.; Chorfi, Y.; Godbout, S. Alternatives to antibiotics in poultry feed: Molecular perspectives. Crit. Rev. Microbiol. 2018, 44, 318–335. [Google Scholar] [CrossRef]
- Mo, W.Y.; Chen, Z.; Leung, H.M.; Leung, A.O. Application of veterinary antibiotics in China’s aquaculture industry and their potential human health risks. Environ. Sci. Pollut. Res. Int. 2017, 24, 8978–8989. [Google Scholar] [CrossRef]
- Aguilar-Perez, K.M.; Medina, D.I.; Narayanan, J.; Parra-Saldivar, R.; Iqbal, H.M.N. Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities. Molecules 2021, 26, 2880. [Google Scholar] [CrossRef]
- Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Adaszynska-Skwirzynska, M.; Szczerbinska, D. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult. Sci. 2019, 98, 358–365. [Google Scholar] [CrossRef]
- Bauer, B.W.; Radovanovic, A.; Willson, N.L.; Bajagai, Y.S.; Hao Van, T.T.; Moore, R.J.; Stanley, D. Oregano: A potential prophylactic treatment for the intestinal microbiota. Heliyon 2019, 5, e02625. [Google Scholar] [CrossRef]
- Sai, C.; Li, D.; Li, S.; Han, T.; Guo, Y.; Li, Z.; Hua, H. LC-MS guided isolation of three pairs of enantiomeric alkaloids from Macleaya cordata and their enantioseparations, antiproliferative activity, apoptosis-inducing property. Sci. Rep. 2017, 7, 15410. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, N.; Kou, S.; Gao, L.; Peng, B.; Dai, Y.; Zheng, J. Sanguinarine synergistically potentiates aminoglycoside-mediated bacterial killing. Microb. Biotechnol. 2022, 15, 2055–2070. [Google Scholar] [CrossRef]
- Kumari, P.; Choi, H.L. Seasonal variability in airborne biotic contaminants in swine confinement buildings. PLoS ONE 2014, 9, e112897. [Google Scholar] [CrossRef]
- Hamoud, R.; Reichling, J.; Wink, M. Synergistic antimicrobial activity of combinations of sanguinarine and EDTA with vancomycin against multidrug resistant bacteria. Drug Metab. Lett. 2014, 8, 119–128. [Google Scholar] [CrossRef]
- Xue, G.D.; Wu, S.B.; Choct, M.; Pastor, A.; Steiner, T.; Swick, R.A. Impact of a Macleaya cordata-derived alkaloid extract on necrotic enteritis in broilers. Poult. Sci. 2017, 96, 3581–3585. [Google Scholar] [CrossRef]
- Liu, G.; Martínez, Y.; Zhang, L.; Ren, W.; Chen, S.; Guan, G.P.; Xiong, X.; Liao, P.; Li, T.; Huang, R.; et al. Dietary supplementation with sanguinarine enhances serum metabolites and antibodies in growing pigs. J. Anim. Sci. 2016, 94, 75–78. [Google Scholar] [CrossRef][Green Version]
- Li, W.H.; Sun, Y.Y.; Liu, J.X. Preparation of Oregano Oil Macleaya cordata Oral Solution and Its Antibacterial Activity in vitro. J. China Anim. Hus. Vet. Med. 2022, 49, 718–730. [Google Scholar] [CrossRef]
- Dale, N. National Research Council Nutrient Requirements of Poultry—Ninth Revised Edition (1994). J. Appl. Poult. Res. 1994, 3, 101. [Google Scholar] [CrossRef]
- Ruan, D.; Fan, Q.; Fouad, A.M.; Sun, Y.; Huang, S.; Wu, A.; Lin, C.; Kuang, Z.; Zhang, C.; Jiang, S. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity, and intestinal microbiota in yellow-feathered chickens. J. Anim. Sci. 2021, 99, skab033. [Google Scholar] [CrossRef]
- Amer, S.A.; Tolba, S.A.; AlSadek, D.M.M.; Abdel Fattah, D.M.; Hassan, A.M.; Metwally, A.E. Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Vet. Res. 2021, 17, 312. [Google Scholar] [CrossRef]
- Kang, P.; Xiao, H.L.; Hou, Y.Q.; Ding, B.Y.; Liu, Y.L.; Zhu, H.L.; Hu, Q.Z.; Hu, Y.; Yin, Y.L. Effects of Astragalus Polysaccharides, Achyranthes bidentata Polysaccharides, and Acantbepanax senticosus Saponin on the Performance and Immunity in Weaned Pigs. Asian Austral. J. Anim. Sci. 2010, 23, 750–756. [Google Scholar] [CrossRef]
- Lee, K.-W.; Kim, J.-S.; Oh, S.-T.; Kang, C.-W.; An, B.-K. Effects of Dietary Sanguinarine on Growth Performance, Relative Organ Weight, Cecal Microflora, Serum Cholesterol Level and Meat Quality in Broiler Chickens. J. Poult. Sci. 2015, 52, 15–22. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, X.L.; Ou, S.Q.; Hou, D.X.; He, J.H. Sanguinarine modulate gut microbiome and intestinal morphology to enhance growth performance in broilers. PLoS ONE 2020, 15, e0234920. [Google Scholar] [CrossRef]
- Vieira, S.L.; Oyarzabal, O.A.; Freitas, D.M.; Berres, J.; Peña, J.E.M.; Torres, C.A.; Coneglian, J.L.B. Performance of Broilers Fed Diets Supplemented with Sanguinarine-Like Alkaloids and Organic Acids. J. Appl. Poult. Res. 2008, 17, 128–133. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S.; Zhang, J.; Song, Y.; Bao, X.; Xu, F.; Zhang, J. Analysis of Serum Biochemical Indexes, Egg Quality, and Liver Transcriptome in Laying Hens Fed Diets Supplemented with Gynostemma pentaphyllum Powder. Genes 2021, 12, 1942. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Zhao, Y.; Tao, L.; Liu, H.; Dong, W.; Yang, G.; Li, L. Effects of dietary supplementation with itaconic acid on the growth performance, nutrient digestibility, slaughter variables, blood biochemical parameters, and intestinal morphology of broiler chickens. Poult. Sci. 2022, 101, 101732. [Google Scholar] [CrossRef]
- Fathi, M.; Tanha, T.; Saeedyan, S. Influence of dietary lycopene on growth performance, antioxidant status, blood parameters and mortality in broiler chicken with cold-induced ascites. Arch. Anim. Nutr. 2022, 76, 50–60. [Google Scholar] [CrossRef]
- Dang, X.; Zhou, H.; Lou, Y.; Li, D. Effects of in ovo feeding of disaccharide and/or methionine on hatchability, growth performance, blood hematology, and serum antioxidant parameters in geese. J. Anim. Sci. 2022, 100, skac014. [Google Scholar] [CrossRef]
- Yang, C.; Tang, X.W.; Liu, X.; Yang, H.; Bin, D.M.; Liu, H.J.; Tang, Q.H.; Tang, J.Y. Effects of dietary oligosaccharides on serum biochemical index, intestinal morphology, and antioxidant status in broilers. Anim. Sci. J. 2022, 93, e13679. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, C.H. Effects of dietary supplementation with rice bran oil on the growth performance, blood parameters, and immune response of broiler chickens. J. Anim. Sci. Technol. 2016, 58, 12. [Google Scholar] [CrossRef]
- Qiu, K.; Li, C.L.; Wang, J.; Qi, G.H.; Gao, J.; Zhang, H.J.; Wu, S.G. Effects of Dietary Supplementation With Bacillus subtilis, as an Alternative to Antibiotics, on Growth Performance, Serum Immunity, and Intestinal Health in Broiler Chickens. Front. Nutr. 2021, 8, 786878. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lei, J.; Liu, L.; Qu, X.; Li, P.; Liu, X.; Guo, Y.; Gao, Q.; Lan, F.; Xiao, B.; et al. Effects of Macleaya cordata extract on laying performance, egg quality, and serum indices in Xuefeng black-bone chicken. Poult. Sci. 2021, 100, 101031. [Google Scholar] [CrossRef] [PubMed]
- van Dullemen, H.M.; van Deventer, S.J.; Hommes, D.W.; Bijl, H.A.; Jansen, J.; Tytgat, G.N.; Woody, J. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 1995, 109, 129–135. [Google Scholar] [CrossRef]
- Webel, D.M.; Finck, B.N.; Baker, D.H.; Johnson, R.W. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 1997, 75, 1514–1520. [Google Scholar] [CrossRef]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Scholmerich, J.; Gross, V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]
- Liu, W.C.; Ou, B.H.; Liang, Z.L.; Zhang, R.; Zhao, Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-kappaB signaling pathway in broilers. Poult. Sci. 2021, 100, 101139. [Google Scholar] [CrossRef]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef]
- Khadem, A.; Soler, L.; Everaert, N.; Niewold, T.A. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory properties. Br. J. Nutr. 2014, 112, 1110–1118. [Google Scholar] [CrossRef]
- Chen, J.; Kang, B.; Zhao, Y.; Yao, K.; Fu, C. Effects of natural dietary supplementation with Macleaya cordata extract containing sanguinarine on growth performance and gut health of early-weaned piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1666–1674. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Yin, Q.Q.; Yang, Y.R. Effect of soybean peptides on growth performance, intestinal structure and mucosal immunity of broilers. J. Anim. Physiol. Anim. Nutr. 2009, 93, 754–760. [Google Scholar] [CrossRef]
- Wakita, Y.; Saiki, A.; Kaneda, H.; Segawa, S.; Tsuchiya, Y.; Kameya, H.; Okamoto, S. Analysis of free radical production capacity in mouse faeces and its possible application in evaluating the intestinal environment: A pilot study. Sci. Rep. 2019, 9, 19533. [Google Scholar] [CrossRef]
- Jang, I.S.; Ko, Y.H.; Kang, S.Y.; Lee, C.Y. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 2007, 134, 304–315. [Google Scholar] [CrossRef]
- Yang, Y.; Iji, P.A.; Choct, M.J.W.s.P.S.J. Dietary modulation of gut microflora in broiler chickens: A review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult. Sci. J. 2009, 65, 114–197. [Google Scholar] [CrossRef]
- Kolluri, G.; Marappan, G.; Yadav, A.S.; Kumar, A.; Mariappan, A.K.; Tyagi, J.S.; Rokade, J.J.; Govinthasamy, P. Effects of Spirulina (Arthrospira platensis) as a drinking water supplement during cyclical chronic heat stress on broiler chickens: Assessing algal composition, production, stress, health and immune-biochemical indices. J. Therm. Biol. 2022, 103, 103100. [Google Scholar] [CrossRef]


| Ingredients | % | Nutrient Levels | % 3 |
|---|---|---|---|
| Corn | 60.00 | Metabolic energy, MJ/kg | 13.25 |
| Soybean meal | 28.40 | Crude protein | 21.00 |
| Cottonseed meal | 7.15 | Crude fiber | 7.00 |
| Limestone | 1.80 | Crude ash | 8.00 |
| CaHPO4 | 1.60 | Ca | 1.00 |
| NaCl | 0.35 | Total phosphorus | 0.45 |
| Lysine | 0.35 | Lysine | 0.95 |
| Vitamin premix 1 | 0.20 | ||
| Trace element premix 2 | 0.15 | ||
| Total | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, W.; Chen, L.; Chen, Z.; Wang, X.; Xu, Q.; Zhang, H.; Chen, H.; Liu, J. Oregano Oil Combined with Macleaya Cordata Oral Solution Improves the Growth Performance and Immune Response of Broilers. Animals 2022, 12, 2480. https://doi.org/10.3390/ani12182480
Zhang C, Li W, Chen L, Chen Z, Wang X, Xu Q, Zhang H, Chen H, Liu J. Oregano Oil Combined with Macleaya Cordata Oral Solution Improves the Growth Performance and Immune Response of Broilers. Animals. 2022; 12(18):2480. https://doi.org/10.3390/ani12182480
Chicago/Turabian StyleZhang, Cheng, Weihao Li, Ligong Chen, Zhaoliang Chen, Xuejing Wang, Qianqian Xu, Hailong Zhang, Huan Chen, and Juxiang Liu. 2022. "Oregano Oil Combined with Macleaya Cordata Oral Solution Improves the Growth Performance and Immune Response of Broilers" Animals 12, no. 18: 2480. https://doi.org/10.3390/ani12182480
APA StyleZhang, C., Li, W., Chen, L., Chen, Z., Wang, X., Xu, Q., Zhang, H., Chen, H., & Liu, J. (2022). Oregano Oil Combined with Macleaya Cordata Oral Solution Improves the Growth Performance and Immune Response of Broilers. Animals, 12(18), 2480. https://doi.org/10.3390/ani12182480







