Effects of Long-Term Low-Protein Diets Supplemented with Sodium Dichloroacetate and Glucose on Metabolic Biomarkers and Intestinal Microbiota of Finishing Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Animals and Experimental Design
2.3. Sample Collection
2.4. Analytical Methods
2.4.1. Metabolic Markers and Hormonal Index in Plasma
2.4.2. Hepatic HMGCR and CYP7A1 Activities
2.4.3. Intestinal Morphology
2.4.4. Intestinal Microbiota Analysis
2.5. Statistical Analysis
3. Results
3.1. Plasma Metabolic and Immunological Biomarkers
3.2. Hormone Concentrations in Plasma
3.3. HMGCR and CYP7A1 in Liver
3.4. Ileal Histology
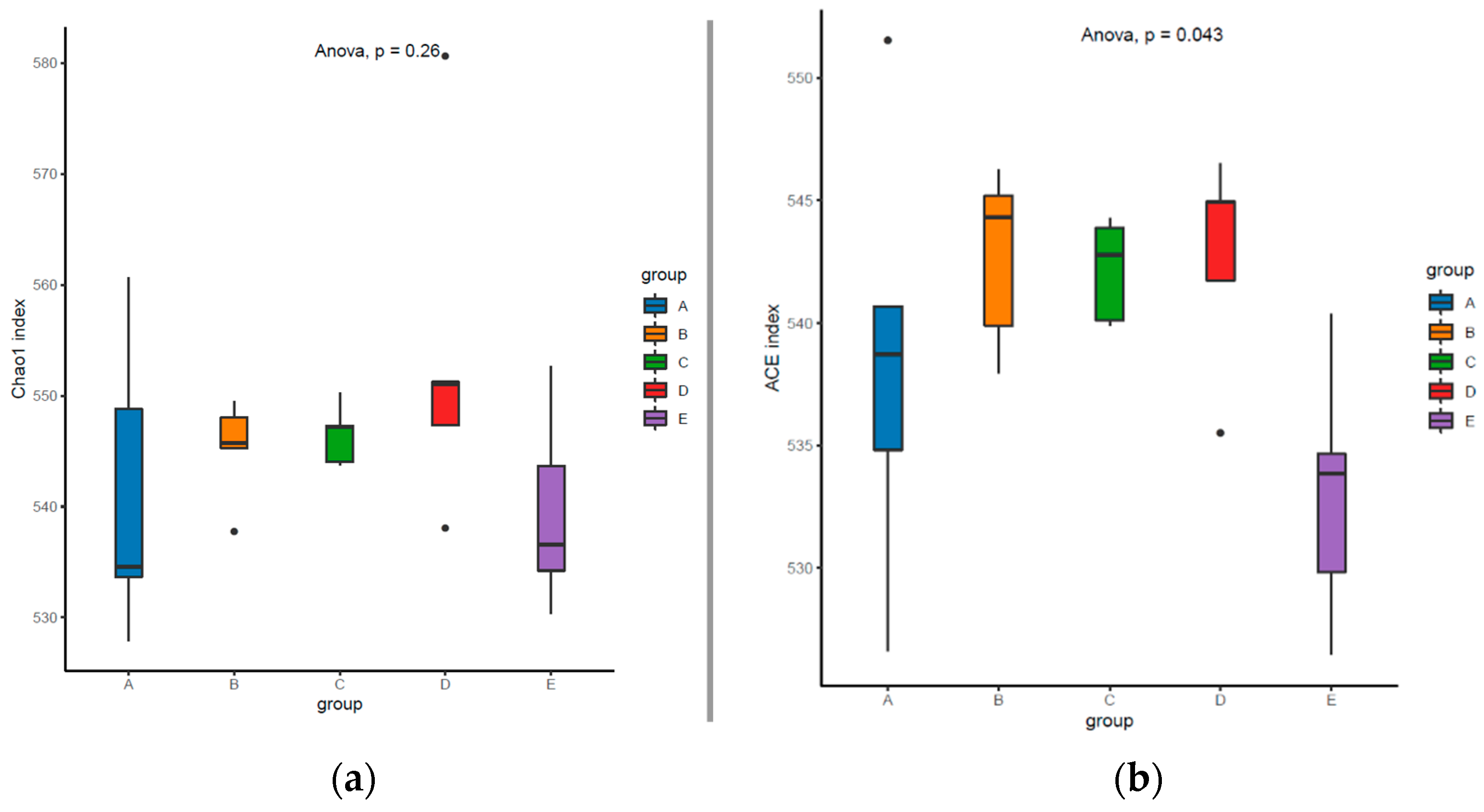
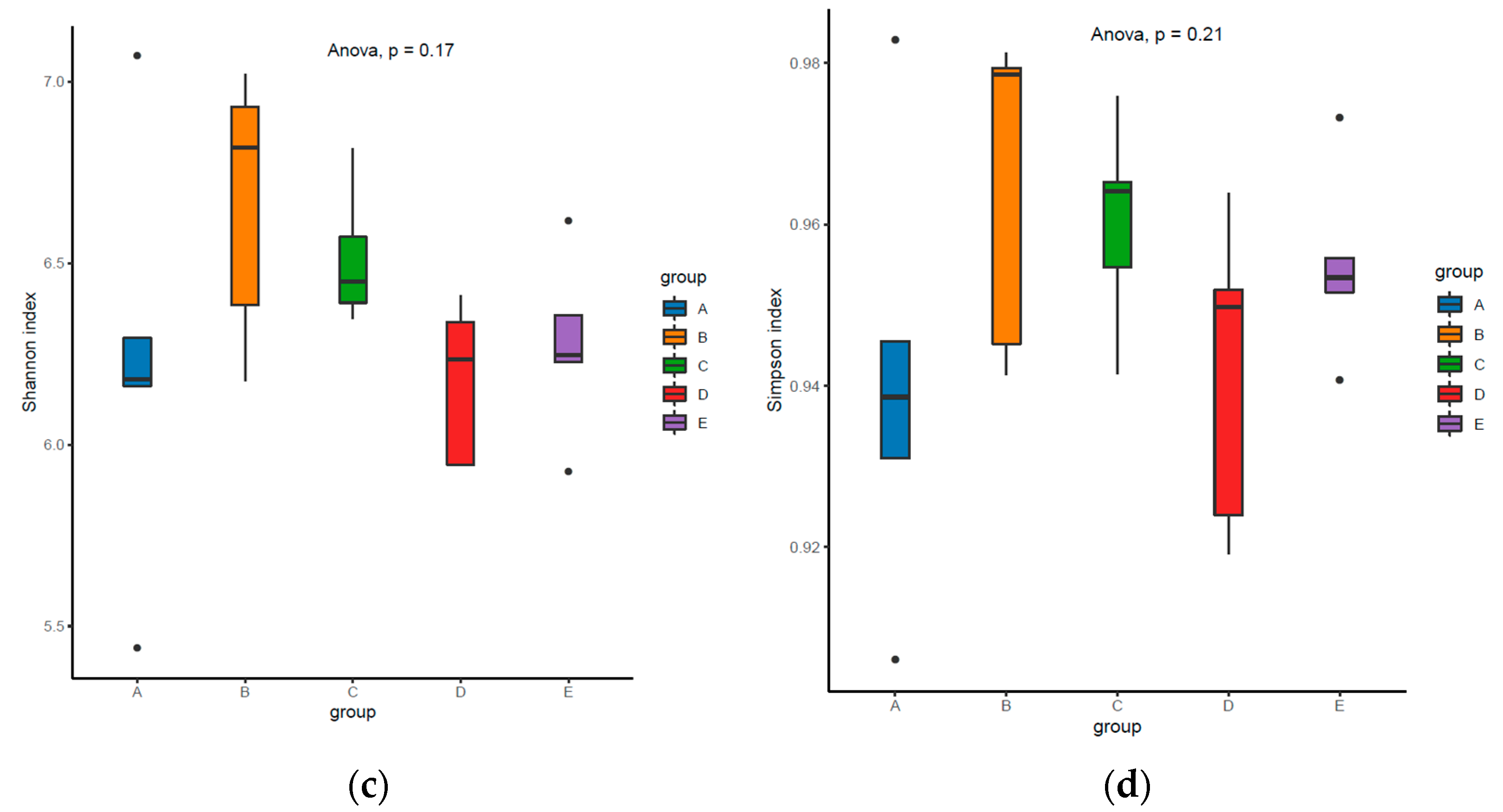
3.5. Colonic Microbial Diversity and Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, W.; Li, Y.; Tang, Z.; Chen, H.; Wan, K.; An, R.; Wu, L.; Sun, Z. Effects of adding sodium dichloroacetate to low-protein diets on nitrogen balance and amino acid metabolism in the portal-drained viscera and liver of pigs. J. Anim. Sci. Biotechnol. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the Interaction between Dietary Protein and Gut Microbiota to Intestinal Health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef]
- Shibata, N.; Kunisawa, J.; Kiyono, H. Dietary and Microbial Metabolites in the Regulation of Host Immunity. Front. Microbiol. 2017, 8, 2171. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef]
- Ma, W.; Mao, P.; Guo, L.; Qiao, S. Crystalline amino acids supplementation improves the performance and carcass traits in late-finishing gilts fed low-protein diets. Anim. Sci. J. 2020, 91, e13317. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, H.; Wan, K.; Zhou, K.; Wang, Y.; Li, J.; Tang, Z.; Sun, W.; Wu, L.; An, R.; et al. Effects of supplementing low-protein diets with sodium dichloroacetate and glucose on growth performance, carcass traits, and meat quality of growing-finishing pigs. J. Anim. Sci. 2022, 100, skab359. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.; Duan, Y.; Guo, Q.; Wang, W.; Wen, C.; Huang, X.; Yin, Y. The Protein and Energy Metabolic Response of Skeletal Muscle to the Low-Protein Diets in Growing Pigs. J. Agric. Food Chem. 2017, 65, 8544–8551. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Tang, Z.; Li, Y.; Li, T.; Xu, Q.; Zhen, J.; Huang, F.; Yang, J.; Chen, C.; et al. Low-Protein Diets Decrease Porcine Nitrogen Excretion but with Restrictive Effects on Amino Acid Utilization. J. Agric. Food Chem. 2018, 66, 8262–8271. [Google Scholar] [CrossRef]
- Kight, C.E.; Fleming, S.E. Oxidation of glucose carbon entering the TCA cycle is reduced by glutamine in small intestine epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1995, 268, G879–G888. [Google Scholar] [CrossRef]
- Shahrzad, S.; Lacombe, K.; Adamcic, U.; Minhas, K.; Coomber, B.L. Sodium dichloroacetate (DCA) reduces apoptosis in colorectal tumor hypoxia. Cancer Lett. 2010, 297, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W.; Kerr, D.S.; Barnes, C.; Bunch, S.T.; Carney, P.R.; Fennell, E.M.; Felitsyn, N.M.; Gilmore, R.L.; Greer, M.; Henderson, G.N.; et al. Controlled Clinical Trial of Dichloroacetate for Treatment of Congenital Lactic Acidosis in Children. Pediatrics 2006, 117, 1519. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef]
- An, R.; Tang, Z.; Li, Y.; Li, T.; Xu, Q.; Zhen, J.; Huang, F.; Yang, J.; Chen, C.; Wu, Z.; et al. Activation of Pyruvate Dehydrogenase by Sodium Dichloroacetate Shifts Metabolic Consumption from Amino Acids to Glucose in IPEC-J2 Cells and Intestinal Bacteria in Pigs. J. Agric. Food Chem. 2018, 66, 3793–3800. [Google Scholar] [CrossRef]
- Wan, K.; Li, Y.; Sun, W.; An, R.; Tang, Z.; Wu, L.; Chen, H.; Sun, Z. Effects of dietary calcium pyruvate on gastrointestinal tract development, intestinal health and growth performance of newly weaned piglets fed low-protein diets. J. Appl. Microbiol. 2020, 128, 355–365. [Google Scholar] [CrossRef]
- Liang, G.; Kao, H.; Wang, T.T.; Guo, Y.; Ping, J.; Wang, H. Optimization, Validation and Application of Spectrophotometric Assay for 3-Hydroxy-3-methylglutarylcoenzyme A Reductase Activity. Trop. J. Pharm. Res. 2015, 14, 671–677. [Google Scholar] [CrossRef][Green Version]
- Guo, J.; Bei, W.; Hu, Y.; Tang, C.; He, W.; Liu, X.; Huang, L.; Cao, Y.; Hu, X.; Zhong, X.; et al. A new TCM formula FTZ lowers serum cholesterol by regulating HMG-CoA reductase and CYP7A1 in hyperlipidemic rats. J. Ethnopharmacol. 2011, 135, 299–307. [Google Scholar] [CrossRef]
- Zhu, H.L.; Liu, Y.L.; Xie, X.L.; Huang, J.J.; Hou, Y.Q. Effect of L-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun. 2013, 19, 242–252. [Google Scholar] [CrossRef]
- Xu, Y.; Curtasu, M.V.; Bendiks, Z.; Marco, M.L.; Nørskov, P.N.; Knudsen, K.E.B.; Hedemann, M.S.; Laerke, H.N. Effects of dietary fibre and protein content on intestinal fibre degradation, short-chain fatty acid and microbiota composition in a high-fat fructose-rich diet induced obese Gottingen Minipig model. Food Funct. 2020, 11, 10758–10773. [Google Scholar] [CrossRef]
- Yu, D.; Zhu, W.; Hang, S. Effects of Long-Term Dietary Protein Restriction on Intestinal Morphology, Digestive Enzymes, Gut Hormones, and Colonic Microbiota in Pigs. Animals 2019, 9, 180. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, J.; Wu, Q.; Chen, J.; Yang, X.; Wang, L.; Jiang, Z. Low Protein Diet Improves Meat Quality and Modulates the Composition of Gut Microbiota in Finishing Pigs. Front. Vet. Sci. 2022, 9, 843957. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Arch. Anim. Nutr. 2008, 62, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Hu, L.; Liu, Y.; Yan, C.; Fang, Z.F.; Lin, Y.; Xu, S.Y.; Li, J.; Wu, C.M.; Chen, D.W.; et al. Effects of low-protein diets supplemented with indispensable amino acids on growth performance, intestinal morphology and immunological parameters in 13 to 35 kg pigs. Animal 2016, 10, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. Effects of varying levels of dietary protein and net energy on growth performance, nitrogen balance and faecal characteristics of growing-finishing pigs. Rev. Bras. Zootec. 2019, 48, e20180021. [Google Scholar] [CrossRef]
- Yang, Q.; Ji, G.; Pan, R.; Zhao, Y.; Yan, P. Protective effect of hydrogen-rich water on liver function of colorectal cancer patients treated with mFOLFOX6 chemotherapy. Mol. Clin. Oncol. 2017, 7, 891–896. [Google Scholar] [CrossRef]
- Fisher, K.D.; Scheffler, T.L.; Kasten, S.C.; Reinholt, B.M.; van Eyk, G.R.; Escobar, J.; Scheffler, J.M.; Gerrard, D.E. Energy dense, protein restricted diet increases adiposity and perturbs metabolism in young, genetically lean pigs. PLoS ONE 2013, 8, e72320. [Google Scholar] [CrossRef]
- Pałkowska-Goździk, E.; Lachowicz, K.; Rosołowska-Huszcz, D. Effects of Dietary Protein on Thyroid Axis Activity. Nutrients 2017, 10, 5. [Google Scholar] [CrossRef]
- Saggau, E.; Beyer, M.; Klein, M.; Schadereit, R.; Derno, M.; Jentsch, W.; Scholze, H. Effects of dietary protein quality on energy metabolism and thyroid hormone Status in growing pigs. Arch. Anim. Breed. 2000, 43, 633–648. [Google Scholar] [CrossRef]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1476. [Google Scholar] [CrossRef]
- Moller, N.; Jorgensen, J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef]
- Tejeda, J.F.; Hernandez-Matamoros, A.; Gonzalez, E. Free-Range and Low-Protein Concentrated Diets in Iberian Pigs: Effect on Plasma Insulin and Leptin Concentration, Lipogenic Enzyme Activity, and Fatty Acid Composition of Adipose Tissue. Animals 2020, 10, 1917. [Google Scholar] [CrossRef] [PubMed]
- Prieur, X.; Tung, Y.C.; Griffin, J.L.; Farooqi, I.S.; O’Rahilly, S.; Coll, A.P. Leptin regulates peripheral lipid metabolism primarily through central effects on food intake. Endocrinology 2008, 149, 5432–5439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonné, A.C.M.; den Bieman, M.G.; Gillissen, G.F.; van Lith, H.A.; van Zutphen, L.F.M. Chromosomal localization of genes involved in biosynthesis, metabolism or transport of cholesterol in the rat. Cytogenet. Genome Res. 2002, 97, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004, 40, 539–551. [Google Scholar] [CrossRef]
- Chen, X.; Song, P.; Fan, P.; He, T.; Jacobs, D.; Levesque, C.L.; Johnston, L.J.; Ji, L.; Ma, N.; Chen, Y.; et al. Moderate Dietary Protein Restriction Optimized Gut Microbiota and Mucosal Barrier in Growing Pig Model. Front. Cell. Infect. Microbiol. 2018, 8, 246. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; et al. Dietary protein levels and amino acid supplementation patterns alter the composition and functions of colonic microbiota in pigs. Anim. Nutr. 2020, 6, 143–151. [Google Scholar] [CrossRef]
- Masuoka, H.; Suda, W.; Tomitsuka, E.; Shindo, C.; Takayasu, L.; Horwood, P.; Greenhill, A.R.; Hattori, M.; Umezaki, M.; Hirayama, K. The influences of low protein diet on the intestinal microbiota of mice. Sci. Rep. 2020, 10, 17077. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Sun, L.; Cheng, Q.M.; Li, Y.C.; Chen, J.X.; Zhao, B.; Qian, C.; Li, B.; Yu, H.R.; Liu, M.; et al. Effect of pelleted alfalfa or native grass total mixed ration on the rumen bacterial community and growth performance of lambs on the Mongolian Plateau. Small Rumin. Res. 2022, 207, 106610. [Google Scholar] [CrossRef]
- Hsu, C.K.; Su, S.C.; Chang, L.C.; Shao, S.C.; Yang, K.J.; Chen, C.Y.; Chen, Y.T.; Wu, I.W. Effects of Low Protein Diet on Modulating Gut Microbiota in Patients with Chronic Kidney Disease: A Systematic Review and Meta-analysis of International Studies. Int. J. Med. Sci. 2021, 18, 3839–3850. [Google Scholar] [CrossRef]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J.; Streutker, C.; Maughan, H.; Rubino, S.; Moriyama, E.H.; Copeland, J.K.; Surendra, A.; Kumar, S.; et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014, 158, 288–299. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Chen, J.; Kang, B.; Jiang, Q.; Han, M.; Zhao, Y.; Long, L.; Fu, C.; Yao, K. Alpha-Ketoglutarate in Low-Protein Diets for Growing Pigs: Effects on Cecal Microbial Communities and Parameters of Microbial Metabolism. Front. Microbiol. 2018, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Spring, S.; Premathilake, H.; Bradway, C.; Shili, C.; DeSilva, U.; Carter, S.; Pezeshki, A. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci. Rep. 2020, 10, 15859. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Akasaka, H.; Suzuki, D.; Ueki, K. Paludibacter propionicigenes gen. nov., sp. nov., a novel strictly anaerobic, Gram-negative, propionate-producing bacterium isolated from plant residue in irrigated rice-field soil in Japan. Int. J. Syst. Evol. Microbiol. 2006, 56, 39–44. [Google Scholar] [CrossRef] [PubMed]


| Item | Treatments | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + DCA | LP + GLUC | LP + DCA + GLUC | |||
| HDL (mg/mL) | 0.98 b | 1.34 a | 1.31 a | 1.25 a | 1.20 a | 0.05 | <0.001 |
| LDL (mg/mL) | 0.47 | 0.47 | 0.56 | 0.52 | 0.52 | 0.03 | 0.104 |
| VLDL (mg/mL) | 6.97 ab | 10.42 a | 4.95 b | 6.95 ab | 4.33 b | 1.30 | 0.026 |
| TG (mmol/L) | 1.48 b | 1.88 a | 1.97 a | 1.51 b | 1.15 c | 0.07 | <0.001 |
| TC (mmol/L) | 4.20 d | 6.28 ab | 6.49 a | 5.42 bc | 5.18 c | 0.30 | <0.001 |
| GLUC (mmol/L) | 17.9 | 19.0 | 20.0 | 23.9 | 18.1 | 2.36 | 0.388 |
| TBA (μmol/L) | 37.3 b | 27.7 c | 32.6 bc | 27.8 c | 42.5 a | 1.73 | <0.001 |
| BUN (mmol/L) | 5.33 a | 3.97 b | 4.88 a | 3.51 b | 3.53 b | 0.29 | <0.001 |
| ALB (g/L) | 39.2 a | 37.1 b | 40.0 a | 39.3 a | 38.9 ab | 0.49 | 0.017 |
| TP (mg/mL) | 95.4 a | 78.1 b | 90.2 ab | 95.4 a | 77.5 b | 3.95 | 0.004 |
| DBil (μmol/L) | 1.93 b | 2.72 a | 2.69 a | 0.89 c | 1.31 bc | 0.21 | <0.001 |
| TBil (μmol/L) | 13.1 | 13.3 | 10.6 | 14.7 | 11.3 | 1.33 | 0.226 |
| LDH (U/L) | 491 a | 467 ab | 393 b | 460 ab | 450 ab | 20.7 | 0.032 |
| PDH (U/L) | 16.4 | 34.3 | 19.7 | 25.1 | 16.0 | 3.33 | 0.194 |
| GDH (U/L) | 2.49 | 2.93 | 2.27 | 2.35 | 2.26 | 0.32 | 0.564 |
| ADA (U/L) | 1.79 | 1.56 | 2.31 | 1.85 | 1.78 | 0.25 | 0.323 |
| ChE (U/L) | 305 | 333 | 238 | 276 | 316 | 36.3 | 0.398 |
| GGT (U/L) | 28.1 b | 30.5 ab | 36.2 a | 27.0 b | 32.5 ab | 1.76 | 0.008 |
| ALP (U/L) | 12.8 c | 19.8 a | 16.6 b | 17.2 ab | 18.6 ab | 0.77 | <0.001 |
| ALT (U/L) | 13.7 | 12.3 | 14.7 | 15.0 | 12.7 | 1.38 | 0.550 |
| AST (U/L) | 10.7 | 10.7 | 9.45 | 12.7 | 11.6 | 1.19 | 0.420 |
| IgA (μg/mL) | 403 | 385 | 381 | 383 | 433 | 63.0 | 0.973 |
| IgG (μg/mL) | 9.35 a | 4.85 b | 7.79 ab | 9.86 a | 7.38 ab | 0.85 | 0.003 |
| IgM (μg/mL) | 5.94 | 6.05 | 6.29 | 5.21 | 6.38 | 1.36 | 0.976 |
| Item | Treatments | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + DCA | LP + GLUC | LP + DCA + GLUC | |||
| T3 (pmol/L) | 1.93 c | 2.81 b | 3.93 a | 4.14 a | 3.95 a | 0.19 | <0.001 |
| T4 (pmol/L) | 3.01 c | 5.68 b | 7.96 a | 7.49 a | 5.74 b | 0.47 | <0.001 |
| Growth hormone (ng/mL) | 2.26 d | 4.68 c | 7.18 a | 7.65 a | 5.95 b | 0.36 | <0.001 |
| Insulin (mIU/L) | 2.71 c | 4.67 b | 8.38 a | 7.81 a | 4.79 b | 0.21 | <0.001 |
| Glucagon (pg/mL) | 30.6 c | 51.4 b | 76.4 a | 69.3 a | 45.5 b | 2.88 | <0.001 |
| Leptin (ng/mL) | 2.03 c | 2.61 b | 3.18 a | 2.94 ab | 2.72 b | 0.10 | <0.001 |
| Melanin (pg/mL) | 119 a | 66.7 b | 68.0 b | 114 a | 100 a | 6.02 | <0.001 |
| Items | Treatments | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + DCA | LP + GLUC | LP + DCA + GLUC | |||
| HMGCR (ng/mL) | 238 a | 230 a | 161 b | 173 b | 198 b | 10.4 | <0.001 |
| CYP7A1 (U/L) | 0.02 d | 0.03 cd | 0.05 a | 0.04 b | 0.03 bc | 0.001 | <0.001 |
| Item | Treatments | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + DCA | LP + GLUC | LP + DCA + GLUC | |||
| Villus height (μm) | 495 | 509 | 409 | 447 | 353 | 47.2 | 0.164 |
| Crypt depth (μm) | 302 | 318 | 289 | 289 | 257 | 27.6 | 0.623 |
| Villus height/Crypt depth | 1.64 | 1.63 | 1.54 | 1.60 | 1.41 | 0.19 | 0.911 |
| Lymphocytes amount per villus | 72.6 | 61.8 | 56.8 | 79.2 | 97.8 | 19.2 | 0.600 |
| Goblet cells per crypt | 10.2 | 10.4 | 13.4 | 5.2 | 3.2 | 2.47 | 0.051 |
| Item | Treatments | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + DCA | LP + GLUC | LP + DCA + GLUC | |||
| Streptococcus | 0.19 | 0.11 | 0.14 | 0.19 | 0.13 | 0.08 | 0.370 |
| Lactobacillus | 0.08 | 0.11 | 0.08 | 0.12 | 0.11 | 0.05 | 0.697 |
| Lachnospiraceae_XPB1014_group | 0.06 | 0.04 | 0.06 | 0.16 | 0.11 | 0.04 | 0.127 |
| Prevotellaceae_NK3B31_group | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.007 | 0.072 |
| uncultured_bacterium_f_Muribaculaceae | 0.05 | 0.04 | 0.05 | 0.06 | 0.04 | 0.03 | 0.721 |
| Lachnospiraceae_NK4A136_group | 0.03 | 0.05 | 0.05 | 0.04 | 0.05 | 0.02 | 0.228 |
| Ruminococcaceae_UCG-005 | 0.04 ab | 0.05 a | 0.04 ab | 0.04 ab | 0.03 b | 0.01 | 0.048 |
| Rikenellaceae_RC9_gut_group | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.01 | 0.693 |
| Prevotellaceae_UCG-003 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.007 | 0.072 |
| Prevotellaceae_UCG-001 | 0.05 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 | 0.264 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Chen, H.; Wan, K.; Tang, Z.; Sun, W.; Wu, L.; Ren, Z.; Ding, Q.; Liang, K.; Sun, Z. Effects of Long-Term Low-Protein Diets Supplemented with Sodium Dichloroacetate and Glucose on Metabolic Biomarkers and Intestinal Microbiota of Finishing Pigs. Animals 2022, 12, 2522. https://doi.org/10.3390/ani12192522
Xu Y, Chen H, Wan K, Tang Z, Sun W, Wu L, Ren Z, Ding Q, Liang K, Sun Z. Effects of Long-Term Low-Protein Diets Supplemented with Sodium Dichloroacetate and Glucose on Metabolic Biomarkers and Intestinal Microbiota of Finishing Pigs. Animals. 2022; 12(19):2522. https://doi.org/10.3390/ani12192522
Chicago/Turabian StyleXu, Yetong, Huiyuan Chen, Ke Wan, Zhiru Tang, Weizhong Sun, Liuting Wu, Zhongxiang Ren, Qi Ding, Kaiyang Liang, and Zhihong Sun. 2022. "Effects of Long-Term Low-Protein Diets Supplemented with Sodium Dichloroacetate and Glucose on Metabolic Biomarkers and Intestinal Microbiota of Finishing Pigs" Animals 12, no. 19: 2522. https://doi.org/10.3390/ani12192522
APA StyleXu, Y., Chen, H., Wan, K., Tang, Z., Sun, W., Wu, L., Ren, Z., Ding, Q., Liang, K., & Sun, Z. (2022). Effects of Long-Term Low-Protein Diets Supplemented with Sodium Dichloroacetate and Glucose on Metabolic Biomarkers and Intestinal Microbiota of Finishing Pigs. Animals, 12(19), 2522. https://doi.org/10.3390/ani12192522






