Video Validation of Tri-Axial Accelerometer for Monitoring Zoo-Housed Tamandua tetradactyla Activity Patterns in Response to Changes in Husbandry Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
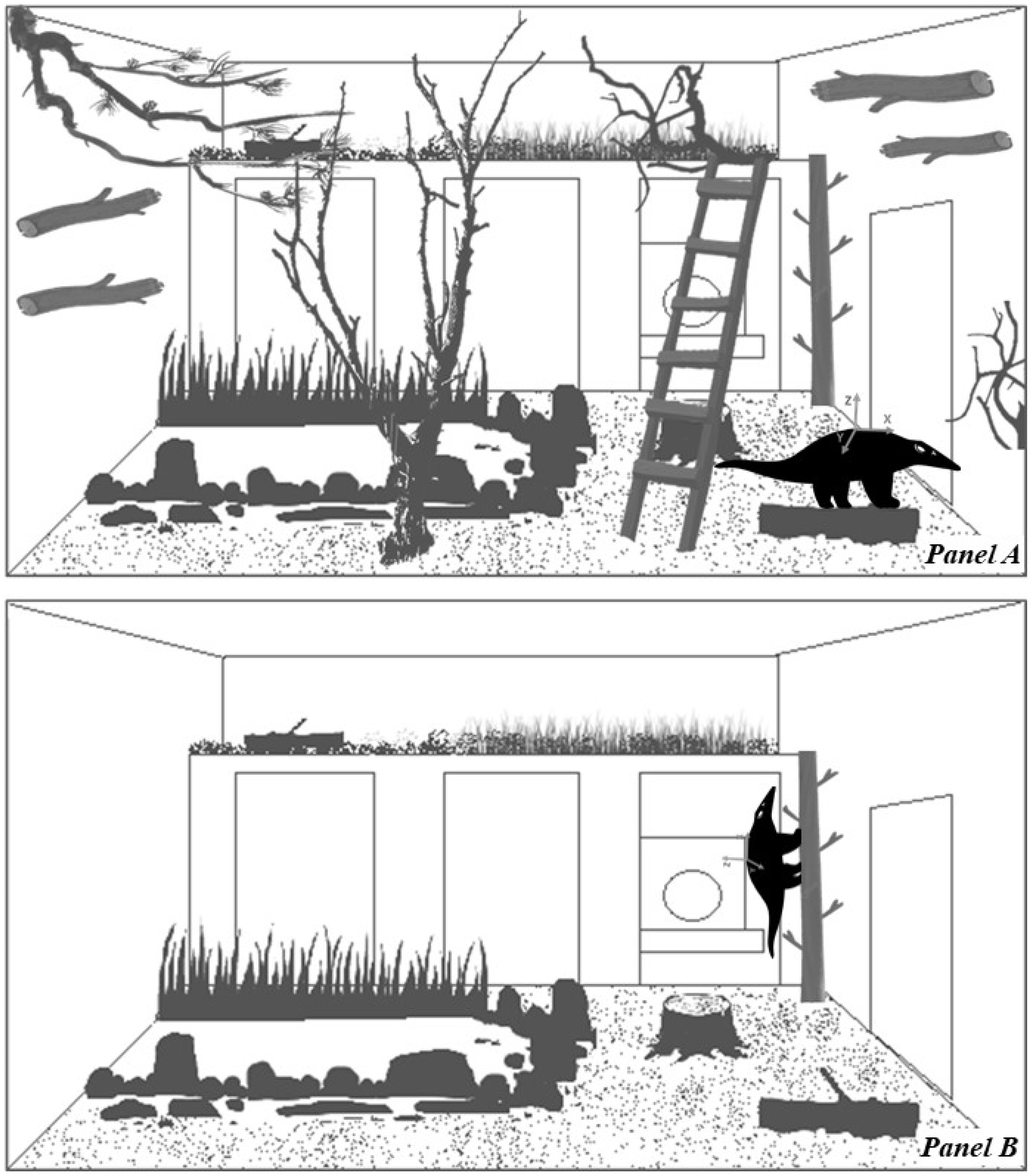
2.1. Studied Animals and Housing Conditions
2.2. Video Cameras and Tri-Axial Accelerometer
2.3. Study 1—Effects of a Vest on Activity Pattern
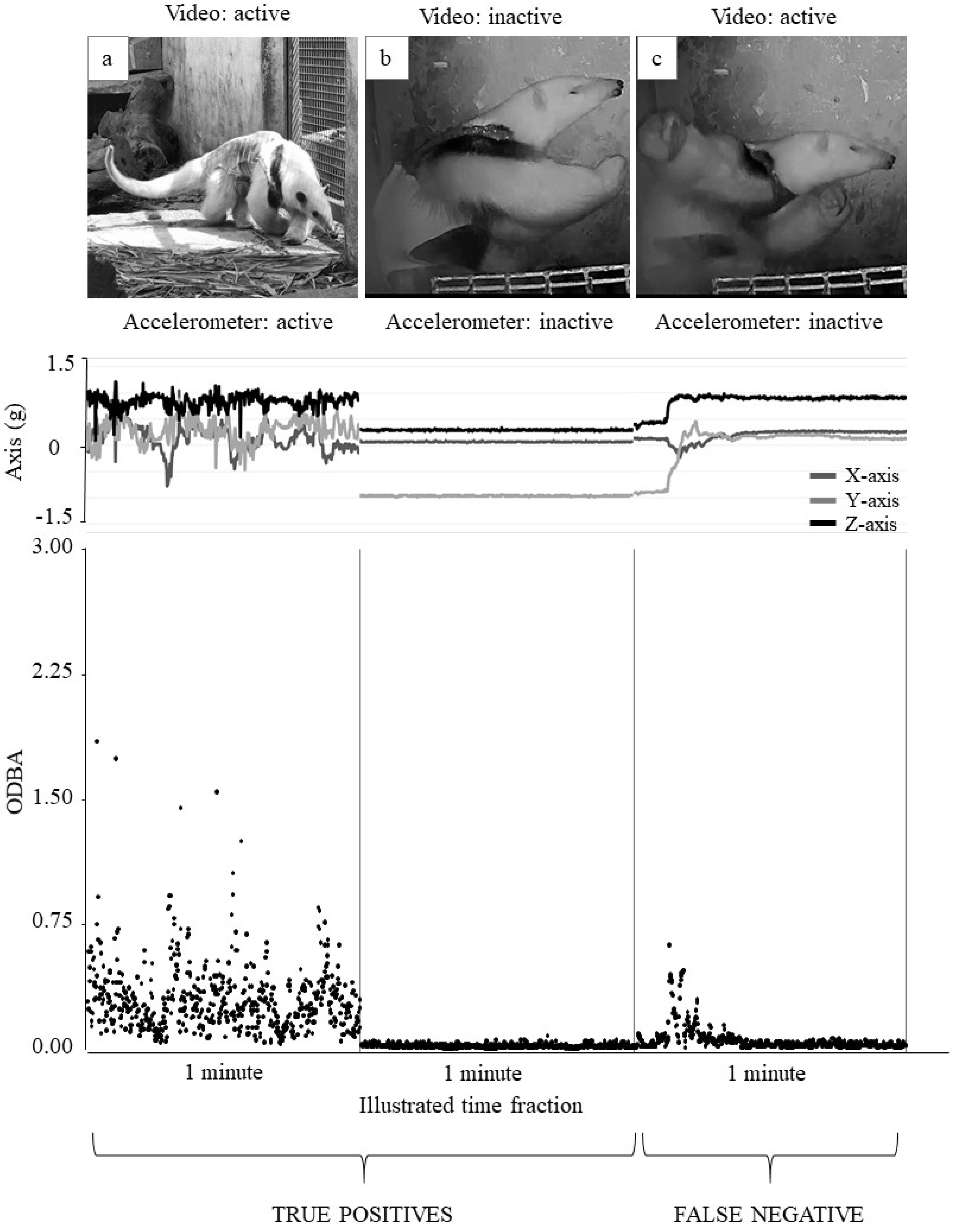
2.4. Study 2—Video Analysis Validation of the Accelerometer
2.5. Study 3—Assessment of Biological Relevance of Using Accelerometer to Monitor Activity Pattern
2.6. Statistical Analysis
3. Results
3.1. Study 1—Effects of Accelerometer Mounting Using a Vest
3.2. Study 2—Correlations between Data from Accelerometer and Video Recordings
3.3. Study 3—Characterisation of Behavioural Response to Changes in Enclosure Complexity Using an Accelerometer
4. Discussion
5. Conclusions
- -
- The use of a vest for mounting the accelerometer did not influence the total activity or activity pattern.
- -
- The pattern of fluctuations in the accelerometer validated by video recordings made it possible to clearly discriminate resting behaviour from active behaviours.
- -
- The use of the accelerometer was biologically relevant to the characterization of the response to changes in environmental complexity, employing ODBA variance as a parameter.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemente, C.J.; Cooper, C.E.; Withers, P.C.; Freakley, C.; Singh, S.; Terrill, P. The private life of echidnas: Using accelerometry and GPS to examine field biomechanics and assess the ecological impact of a widespread, semi-fossorial monotreme. J. Exp. Biol. 2016, 219, 3271–3283. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.T.; Barnes, B.M.; Buck, C.L. Integrating physiology, behavior, and energetics: Biologging in a free-living arctic hibernator. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.M.; Rode, K.D.; Cutting, A.; Owen, M.A.; Jensen, S.; Ware, J.V.; Robbins, C.T.; Durner, G.M.; Atwood, T.C.; Obbard, M.E.; et al. Using tri-axial accelerometers to identify wild polar bear behaviors. Endanger. Species Res. 2017, 32, 19–33. [Google Scholar] [CrossRef]
- Hughey, L.F.; Hein, A.M.; Strandburg-Peshkin, A.; Jensen, F.H. Challenges and solutions for studying collective animal behaviour in the wild. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170005. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, K.D.; Vyazovskiy, V.V.; Hernandez-Aguilar, R.A.; Imron, M.A.; Nekaris, K.A.I. Environment shapes sleep patterns in a wild nocturnal primate. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gleiss, A.C.; Wilson, R.P.; Shepard, E.L. Making overall dynamic body acceleration work: On the theory of acceleration as a proxy for energy expenditure. Methods Ecol. Evol. 2011, 2, 23–33. [Google Scholar] [CrossRef]
- Gervasi, V.; Brunberg, S.; Swenson, J.E. An individual-based method to measure animal activity levels: A test on brown bears. Wildl. Soc. Bull. 2006, 34, 1314–1319. [Google Scholar] [CrossRef]
- Shepard, E.L.; Wilson, R.P.; Quintana, F.; Laich, A.G.; Liebsch, N.; Albareda, D.A.; Halsey, L.G.; Gleiss, A.; Morgan, D.T.; Myers, A.E.; et al. Identification of animal movement patterns using tri-axial accelerometry. Endanger. Species Res. 2008, 10, 47–60. [Google Scholar] [CrossRef]
- Withey, J.C.; Bloxton, T.D.; Marzluff, J.M. Effects of Tagging and Location Error in Wildlife Radiotelemetry Studies. In Radio tracking and Animal Populations; Academic Press: Cambridge, MA, USA, 2001; pp. 43–75. [Google Scholar] [CrossRef]
- Herbst, L.; Redford, K. Home-range size and social spacing among female common long-nosed armadillos (Dasypus novemcinctus). Natl. Geogr. Res. Explor. 1991, 7, 236–237. [Google Scholar]
- Golabek, K.A.; Jordan, N.R.; Clutton-Brock, T.H. Radiocollars do not affect the survival or foraging behaviour of wild meerkats. J. Zool. 2008, 274, 248–253. [Google Scholar] [CrossRef]
- Swenson, J.E.; Wallin, K.; Ericsson, G.; Cederlund, G.; Sandegren, F. Effects of ear-tagging with radiotransmitters on survival of moose calves. J. Wildl. Manag. 1999, 63, 354–358. [Google Scholar] [CrossRef]
- Brown, D.D.; Kays, R.; Wikelski, M.; Wilson, R.; Klimley, A.P. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelemetry 2013, 1, 20. [Google Scholar] [CrossRef]
- Kröschel, M.; Reineking, B.; Werwie, F.; Wildi, F.; Storch, I. Remote monitoring of vigilance behavior in large herbivores using acceleration data. Anim. Biotelemetry 2017, 5, 10. [Google Scholar] [CrossRef]
- Redford, K.H.; Eisenberg, J.F. Order Xenarthra (Edentata). Mammals of the Neotropics: The Southern Cone; The University of Chicago Press: Chicago, IL, USA, 1992; pp. 46–68. [Google Scholar]
- Torres, R.; Monguillot, J.; Bruno, G.; Michelutti, P.; Ponce, A. Ampliación del límite austral de la distribución del oso melero (Tamandua tetradactyla) en la Argentina. Nótulas Faunísticas 2009, 39, 1–5. [Google Scholar]
- Hayssen, V. Tamandua tetradactyla (Pilosa: Myrmecophagidae). Mamm. Species 2011, 43, 64–74. [Google Scholar] [CrossRef]
- Tamburini, D. Orden Cingulata. In Mamíferos de Córdoba y su Estado de Conservación; Torres, R., Tamburini, D., Eds.; Editorial de la Universidad Nacional de Córdoba: Córdoba, Argentina, 2018; pp. 87–111. [Google Scholar]
- Varela, D.; Cirignoli, S.; Torres, R.M.; Superina, M. Tamandua Tetradactyla. en Categorización 2019 de los Mamíferos de Argentina Según su Riesgo de Extinción; SAyDS–SAREM, Ed.; Secretaría de Gobierno de Ambiente y Desarrollo Sustentable: Buenos Aires, Argentina, 2019. [Google Scholar] [CrossRef]
- Miranda, F.; Fallabrino, A.; Arteaga, M.; Tirira, D.G.; Meritt, D.A.; Superina, M. Tamandua tetradactyla. The IUCN Red List of Threatened Species 2014: E.T21350A47442916. 2014. Available online: https://www.iucnredlist.org/species/21350/47442916 (accessed on 20 October 2020).
- Superina, M.; Miranda, F.; Plese, T. Maintenance of Xenarthra in Captivity. In The Biology of the Xenarthra; Vizcaíno, S.F., Loughry, W.J., Eds.; University Press of Florida: Gainesville, FL, USA, 2008; pp. 232–243. [Google Scholar]
- Aguilar, R.F.; Superina, M. Xenarthra: Edentata (Anteaters, Armadillos, Sloths). In Fowler’s Zoo and Wild Animal Medicine; Miller, R.E., Fowler, M.E., Eds.; Elsevier: St. Louis, MO, USA, 2015; Volume 8, pp. 355–369. [Google Scholar] [CrossRef]
- Navarrete, D.; Ortega, J. Tamandua mexicana (Pilosa: Myrmecophagidae). Mamm. Species 2011, 43, 56–63. [Google Scholar] [CrossRef]
- Montgomery, G.G. Movements, foraging and food habits of the four extant species of Neotropical vermilinguas (Mammalia: Myrmecophagidae). In The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas; Montgomery, G.G., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1985; pp. 365–377. [Google Scholar]
- Fernandes, T.N.; Young, R.J. Fluctuations in the tympanic membrane temperatures of non-restrained captive giant anteaters and southern tamanduas. J. Zool. 2008, 274, 94–98. [Google Scholar] [CrossRef]
- Eguizábal, G.V.; Palme, R.; Superina, M.; Asencio, C.J.; García Capocasa, M.C.; Busso, J.M. Characterization and correlations of behavioral and adrenocortical activities of zoo-housed lesser anteaters (Tamandua tetradactyla). Zoo Biol. 2019, 38, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Eguizábal, G.V.; Superina, M.; Palme, R.; Asencio, C.J.; Villarreal, D.P.; Borrelli, L.; Busso, J.M. Non-Invasive Assessment of the Seasonal Stress Response to Veterinary Procedures and Transportation of Zoo-Housed Lesser Anteater (Tamandua tetradactyla). Animals 2022, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Dierenfeld, E.S.; Graffam, W.S. Manual de Nutrición y Dietas para Animales Silvestres en Cautiverio (Ejemplos para Animales de América Latina); Zoo Conservation Outreach Group: New Orleans, LA, USA, 1996. [Google Scholar]
- Eguizábal, G.V.; Palme, R.; Villarreal, D.; Dal Borgo, C.; Di Rienzo, J.A.; Busso, J.M. Assessment of adrenocortical activity and behaviour of the collared anteater (Tamandua tetradactyla) in response to food-based environmental enrichment. Zoo Biol. 2013, 32, 632–640. [Google Scholar] [CrossRef]
- Rojano-Bolaño, C.; Miranda-Cortés, L.; Ávila-Avilán, R.; Álvarez-Otero, G. Parámetros hematológicos de Hormigueros gigantes (Myrmecophaga tridactyla Linnaeus, 1758) de vida libre en Pore, Colombia. Vet. Zootec. 2014, 8, 85–99. [Google Scholar] [CrossRef]
- TechnoSmArt Europe. GPS Tracking Sistems for Animals. Available online: https://www.technosmart.eu/ (accessed on 5 September 2019).
- Saudargas, R.A.; Drummer, L.C. Single subject (small N) research designs and zoo research. Zoo Biol. 1996, 15, 173–181. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour. An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Zárate, V.; Mufari, J.R.; Abalos Luna, L.G.; Villarreal, D.P.; Busso, J.M. Assessment of Feeding Behavior of the Zoo-Housed Lesser Anteater (Tamandua tetradactyla) and Nutritional Values of Natural Prey. J. Zool. Bot. Gard. 2022, 3, 19–31. [Google Scholar] [CrossRef]
- Sherwin, C.M.; Christiansen, S.B.; Duncan, I.J.H.; Erhard, H.W.; Lay, D.C.; Mench, J.A.; O’Connor, C.E.; Petherick, C.J. Guidelines for the ethical use of animals in applied animal behaviour research. Appl. Anim. Behav. Sci. 2003, 81, 291–305, (updated 2017, International Society for Applied Ethology). [Google Scholar] [CrossRef]
- Ladds, M.A.; Thompson, A.P.; Kadar, J.P.; Slip, D.J.; Hocking, D.P.; Harcourt, R.G. Super machine learning: Improving accuracy and reducing variance of behaviour classification from accelerometry. Anim. Biotelemetry 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Shepard, E.L.; Wilson, R.P.; Halsey, L.G.; Quintana, F.; Laich, A.G.; Gleiss, A.C.; Liebsch, N.; Myers, A.E.; Norman, B. Derivation of body motion via appropriate smoothing of acceleration data. Aquat. Biol. 2008, 4, 235–241. [Google Scholar] [CrossRef]
- Vázquez Diosdado, J.A.; Barker, Z.E.; Hodges, H.R.; Amory, J.R.; Croft, D.P.; Bell, N.J.; Codling, E.A. Classification of behaviour in housed dairy cows using an accelerometer-based activity monitoring system. Anim. Biotelemetry 2015, 3, 15. [Google Scholar] [CrossRef]
- Fehlmann, G.; O’Riain, M.J.; Hopkins, P.W.; O’Sullivan, J.; Holton, M.D.; Shepard, E.L.; King, A.J. Identification of behaviours from accelerometer data in a wild social primate. Anim. Biotelem. 2017, 5, 6. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behaviour: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [Green Version]
- Chiapero, F.; Ferrari, R.H.; Guglielmetti, A.; García Capocasa, M.C.; Busso, J.M. Visitors’ perceptions of zoo-housed lesser anteater (Tamandua tetradactyla) welfare: Observation plays a larger role than a brief informative talk. Zoo Biol. 2021, 40, 33–43. [Google Scholar] [CrossRef]
- Schmid, B.; Helfrich-Förster, C.; Yoshii, T. A new ImageJ plug-in “ActogramJ” for chronobiological analyses. J. Biol. Rhythm. 2011, 26, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat versión 2019. Grupo InfoStat, Faculty of Agricultural Sciences, National University of Córdoba, Argentina, 2019. Available online: http://www.infostat.com.ar (accessed on 16 March 2022).
- Gleiss, A.C.; Dale, J.J.; Holland, K.N.; Wilson, R.P. Accelerating estimates of activity-specific metabolic rate in fishes: Testing the applicability of acceleration data-loggers. J. Exp. Mar. Biol. Ecol. 2010, 385, 85–91. [Google Scholar] [CrossRef]
- Brown, D.D. Activity Patterns and Space Use of Northern Tamandua Anteaters (Tamandua mexicana) on Barro Colorado Island, Panamá; University of California: Davis, CA, USA, 2011. [Google Scholar]
- Di Blanco, Y.E.; Pérez, I.J.; Díaz, P.; Spørring, Y.K. Cinco años de radiomarcaje de osos hormigueros (Myrmecophaga tridactyla): Mejoras implementadas y lecciones aprendidas. Edentata 2012, 13, 49–55. [Google Scholar] [CrossRef]
- Cooke, S.J.; Hinch, S.G.; Wikelski, M.; Andrews, R.D.; Kuchel, L.J.; Wolcott, T.G.; Butler, P.J. Biotelemetry: A mechanistic approach to ecology. Trends Ecol. Evol. 2004, 19, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Spiegel, O.; Fortmann-Roe, S.; Harel, R.; Wikelski, M.; Getz, W.M. Using tri-axial acceleration data to identify behavioral modes of free-ranging animals: General concepts and tools illustrated for griffon vultures. J. Exp. Biol. 2012, 215, 986–996. [Google Scholar] [CrossRef]
- Halsey, L.G.; Shepard, E.L.; Wilson, R.P. Assessing the development and application of the accelerometry technique for estimating energy expenditure. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 305–314. [Google Scholar] [CrossRef]
- McClune, D.W.; Marks, N.J.; Wilson, R.P.; Houghton, J.D.R.; Montgomery, I.W.; McGowan NEGormley, E.; Scantlebury, M. Tri-axial accelerometers quantify behaviour in the Eurasian badger (Meles meles): Towards an automated interpretation of field data. Anim. Biotelemetry 2014, 2, 5. [Google Scholar] [CrossRef]
- Kumpulainen, P.; Valldeoriola Cardó, A.; Somppi, S.; Törnqvist, H.; Väätäjä, H.; Majaranta, P.; Gizatdinova, Y.; Hoog Antink, C.; Surakka, V.; Kujala, M.V.; et al. Dog behaviour classification with movement sensors placed on the harness and the collar. Appl. Anim. Behav. Sci. 2021, 241, 105393. [Google Scholar] [CrossRef]
- Barwick, J.; Lamb, D.W.; Dobos, R.; Welch, M.; Trotter, M. Categorising sheep activity using a tri-axial accelerometer. Comput. Electron. Agric. 2018, 145, 289–297. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Campera, M.; Chimienti, M.; Murray, C.; Balestri, M.; Showell, Z. Training in the Dark: Using Target Training for Non-Invasive Application and Validation of Accelerometer Devices for an Endangered Primate (Nycticebus bengalensis). Animals 2022, 12, 411. [Google Scholar] [CrossRef]
- De Godoy Peixoto, F.B.; Ambrózio, M.T.G.; Colbachini, H.; Padilha, F.L.A.; Costa, F.R. Enriquecimento ambiental aplicado a tamanduás-mirins (Tamandua tetradactyla) no Aquário de São Paulo: Estudo de caso. Pesqui. Ensino Ciências Exatas Nat. 2019, 3, 119–124. [Google Scholar] [CrossRef]
- Mendes, C.P.; Carreira, D.; Pedrosa, F.; Beca, G.; Lautenschlager, L.; Akkawi, P.; Berce, W.; Ferraz, K.M.M.B.; Galetti, M. Landscape of human fear in Neotropical rainforest mammals. Biol. Conserv. 2020, 241, 108257. [Google Scholar] [CrossRef]
- Norris, D.; Michalski, F.; Peres, C.A. Habitat patch size modulates terrestrial mammal activity patterns in Amazonian forest fragments. J. Mammal. 2010, 91, 551–560. [Google Scholar] [CrossRef]
- Wang, Y.; Nickel, B.; Rutishauser, M.; Bryce, C.M.; Williams, T.M.; Elkaim, G.; Wilmers, C.C. Movement, resting, and attack behaviors of wild pumas are revealed by tri-axial accelerometer measurements. Mov. Ecol. 2015, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Nogoy, K.M.C.; Chon S-i Park J-h Sivamani, S.; Lee, D.-H.; Choi, S.H. High Precision Classification of Resting and Eating Behaviors of Cattle by Using a Collar-Fitted Triaxial Accelerometer Sensor. Sensors 2022, 22, 5961. [Google Scholar] [CrossRef]
- Sprinkle, J.E.; Sagers, J.K.; Hall, J.B.; Ellison, M.J.; Yelich, J.V.; Brennan, J.R.; Taylor, J.B.; Lamb, J.B. Predicting Cattle Grazing Behavior on Rangeland using Accelerometers. Rangel. Ecol. Manag. 2021, 76, 157–170. [Google Scholar] [CrossRef]
- Hammond, T.T.; Springthorpe, D.; Walsh, R.E.; Berg-Kirpatrick, T. Using accelerometers to remotely and automatically characterize behavior in small animals. J. Exp. Biol. 2016, 219, 1618–1624. [Google Scholar] [CrossRef]
- Chen, J.; Brown, G.; Fudickar, A. Simulation-based validation of activity logger data for animal behavior studies. Anim. Biotelemetry 2021, 9, 31. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavese, S.; Centeno, C.; Von Fersen, L.; Eguizábal, G.V.; Donet, L.; Asencio, C.J.; Villarreal, D.P.; Busso, J.M. Video Validation of Tri-Axial Accelerometer for Monitoring Zoo-Housed Tamandua tetradactyla Activity Patterns in Response to Changes in Husbandry Conditions. Animals 2022, 12, 2516. https://doi.org/10.3390/ani12192516
Pavese S, Centeno C, Von Fersen L, Eguizábal GV, Donet L, Asencio CJ, Villarreal DP, Busso JM. Video Validation of Tri-Axial Accelerometer for Monitoring Zoo-Housed Tamandua tetradactyla Activity Patterns in Response to Changes in Husbandry Conditions. Animals. 2022; 12(19):2516. https://doi.org/10.3390/ani12192516
Chicago/Turabian StylePavese, Sofía, Carlos Centeno, Lorenzo Von Fersen, Gabina V. Eguizábal, Luis Donet, Camila J. Asencio, Daniel P. Villarreal, and Juan Manuel Busso. 2022. "Video Validation of Tri-Axial Accelerometer for Monitoring Zoo-Housed Tamandua tetradactyla Activity Patterns in Response to Changes in Husbandry Conditions" Animals 12, no. 19: 2516. https://doi.org/10.3390/ani12192516




