Basal Reactivity Evaluated by Infrared Thermography in the “Caballo de Deporte Español” Horse Breed According to Its Coat Color
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Physiological Data
2.4. Statistical and Genetic Analyses
2.5. Ethic Statement
3. Results
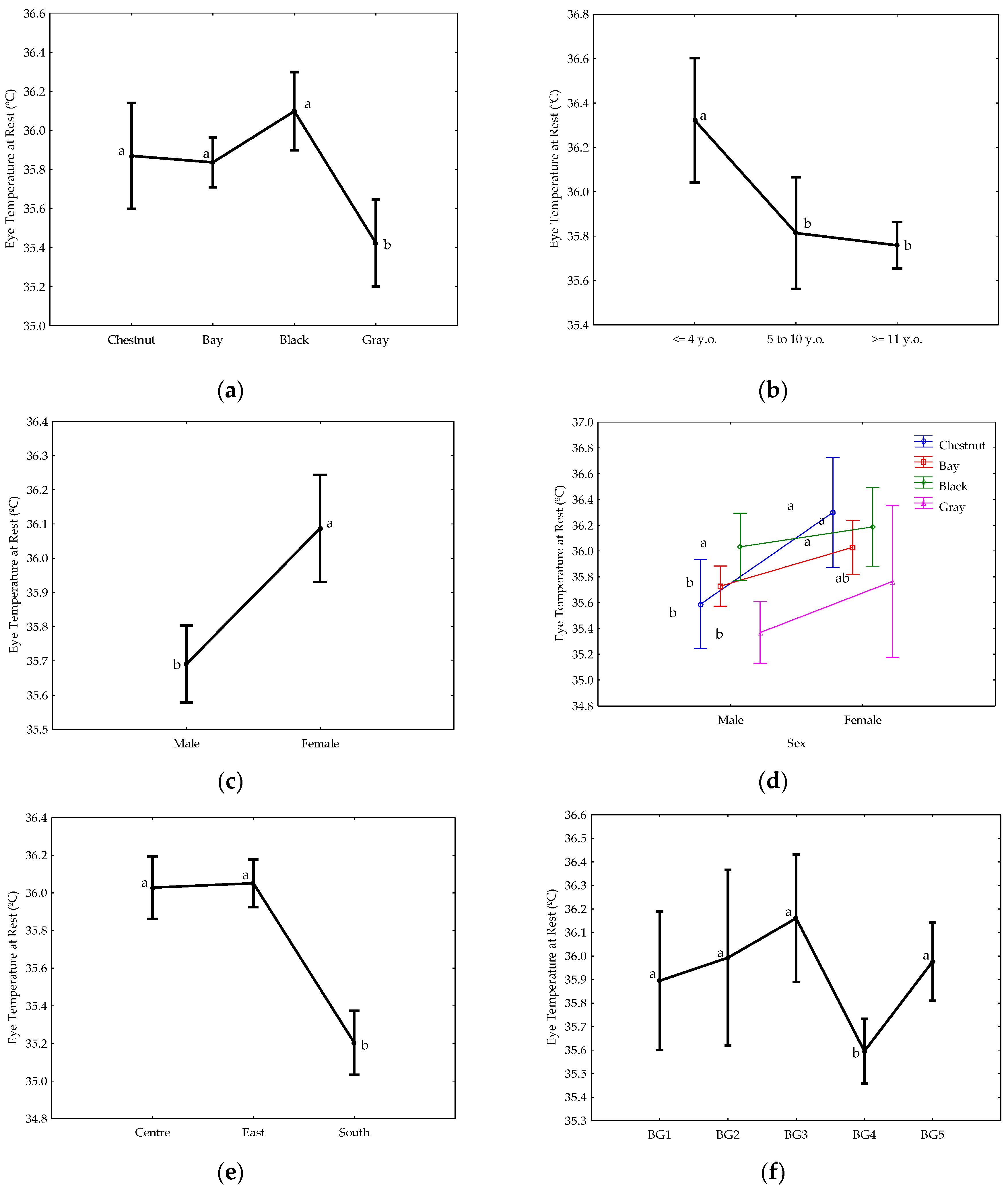
3.1. Statistical Analyses
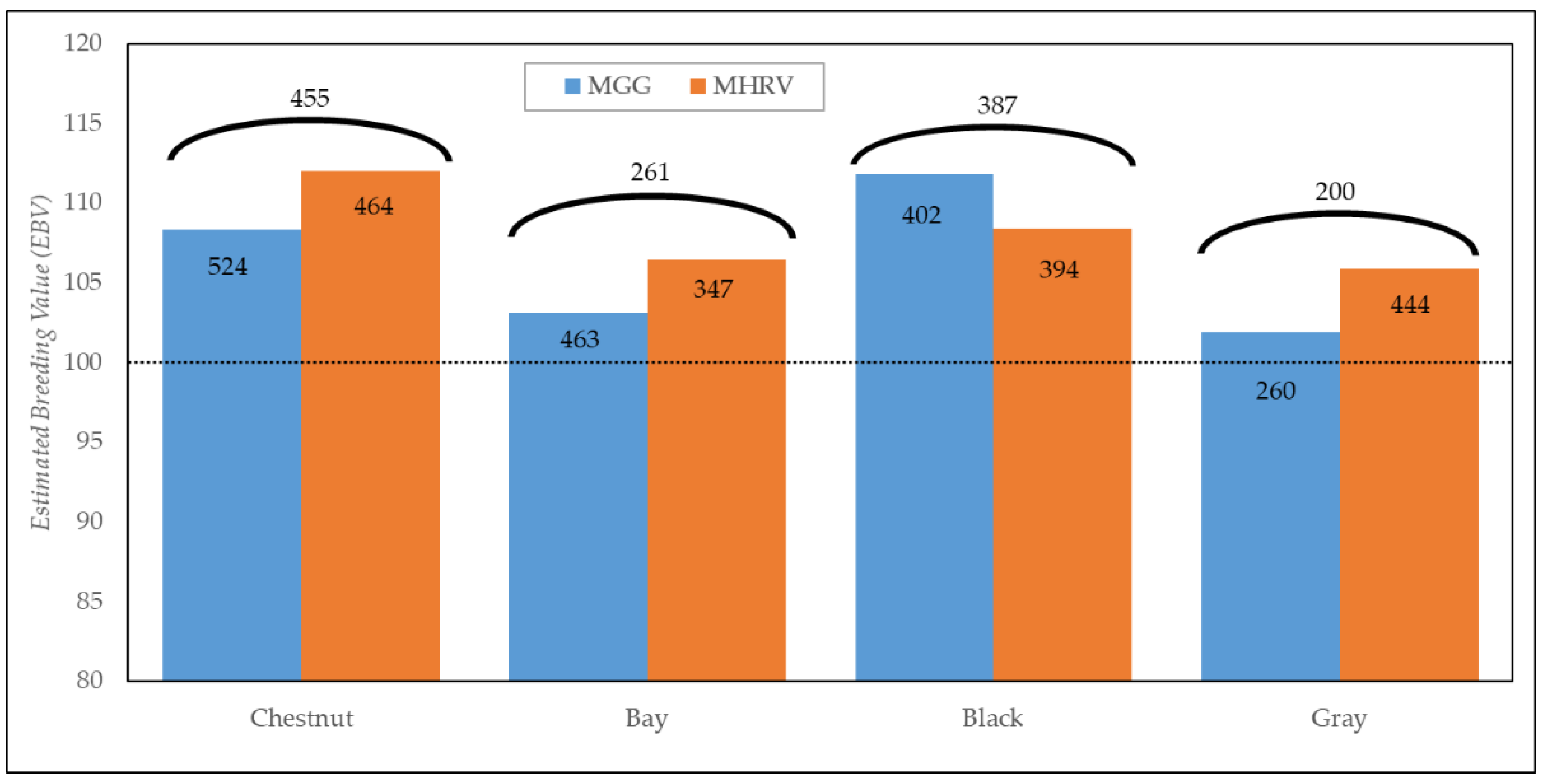
3.2. Genetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wutke, S.; Benecke, N.; Sandoval-Castellanos, E.; Döhle, H.-J.; Friederich, S.; Gonzalez, J.; Hallsson, J.H.; Hofreiter, M.; Lõugas, L.; Magnell, O.; et al. Spotted phenotypes in horses lost attractiveness in the Middle Ages. Sci. Rep. 2016, 6, 38548. [Google Scholar] [CrossRef]
- Ludwig, A.; Reissmann, M.; Benecke, N.; Bellone, R.; Sandoval-Castellanos, E.; Cieslak, M.; Fortes, G.G.; Morales-Muñiz, A.; Hofreiter, M.; Pruvost, M. Twenty-five thousand years of fluctuating selection on leopard complex spotting and congenital night blindness in horses. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130386. [Google Scholar] [CrossRef]
- Ludwig, A.; Pruvost, M.; Reissmann, M.; Benecke, N.; Brockmann, G.A.; Castaños, P.; Cieslak, M.; Lippold, S.; Llorente, L.; Malaspinas, A.-S.; et al. Coat Color Variation at the Beginning of Horse Domestication. Science 2009, 324, 485. [Google Scholar] [CrossRef]
- Pruvost, M.; Bellone, R.; Benecke, N.; Sandoval-Castellanos, E.; Cieslak, M.; Kuznetsova, T.; Morales-Muñiz, A.; O’Connor, T.; Reissmann, M.; Hofreiter, M.; et al. Genotypes of predomestic horses match phenotypes painted in Paleolithic works of cave art. Proc. Natl. Acad. Sci. USA 2011, 108, 18626–18630. [Google Scholar] [CrossRef]
- Sánchez-Guerrero, M.J.; Solé, M.; Azor, P.J.; Sölkner, J.; Valera, M. Genetic and environmental risk factors for vitiligo and melanoma in Pura Raza Español horses. Equine Vet.- J. 2019, 51, 606–611. [Google Scholar] [CrossRef]
- Sundström, E.; Imsland, F.; Mikko, S.; Wade, C.; Sigurdsson, S.; Pielberg, G.R.; Golovko, A.; Curik, I.; Seltenhammer, M.H.; Sölkner, J.; et al. Copy number expansion of the STX17 duplication in melanoma tissue from Grey horses. BMC Genom. 2012, 13, 365. [Google Scholar] [CrossRef]
- Marklund, S.; Moller, M.; Sandberg, K.; Andersson, L. Close association between sequence polymorphism in the KIT gene and the roan coat color in horses. Mamm. Genome 1999, 10, 283–288. [Google Scholar] [CrossRef]
- Hintz, H.F.; Van Vleck, L.D. Lethal dominant roan in horses. J. Hered. 1979, 70, 145–146. [Google Scholar] [CrossRef]
- Grahn, B.H.; Pinard, C.; Archer, S.; Bellone, R.; Forsyth, G.; Sandmeyer, L.S. Congenital ocular anomalies in purebred and crossbred Rocky and Kentucky Mountain horses in Canada. Can. Vet.- J. La Rev. Vet.- Can. 2008, 49, 675–681. [Google Scholar]
- Andersson, L.S.; Axelsson, J.; Dubielzig, R.R.; Lindgren, G.; Ekesten, B. Multiple congenital ocular anomalies in Icelandic horses. BMC Vet.-Res. 2011, 7, 21. [Google Scholar] [CrossRef]
- Ripollés-Lobo, M.; Perdomo-González, D.; Sánchez-Guerrero, M.; Bartolomé, E.; Valera, M. Genetic relationship between free movement and under rider gaits in young Pura Raza Española horses. Livest. Sci. 2022, 263, 105031. [Google Scholar] [CrossRef]
- Stachurska, A.; Pięta, M.; Łojek, J.; Szulowska, J. Performance in racehorses of various colours. Livest. Sci. 2007, 106, 282–286. [Google Scholar] [CrossRef]
- de Mira, M.C.; Lamy, E.; Santos, R.; Williams, J.; Pinto, M.V.; Martins, P.S.; Rodrigues, P.; Marlin, D. Salivary cortisol and eye temperature changes during endurance competitions. BMC Vet.-Res. 2021, 17, 1–12. [Google Scholar] [CrossRef]
- Jacobs, L.N.; Staiger, E.A.; Albright, J.D.; Brooks, S.A. The MC1R and ASIP Coat Color Loci May Impact Behavior in the Horse. J. Hered. 2016, 107, 214–219. [Google Scholar] [CrossRef]
- Brunberg, E.; Gille, S.; Mikko, S.; Lindgren, G.; Keeling, L.J. Icelandic horses with the Silver coat colour show altered behaviour in a fear reaction test. Appl. Anim. Behav. Sci. 2013, 146, 72–78. [Google Scholar] [CrossRef]
- Abdel-Azeem, N.M.; Emeash, H.H. Relationship of horse temperament with breed, age, sex, and body characteristics: A questionnaire-based study. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 1–6. [Google Scholar] [CrossRef]
- Masko, M.; Domino, M.; Lewczuk, D.; Jasinski, T.; Gajewski, Z. Horse Behavior, Physiology and Emotions during Habituation to a Treadmill. Animals 2020, 10, 921. [Google Scholar] [CrossRef]
- Finn, J.L.; Haase, B.; Willet, C.; van Rooy, D.; Chew, T.; Wade, C.M.; Hamilton, N.A.; Velie, B.D. The relationship between coat colour phenotype and equine behaviour: A pilot study. Appl. Anim. Behav. Sci. 2016, 174, 66–69. [Google Scholar] [CrossRef]
- Wickens, C.; Brooks, S.A. Genetics of Equine Behavioral Traits. Vet.-Clin. Equine Pract. 2020, 36, 411–424. [Google Scholar] [CrossRef]
- Topczewska, J.; Krupa, W.; Sokołowicz, Z.; Lechowska, J. Does Experience Make Hucul Horses More Resistant to Stress? A Pilot Study. Animals 2021, 11, 3345. [Google Scholar] [CrossRef]
- Bukhari, S.; McElligott, A.; Parkes, R. Quantifying the Impact of Mounted Load Carrying on Equids: A Review. Animals 2021, 11, 1333. [Google Scholar] [CrossRef] [PubMed]
- Safryghin, A.; Hebesberger, D.V.; Wascher, C.A.F. Testing for Behavioral and Physiological Responses of Domestic Horses (Equus caballus) Across Different Contexts—Consistency Over Time and Effects of Context. Front. Psychol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.L. Physiology of equine performance and associated tests of function. Equine Vet.-J. 2007, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Bitschnau, C.; Wiestner, T.; Trachsel, D.S.; Auer, J.A.; Weishaupt, M.A. Performance parameters and post exercise heart rate recovery in Warmblood sports horses of different performance levels. Equine Vet.-J. 2010, 42, 17–22. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Grzędzicka, J.; Seń, J.; Czopowicz, M.; Żmigrodzka, M.; Winnicka, A.; Cywińska, A.; Carter, C. Stress response after race and endurance training sessions and competitions in Arabian horses. Prev. Vet.-Med. 2021, 188, 105265. [Google Scholar] [CrossRef]
- Gardela, J.; Carbajal, A.; Tallo-Parra, O.; Olvera-Maneu, S.; Álvarez-Rodríguez, M.; Jose-Cunilleras, E.; López-Béjar, M. Temporary Relocation during Rest Periods: Relocation Stress and Other Factors Influence Hair Cortisol Concentrations in Horses. Animals 2020, 10, 642. [Google Scholar] [CrossRef]
- Janczarek, I.; Bereznowski, A.; Strzelec, K. The influence of selected factors and sport results of endurance horses on their saliva cortisol concentration. Pol. J. Vet.-Sci. 2013, 16, 533–541. [Google Scholar] [CrossRef]
- Kędzierski, W.; Cywińska, A.; Strzelec, K.; Kowalik, S. Changes in salivary and plasma cortisol levels in Purebred Arabian horses during race training session. Anim. Sci. J. 2013, 85, 313–317. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Pingwara, R.; Winnicka, A. The Effect of Physical Training on Peripheral Blood Mononuclear Cell Ex Vivo Proliferation, Differentiation, Activity, and Reactive Oxygen Species Production in Racehorses. Antioxidants 2020, 9, 1155. [Google Scholar] [CrossRef]
- Cappelli, K.; Mecocci, S.; Gioiosa, S.; Giontella, A.; Silvestrelli, M.; Cherchi, R.; Valentini, A.; Chillemi, G.; Capomaccio, S. Gallop Racing Shifts Mature mRNA towards Introns: Does Exercise-Induced Stress Enhance Genome Plasticity? Genes 2020, 11, 410. [Google Scholar] [CrossRef]
- Kim, W.-S.; Nejad, J.G.; Roh, S.-G.; Lee, H.-G. Heat-Shock Proteins Gene Expression in Peripheral Blood Mononuclear Cells as an Indicator of Heat Stress in Beef Calves. Animals 2020, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.J.; Schaefer, A.L.; Warren, L.; Burwash, L.; Anderson, M.; Baron, V. Adrenocortical and metabolic responses to ACTH injection in horses: An assessment by salivary cortisol and infrared thermography of the eye. Can. J. Anim. Sci. 2001, 81, 621. [Google Scholar]
- Valera, M.; Bartolomé, E.; Sánchez, M.J.; Molina, A.; Cook, N.; Schaefer, A. Changes in Eye Temperature and Stress Assessment in Horses During Show Jumping Competitions. J. Equine Vet.-Sci. 2012, 32, 827–830. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Maśko, M.; Domino, M.; Winnicka, A. Infrared Thermography Correlates with Lactate Concentration in Blood during Race Training in Horses. Animals 2020, 10, 2072. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.; Verkerk, G.; Schaefer, A.; Colyn, J.; Stafford, K. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef]
- Bartolomé, E.; Sánchez, M.J.; Molina, A.; Schaefer, A.L.; Cervantes, I.; Valera, M. Using eye temperature and heart rate for stress assessment in young horses competing in jumping competitions and its possible influence on sport performance. Animal 2013, 7, 2044–2053. [Google Scholar] [CrossRef]
- Bartolomé, E.; Perdomo-González, D.; Sánchez-Guerrero, M.; Valera, M. Genetic Parameters of Effort and Recovery in Sport Horses Assessed with Infrared Thermography. Animals 2021, 11, 832. [Google Scholar] [CrossRef]
- Sánchez, M.J.; Bartolomé, E.; Valera, M. Genetic study of stress assessed with infrared thermography during dressage competitions in the Pura Raza Español horse. Appl. Anim. Behav. Sci. 2016, 174, 58–65. [Google Scholar] [CrossRef]
- Bartolomé, E.; Cervantes, I.; Valera, M.; Gutiérrez, J. Influence of foreign breeds on the genetic structure of the Spanish Sport Horse population. Livest. Sci. 2011, 142, 70–79. [Google Scholar] [CrossRef]
- Bartolomé, E.; Valera, M.; Fernández, J.; Rodríguez-Ramilo, S.T. Effects of Selection on Breed Contribution in the Caballo de Deporte Español. Animals 2022, 12, 1635. [Google Scholar] [CrossRef]
- Bowling, A.T.; Ruvinsky, A. Genetics of colour variation. In The Genetics of the Horse; Bowling, A.T., Ed.; CABI Publishing, CAB International: Wallingford, UK, 2000; pp. 53–70. ISBN 0851994296. [Google Scholar]
- Misztal, I.; Tsuruta, S.; Lourenco, D.; Aguilar, I.; Legarra, A. Manual for BLUPF90 Family of Programs; Athen University: Athen, GA, USA, 2014. [Google Scholar]
- Plummer, M.; Best, N.; Cowles, K.; Vines, K. CODA: Convergence diagnosis and output analysis for MCMC. R News 2006, 6, 7–11. [Google Scholar]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 1–21. ISBN 0-85199-359-1. [Google Scholar]
- Lloyd, A.S.; Martin, J.E.; Gauci, H.; Wilkinson, R.G. Horse personality: Variation between breeds. Appl. Anim. Behav. Sci. 2008, 112, 369–383. [Google Scholar] [CrossRef]
- Naccache, F.; Metzger, J.; Distl, O. Genetic risk factors for osteochondrosis in various horse breeds. Equine Vet.-J. 2018, 50, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pielberg, G.R.; Golovko, A.; Sundström, E.; Curik, I.; Lennartsson, J.; Seltenhammer, M.H.; Druml, T.; Binns, M.; Fitzsimmons, C.; Lindgren, G.; et al. A cis-acting regulatory mutation causes premature hair graying and susceptibility to melanoma in the horse. Nat. Genet. 2008, 40, 1004–1009. [Google Scholar] [CrossRef]
- Perdomo-González, D.I.; Molina, A.; Sánchez-Guerrero, M.J.; Bartolomé, E.; Varona, L.; Valera, M. Genetic inbreeding depression load for fertility traits in Pura Raza Española mares. J. Anim. Sci. 2021, 99, skab316. [Google Scholar] [CrossRef]
- Bartolomé, E.; Goyache, F.; Molina, A.; Cervantes, I.; Valera, M.; Gutiérrez, J. Pedigree estimation of the (sub) population contribution to the total gene diversity: The horse coat colour case. Animal 2010, 4, 867–875. [Google Scholar] [CrossRef]
- Pérez-Guisado, J.; Lopez-Rodríguez, R.; Muñoz-Serrano, A. Heritability of dominant–aggressive behaviour in English Cocker Spaniels. Appl. Anim. Behav. Sci. 2006, 100, 219–227. [Google Scholar] [CrossRef]
- Reissmann, M.; Ludwig, A. Pleiotropic effects of coat colour-associated mutations in humans, mice and other mammals. Semin. Cell Dev. Biol. 2013, 24, 576–586. [Google Scholar] [CrossRef]
- Sánchez-Martín, M.; Rodríguez-García, A.; Pérez-Losada, J.; Sagrera, A.; Read, A.P.; Sánchez-García, I. SLUG (SNAI2) deletions in patients with Waardenburg disease. Hum. Mol. Genet. 2002, 11, 3231–3236. [Google Scholar] [CrossRef]
- Pérez-Losada, J.; Sanchez-Martin, M.; Rodrı́guez-Garcı́a, A.; Sánchez, M.L.; Orfao, A.; Flores, T.; Sanchez-Garcia, I. Zinc-finger transcription factor Slug contributes to the function of the stem cell factor c-kit signaling pathway. Blood 2002, 100, 1274–1286. [Google Scholar] [CrossRef]
- Robinson, K.C.; Kemény, L.V.; Fell, G.L.; Hermann, A.L.; Allouche, J.; Ding, W.; Yekkirala, A.; Hsiao, J.J.; Su, M.Y.; Theodosakis, N.; et al. Reduced MC4R signaling alters nociceptive thresholds associated with red hair. Sci. Adv. 2021, 7, eabd1310. [Google Scholar] [CrossRef] [PubMed]
- Bacon, E.K.; Donnelly, C.G.; Bellone, R.R.; Finno, C.J.; Velie, B.D. Melanocortin-1 receptor influence in equine opioid sensitivity. Equine Vet.- Educ. 2022, 1–11. [Google Scholar] [CrossRef]
- Burattini, B.; Fenner, K.; Anzulewicz, A.; Romness, N.; McKenzie, J.; Wilson, B.; McGreevy, P. Age-Related Changes in the Behaviour of Domestic Horses as Reported by Owners. Animals 2020, 10, 2321. [Google Scholar] [CrossRef] [PubMed]
- Aune, A.; Fenner, K.; Wilson, B.; Cameron, E.; McLean, A.; McGreevy, P. Reported Behavioural Differences between Geldings and Mares Challenge Sex-Driven Stereotypes in Ridden Equine Behaviour. Animals 2020, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Keeler, C.; Ridgway, S.; Lipscomb, L.; Fromm, E. The Genetics of Adrenal Size and Tameness in Colorphase Foxes. J. Hered. 1968, 59, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.S.; Zhou, J.; Shi, M.; Redmann, S., Jr.; Mynatt, R.L.; Ryan, D.H. Overexpression of agouti protein and stress re-sponsiveness in mice. Physiol. Behav. 2001, 73, 599–608. [Google Scholar] [CrossRef]
- Corbin, L.J.; Pope, J.; Sanson, J.; Antczak, D.F.; Miller, D.; Sadeghi, R.; Brooks, S.A. An Independent Locus Upstream of ASIP Controls Variation in the Shade of the Bay Coat Colour in Horses. Genes 2020, 11, 606. [Google Scholar] [CrossRef]
- Solé, M.; Valera, M.; Fernández, J. Genetic structure and connectivity analysis in a large domestic livestock meta-population: The case of the Pura Raza Español horses. J. Anim. Breed. Genet. 2018, 135, 460–471. [Google Scholar] [CrossRef]
- Negro, S.; Guerrero, M.J.S.; Bartolomé, E.; Solé, M.; Gomez, M.D.; Membrillo, A.; Molina, A.; Valera, M. Evidence for the effect of serotoninergic and dopaminergic gene variants on stress levels in horses participating in dressage and harness racing. Anim. Prod. Sci. 2019, 59, 2206. [Google Scholar] [CrossRef]
- Hill, W.G.; Zhang, X.-S. Effects on phenotypic variability of directional selection arising through genetic differences in residual variability. Genet. Res. 2004, 83, 121–132. [Google Scholar] [CrossRef]
- SanCristobal-Gaudy, M.; Elsen, J.-M.; Bodin, L.; Chevalet, C. Prediction of the response to a selection for canalisation of a continuous trait in animal breeding. Genet. Sel. Evol. 1998, 30, 1–29. [Google Scholar] [CrossRef]
- Hill, W.G. Direct effect of selection on phenotypic variability of quantitative traits. In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpelier, France, 19–23 August 2002; pp. 19–02. [Google Scholar]
- SanCristobal-Gaudy, M.; Bodin, L.; Elsen, J.-M.; Chevalet, C. Genetic components of litter size variability in sheep. Genet. Sel. Evol. 2001, 33, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Viveros, J. Parámetros genéticos en la varianza residual de variables de comportamiento en toros de lidia. Arch. Zootec. 2020, 69, 354–358. [Google Scholar] [CrossRef]
- Mulder, H.A.; Hill, W.G.; Vereijken, A.; Veerkamp, R.F. Estimation of genetic variation in residual variance in female and male broiler chickens. Animal 2009, 3, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Fina, M.; Ibáñez-Escriche, N.; Piedrafita, J.; Casellas, J. Canalization analysis of birth weight in Bruna de los Pirineus beef cattle. J. Anim. Sci. 2013, 91, 3070–3078. [Google Scholar] [CrossRef]
- Solé, M.; Bartolomé, E.; Sánchez, M.J.; Molina, A.; Valera, M. Predictability of adult Show Jumping ability from early information: Alternative selection strategies in the Spanish Sport Horse population. Livest. Sci. 2017, 200, 23–28. [Google Scholar] [CrossRef]
- Rowe, S.J.; White, I.M.; Avendaño, S.; Hill, W.G. Genetic heterogeneity of residual variance in broiler chickens. Genet. Sel. Evol. 2006, 38, 1–19. [Google Scholar] [CrossRef]
- Bartolomé, E.; Menéndez-Buxadera, A.; Molina, A.; Valera, M. Plasticity effect of rider-horse interaction on genetic evaluations for Show Jumping discipline in sport horses. J. Anim. Breed. Genet. 2018, 135, 138–148. [Google Scholar] [CrossRef]
- Castro, M.S.M.; de Vasconcelos, A.M.; dos Santos, V.O.; Lopes, A.K.C.; de Farias, M.R.S.; Lima, F.R.G.; Rogério, M.C.P.; Ferreira, J.; Silveira, R.M.F. Thermoregulatory capacity of Santa Inês hair ewes of different genotypes associated with coat colors raised in a hot environment. J. Appl. Anim. Welf. Sci. 2020, 24, 400–410. [Google Scholar] [CrossRef]
 ), minimum (Min;
), minimum (Min;  ) and average temperature (°C) registered within the El1 area. Square down and right of the image indicate the relative humidity (Rh) and the atmospheric temperature (T.atm) registered during the measurement and used to calibrate the results. The color scale on the right indicated maximum and minimum temperatures registered within the photo.
) and average temperature (°C) registered within the El1 area. Square down and right of the image indicate the relative humidity (Rh) and the atmospheric temperature (T.atm) registered during the measurement and used to calibrate the results. The color scale on the right indicated maximum and minimum temperatures registered within the photo.
 ), minimum (Min;
), minimum (Min;  ) and average temperature (°C) registered within the El1 area. Square down and right of the image indicate the relative humidity (Rh) and the atmospheric temperature (T.atm) registered during the measurement and used to calibrate the results. The color scale on the right indicated maximum and minimum temperatures registered within the photo.
) and average temperature (°C) registered within the El1 area. Square down and right of the image indicate the relative humidity (Rh) and the atmospheric temperature (T.atm) registered during the measurement and used to calibrate the results. The color scale on the right indicated maximum and minimum temperatures registered within the photo.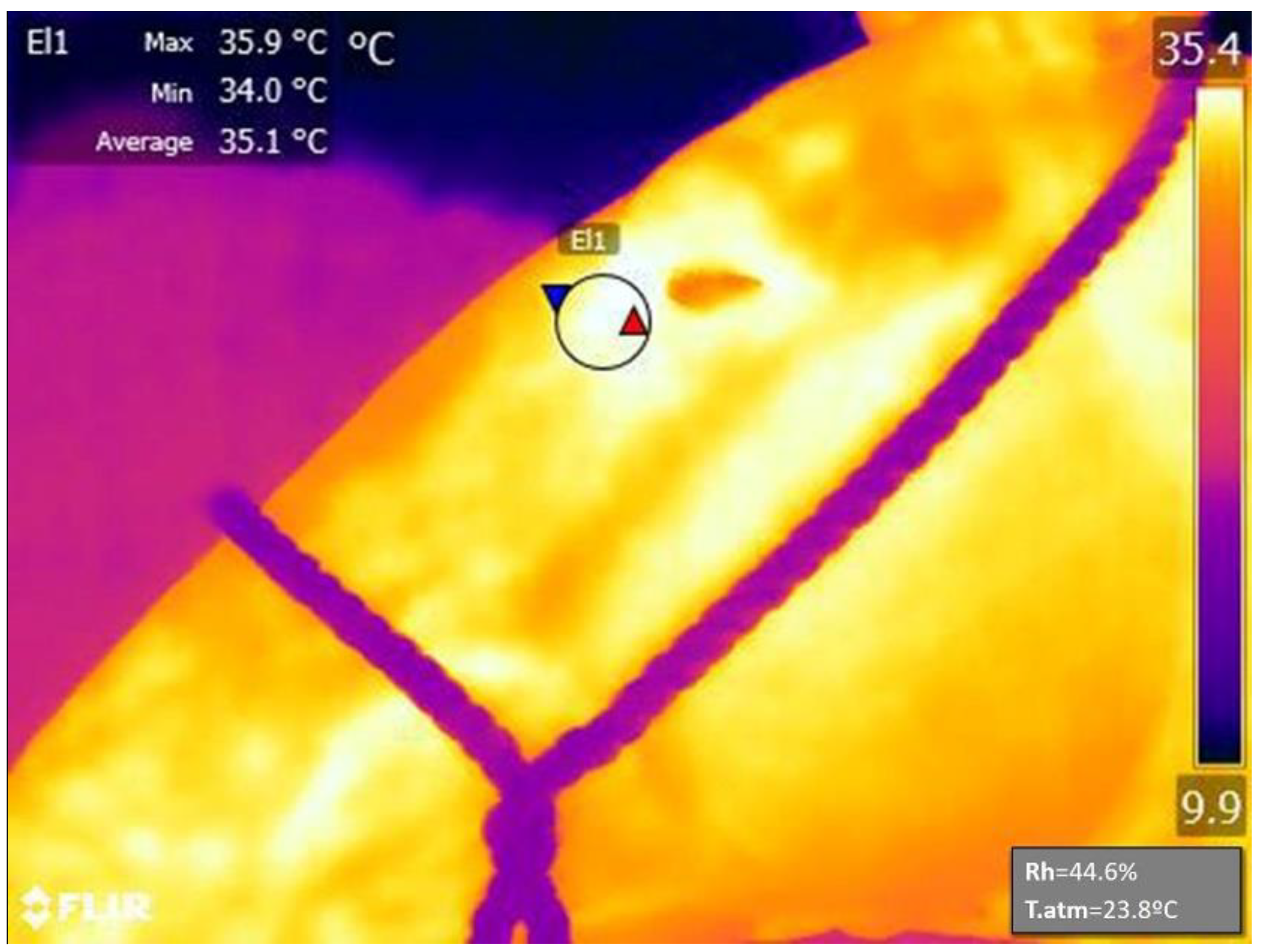
| Breed Group | Name | Description | N (%) |
|---|---|---|---|
| BG1 | German (GE) | More than 50% of the CDE ancestors belonged to German horse breeds: Holsteiner, Hanoverian, Westphalian, Oldenburger, or Trakehner. | 45 (9.6%) |
| BG2 | Netherlands (NH) | More than 50% of the CDE ancestors belonged to Netherland horse breeds: Dutch Warmblood, Belgian Warmblood. | 28 (5.9%) |
| BG3 | Trotter (TR) | More than 50% of the CDE ancestors belonged to Trotter horse breeds. | 141 (29.9%) |
| BG4 | Pura Raza Español (PRE) | More than 50% of the CDE ancestors belonged to Pura Raza Español breed. | 204 (43.3%) |
| BG5 | Other Horse Breeds (OHT) | Included CDE horses with more than 50% of their ancestors from other minority sport horse breeds (Zangersheide, Irish Sport Horse, etc.). | 53 (11.3%) |
| p Value | Effect | ||||
|---|---|---|---|---|---|
| Age | Sex | Location | Breed Group | ||
| Total Population | ** | *** | *** | *** | |
| Coat color *** | Total Interaction | n.s. | ** | n.s. | n.s. |
| Chestnut | n.s. | ** | ** | n.s. | |
| Bay | n.s. | * | n.s. | n.s. | |
| Black | * | n.s. | n.s. | n.s. | |
| Gray | n.s. | n.s. | n.s. | ** | |
| Model | σu | σe | h2 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (s.d.) | Median | HPD 95% | Mean (s.d.) | Median | HPD 95% | |||
| MGG | 0.186 (0.145) | 0.149 | 0.007–0.476 | 0.736 (0.144) | 0.760 | 0.425–0.977 | 0.20 | |
| MHRV | Chestnut | 0.201 (0.092) | 0.190 | 0.056–0.393 | 0.82 (0.221) | 0.799 | 0.358–1.2340 | 0.20 |
| Bay | 0.88 (0.125) | 0.883 | 0.665–1.141 | 0.19 | ||||
| Black | 0.98 (0.175) | 0.973 | 0.667–1.319 | 0.17 | ||||
| Gray | 0.72 (0.180) | 0.706 | 0.394–1.1020 | 0.22 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomé, E.; Perdomo-González, D.I.; Ripollés-Lobo, M.; Valera, M. Basal Reactivity Evaluated by Infrared Thermography in the “Caballo de Deporte Español” Horse Breed According to Its Coat Color. Animals 2022, 12, 2515. https://doi.org/10.3390/ani12192515
Bartolomé E, Perdomo-González DI, Ripollés-Lobo M, Valera M. Basal Reactivity Evaluated by Infrared Thermography in the “Caballo de Deporte Español” Horse Breed According to Its Coat Color. Animals. 2022; 12(19):2515. https://doi.org/10.3390/ani12192515
Chicago/Turabian StyleBartolomé, Ester, Davinia I. Perdomo-González, María Ripollés-Lobo, and Mercedes Valera. 2022. "Basal Reactivity Evaluated by Infrared Thermography in the “Caballo de Deporte Español” Horse Breed According to Its Coat Color" Animals 12, no. 19: 2515. https://doi.org/10.3390/ani12192515
APA StyleBartolomé, E., Perdomo-González, D. I., Ripollés-Lobo, M., & Valera, M. (2022). Basal Reactivity Evaluated by Infrared Thermography in the “Caballo de Deporte Español” Horse Breed According to Its Coat Color. Animals, 12(19), 2515. https://doi.org/10.3390/ani12192515






