Prediction of Liver Triglyceride Content in Early Lactation Multiparous Holstein Cows Using Blood Metabolite, Mineral, and Protein Biomarker Concentrations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Sampling, and Analysis
2.2. Preparation of Data Sets
2.3. Model Training, Evaluation, and Validation
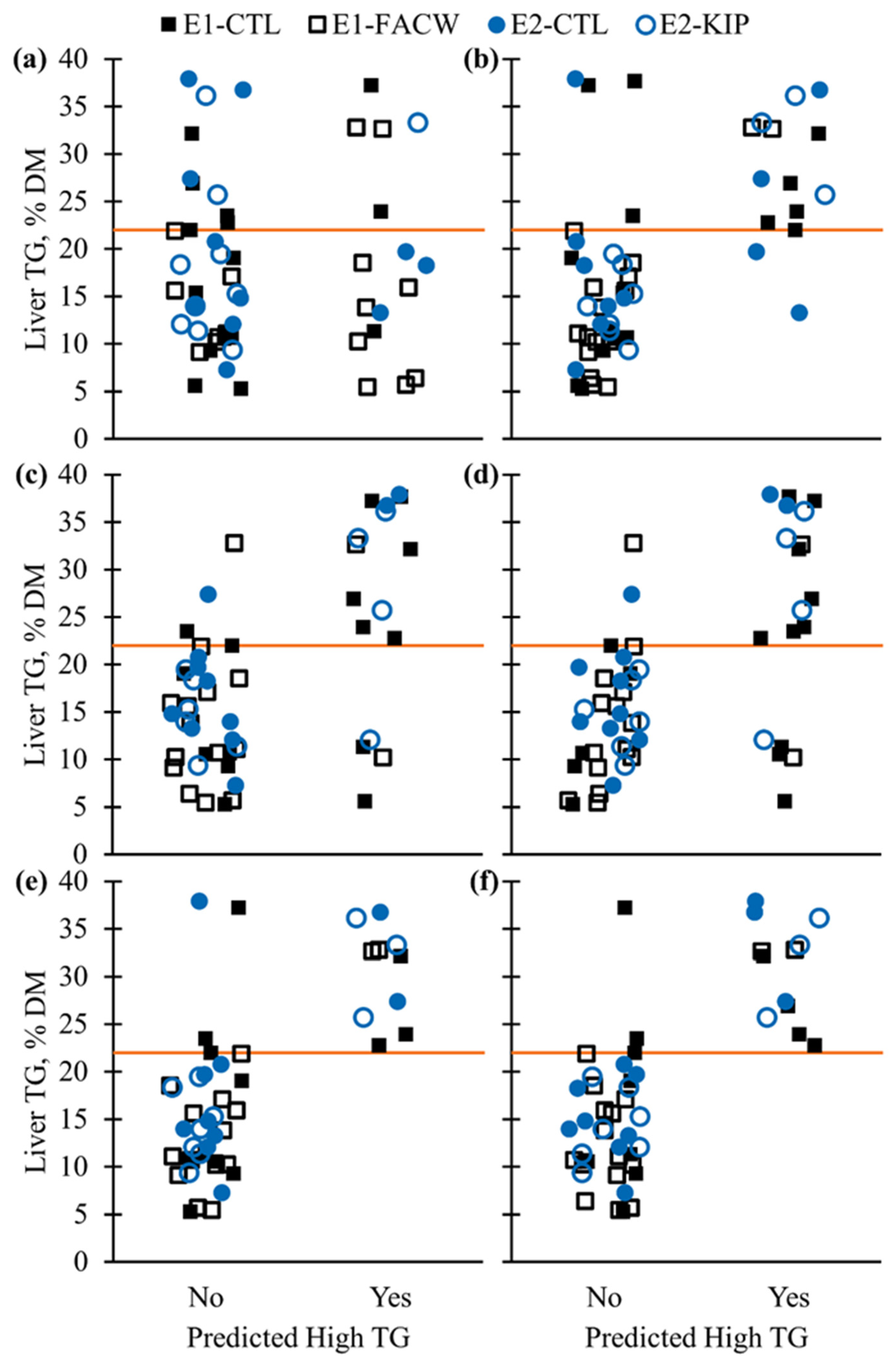
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grummer, R.R. Etiology of Lipid-Related Metabolic Disorders in Periparturient Dairy Cows. J. Dairy Sci. 1993, 76, 3882–3896. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited Review: Pathology, Etiology, Prevention, and Treatment of Fatty Liver in Dairy Cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Bradford, B.J.; Mamedova, L.K.; Minton, J.E.; Drouillard, J.S.; Johnson, B.J. Daily Injection of Tumor Necrosis Factor-α Increases Hepatic Triglycerides and Alters Transcript Abundance of Metabolic Genes in Lactating Dairy Cattle. J. Nutr. 2009, 139, 1451–1456. [Google Scholar] [CrossRef]
- White, H.M. The Role of TCA Cycle Anaplerosis in Ketosis and Fatty Liver in Periparturient Dairy Cows. Animals 2015, 5, 793–802. [Google Scholar] [CrossRef]
- Jorritsma, R.; Jorritsma, H.; Schukken, Y.H.; Bartlett, P.C.; Wensing, T.; Wentink, G.H. Prevalence and Indicators of Post Partum Fatty Infiltration of the Liver in Nine Commercial Dairy Herds in The Netherlands. Livest. Prod. Sci. 2001, 68, 53–60. [Google Scholar] [CrossRef]
- Wensing, T.; Kruip, T.; Geelen, M.J.H.; Wentink, G.H.; van den Top, A.M. Postpartum Fatty Liver in High-Producing Dairy Cows in Practice and in Animal Studies. The Connection with Health, Production and Reproduction Problems. Comp. Haematol. Int. 1997, 7, 167–171. [Google Scholar] [CrossRef]
- Jorritsma, R.; Jorritsma, H.; Schukken, Y.H.; Wentink, G.H. Relationships between Fatty Liver and Fertility and Some Periparturient Diseases in Commercial Dutch Dairy Herds. Theriogenology 2000, 54, 1065–1074. [Google Scholar] [CrossRef]
- Arshad, U.; Santos, J.E.P. Hepatic Triacylglycerol Associations with Production and Health in Dairy Cows. J. Dairy Sci. 2022, 105, 5393–5409. [Google Scholar] [CrossRef] [PubMed]
- Oetzel, G.R. Monitoring and Testing Dairy Herds for Metabolic Disease. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Sailer, K.J.; Pralle, R.S.; Oliveira, R.C.; Erb, S.J.; Oetzel, G.R.; White, H.M. Technical Note: Validation of the BHBCheck Blood β-Hydroxybutyrate Meter as a Diagnostic Tool for Hyperketonemia in Dairy Cows. J. Dairy Sci. 2018, 101, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Couto Serrenho, R.; Bruinjé, T.C.; Morrison, E.I.; Renaud, D.L.; DeVries, T.J.; Duffield, T.F.; LeBlanc, S.J. Validation of a Point-of-Care Handheld Blood Total Calcium Analyzer in Postpartum Dairy Cows. JDS Commun. 2021, 2, 41–45. [Google Scholar] [CrossRef]
- Pralle, R.S.; Li, W.; Murphy, B.N.; Holdorf, H.T.; White, H.M. Novel Facets of the Liver Transcriptome Are Associated with the Susceptibility and Resistance to Lipid-Related Metabolic Disorders in Periparturient Holstein Cows. Animals 2021, 11, 2558. [Google Scholar] [CrossRef] [PubMed]
- van den Top, A.M.; Geelen, M.J.H.; Wensing, T.; Wentink, G.H.; van ’T Klooster, A.T.; Beynen, A.C. Higher Postpartum Hepatic Triacylglycerol Concentrations in Dairy Cows with Free Rather than Restricted Access to Feed during the Dry Period Are Associated with Lower Activities of Hepatic Glycerolphosphate Acyltransferase. J. Nutr. 1996, 126, 76–85. [Google Scholar] [CrossRef]
- Reid, I.M.; Roberts, C.J.; Treacher, R.J.; Williams, L.A. Effect of Body Condition at Calving on Tissue Mobilization, Development of Fatty Liver and Blood Chemistry of Dairy Cows. Anim. Sci. 1986, 43, 7–15. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Bradford, B.J.; Bobe, G.; Nafikov, R.A.; Lu, Y.; Young, J.W.; Beitz, D.C. Strong relationships between mediators of the acute phase response and fatty liver in dairy cows. Can. J. Anim. Sci. 2005, 85, 165–175. [Google Scholar] [CrossRef]
- Sejersen, H.; Sørensen, M.T.; Larsen, T.; Bendixen, E.; Ingvartsen, K.L. Liver protein expression in dairy cows with high liver triglycerides in early lactation. J. Dairy Sci. 2012, 95, 2409–2421. [Google Scholar] [CrossRef] [PubMed]
- Eshraghian, A.; Nikeghbalian, S.; Geramizadeh, B.; Malek-Hosseini, S.A. Serum magnesium concentration is independently associated with non-alcoholic fatty liver and non-alcoholic steatohepatitis. United Eur. Gastroenterol. J. 2018, 6, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, X.; Song, Y.; Fan, L.; Wu, L.; Kabagambe, E.; Hou, L.; Shrubsole, M.H.; Liu, J.; Dai, Q. Intakes of magnesium, calcium and risk of fatty liver disease and prediabetes. Public Health Nutr. 2018, 21, 2088–2095. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, M.J.; Kim, E.S.; Mo, E.Y.; Moon, S.D.; Han, J.H.; Cha, B.Y. Calcium, phosphorus and fatty liver. J. Gastroenterol. Hepatol. 2015, 30, 733–741. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Archetti, I.; Lacetera, N.; Bertoni, G. Inflammatory Response and Acute Phase Proteins in the Transition Period of High-Yielding Dairy Cows. In Acute Phase Proteins as Early Non-Specific Biomarkers of Human and Veterinary Diseases; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E. Use of the Liver Activity Index and Other Metabolic Variables in the Assessment of Metabolic Health in Dairy Herds. Vet. Clin. North Am. Food Anim. Pract. 2013, 29, 413–431. [Google Scholar] [CrossRef]
- Caputo Oliveira, R.; Sailer, K.J.; Holdorf, H.T.; Seely, C.R.; Pralle, R.S.; Hall, M.B.; Bello, N.M.; White, H.M. Postpartum Supplementation of Fermented Ammoniated Condensed Whey Improved Feed Efficiency and Plasma Metabolite Profile. J. Dairy Sci. 2019, 102, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Pralle, R.S.; Erb, S.J.; Holdorf, H.T.; White, H.M. Greater Liver PNPLA3 Protein Abundance in Vivo and in Vitro Supports Lower Triglyceride Accumulation in Dairy Cows. Sci. Rep. 2021, 11, 2839. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, K.; Gobikrushanth, M.; Behrouzi, A.; Hoff, B.; Colazo, M.G. Prevalence of early postpartum health disorders in Holstein cows and associations with production, reproduction, and survival outcomes on Alberta dairy farms. Can. Vet. J. 2021, 62, 273–280. [Google Scholar] [PubMed]
- Pralle, R.S.; Schultz, N.E.; White, H.M.; Weigel, K.A. Hyperketonemia GWAS and Parity Dependent SNP Associations in Holstein Dairy Cows Intensively Sampled for Blood β-Hydroxybutyrate Concentration. Physiol. Genom. 2020, 52, 347–357. [Google Scholar] [CrossRef]
- Caputo Oliveira, R.; Erb, S.J.; Pralle, R.S.; Holdorf, H.T.; Seely, C.R.; White, H.M. Postpartum Supplementation with Fermented Ammoniated Condensed Whey Altered Nutrient Partitioning to Support Hepatic Metabolism. J. Dairy Sci. 2020, 103, 7055–7067. [Google Scholar] [CrossRef]
- Carpenter, A.J.; Ylioja, C.M.; Mamedova, L.K.; Olagaray, K.E.; Bradford, B.J. Effects of Early Postpartum Sodium Salicylate Treatment on Long-Term Milk, Intake, and Blood Parameters of Dairy Cows. J. Dairy Sci. 2018, 101, 1437–1447. [Google Scholar] [CrossRef]
- Michaylova, V.; Ilkova, P. Photometric Determination of Micro Amounts of Calcium with Arsenazo III. Anal. Chim. Acta 1971, 53, 194–198. [Google Scholar] [CrossRef]
- Leon, L.P.; Chu, D.K.; Stasiw, R.O.; Snyder, L.R. New More Specific Methods for SMA 12/60 Multichannel Biochemical Analyzer. Adv. Autom. Anal. Tech. Int. Congr. 1976, 1, 152–156. [Google Scholar]
- Trinder, P. Determination of Glucose in Blood Using Glucose Oxidase with an Alternative Oxygen Acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Ratge, D.; Kohse, K.P.; Wisser, H. Measurement of Magnesium in Serum and Urine with a Random Access Analyzer by Use of a Modified Xylidyl Blue-1 Procedure. Clin. Chim. Acta 1986, 159, 197–203. [Google Scholar] [CrossRef]
- Amador, E.; Urban, J. Simplified Serum Phosphorus Analyses by Continuous-Flow Ultraviolet Spectrophotometry. Clin. Chem. 1972, 18, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.C.; Verkerk, G.A.; Whyte, B.E.; Macdonald, K.A.; Burton, L.; Cursons, R.T.; Roche, J.R.; Holmes, C.W. Somatotropic Axis Components and Nutrient Partitioning in Genetically Diverse Dairy Cows Managed under Different Feed Allowances in a Pasture System. J. Dairy Sci. 2009, 92, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.G.; Crookenden, M.A.; Henty, K.M.; Handley, R.R.; Kuhn-Sherlock, B.; White, H.M.; Donkin, S.S.; Snell, R.G.; Meier, S.; Heiser, A.; et al. Epigenetic Regulation of Pyruvate Carboxylase Gene Expression in the Postpartum Liver. J. Dairy Sci. 2016, 99, 5820–5827. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Foster, L.B.; Dunn, R.T. Stable Reagents for Determination of Serum Triglycerides by a Colorimetric Hantzsch Condensation Method. Clin. Chem. 1973, 19, 338–340. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Rohart, F.; Eslami, A.; Matigian, N.; Bougeard, S.; Lê Cao, K.-A. MINT: A Multivariate Integrative Method to Identify Reproducible Molecular Signatures across Independent Experiments and Platforms. BMC Bioinform. 2017, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Rohart, F.; Gautier, B.; Singh, A.; Cao, K.-A.L. MixOmics: An R Package for ‘omics Feature Selection and Multiple Data Integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Wang, Q.; Bovenhuis, H. Validation strategy can result in an overoptimistic view of the ability of milk infrared spectra to predict methane emission of dairy cattle. J. Dairy Sci. 2019, 102, 6288–6295. [Google Scholar] [CrossRef]
- Qin, L.; Huang, H.; Begg, C.B. Cautionary Note on Using Cross-Validation for Molecular Classification. J. Clinical. Oncol. 2016, 34, 3931–3938. [Google Scholar] [CrossRef]
- Tedeschi, L.O. Assessment of the Adequacy of Mathematical Models. Agric. Syst. 2006, 89, 225–247. [Google Scholar] [CrossRef]
- Liao, J.J.Z. An Improved Concordance Correlation Coefficient. Pharm. Stat. 2003, 2, 253–261. [Google Scholar] [CrossRef]
- Šimundić, A.-M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar] [PubMed]
- Iwersen, M.; Falkenberg, U.; Voigtsberger, R.; Forderung, D.; Heuwieser, W. Evaluation of an Electronic Cowside Test to Detect Subclinical Ketosis in Dairy Cows. J. Dairy Sci. 2009, 92, 2618–2624. [Google Scholar] [CrossRef]
- Bach, K.D.; Heuwieser, W.; McArt, J.A.A. Technical Note: Comparison of 4 Electronic Handheld Meters for Diagnosing Hyperketonemia in Dairy Cows. J. Dairy Sci. 2016, 99, 9136–9142. [Google Scholar] [CrossRef] [PubMed]
- Pralle, R.S.; White, H.M. Symposium Review: Big Data, Big Predictions: Utilizing Milk Fourier-Transform Infrared and Genomics to Improve Hyperketonemia Management. J. Dairy Sci. 2020, 103, 3867–3873. [Google Scholar] [CrossRef] [PubMed]
- Pralle, R.S.; Weigel, K.W.; White, H.M. Predicting Blood β-Hydroxybutyrate Using Milk Fourier Transform Infrared Spectrum, Milk Composition, and Producer-Reported Variables with Multiple Linear Regression, Partial Least Squares Regression, and Artificial Neural Network. J. Dairy Sci. 2018, 101, 4378–4387. [Google Scholar] [CrossRef]
- Chandler, T.L.; Pralle, R.S.; Dórea, J.R.R.; Poock, S.E.; Oetzel, G.R.; Fourdraine, R.H.; White, H.M. Predicting Hyperketonemia by Logistic and Linear Regression Using Test-Day Milk and Performance Variables in Early-Lactation Holstein and Jersey Cows. J. Dairy Sci. 2018, 101, 2476–2491. [Google Scholar] [CrossRef] [PubMed]
- Pralle, R.S.; Amdall, J.D.; Fourdraine, R.H.; Oetzel, G.R.; White, H.M. Hyperketonemia Predictions Provide an On-Farm Management Tool with Epidemiological Insights. Animals 2021, 11, 1291. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, R.B.; Cecava, M.J.; Donkin, S.S. Changes in MRNA Expression for Gluconeogenic Enzymes in Liver of Dairy Cattle during the Transition to Lactation. J. Dairy Sci. 2000, 83, 1228–1236. [Google Scholar] [CrossRef]
- Im, R. Incidence and Severity of Fatty Liver in Dairy Cows. Vet Rec. 1980, 107, 281–284. [Google Scholar] [CrossRef]
- Bollatti, J.M.; Zenobi, M.G.; Barton, B.A.; Staples, C.R.; Santos, J.E.P. Responses to Rumen-Protected Choline in Transition Cows Do Not Depend on Prepartum Body Condition. J. Dairy Sci. 2020, 103, 2272–2286. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of Subclinical Ketosis in Early Lactation Dairy Cattle. J. Dairy Sci. 2012, 95, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.C.; Leno, B.M.; Bach, K.D.; McArt, J.A.A. Epidemiology of Subclinical Hypocalcemia in Early-Lactation Holstein Dairy Cows: The Temporal Associations of Plasma Calcium Concentration in the First 4 Days in Milk with Disease and Milk Production. J. Dairy Sci. 2018, 101, 9321–9331. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R.; Guard, C.L. An Economic Analysis of Hyperketonemia Testing and Propylene Glycol Treatment Strategies in Early Lactation Dairy Cattle. Prev. Vet. Med. 2014, 117, 170–179. [Google Scholar] [CrossRef]
- Kuehne, G.; Llewellyn, R.; Pannell, D.J.; Wilkinson, R.; Dolling, P.; Ouzman, J.; Ewing, M. Predicting Farmer Uptake of New Agricultural Practices: A Tool for Research, Extension and Policy. Agric. Syst. 2017, 156, 115–125. [Google Scholar] [CrossRef]
- Mahrt, A.; Burfeind, O.; Heuwieser, W. Evaluation of Hyperketonemia Risk Period and Screening Protocols for Early-Lactation Dairy Cows. J. Dairy Sci. 2015, 98, 3110–3119. [Google Scholar] [CrossRef]
- White, H. ADSA Foundation Scholar Award: Influencing hepatic metabolism: Can nutrient partitioning be modulated to optimize metabolic health in the transition dairy cow? J. Dairy Sci. 2020, 103, 6741–6750. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Faghihimani, E.; Adibi, P. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 11–26. [Google Scholar] [CrossRef]
- Becker, S.K.; Sponder, G.; Sandhu, M.A.; Trappe, S.; Kolisek, M.; Aschenbach, J. The Combined Influence of Magnesium and Insulin on Central Metabolic Functions and Expression of Genes Involved in Magnesium Homeostasis of Cultured Bovine Adipocytes. Int. J. Mol. Sci. 2021, 22, 5897. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Ospina, P.A.; Oetzel, G.R. A Field Trial on the Effect of Propylene Glycol on Milk Yield and Resolution of Ketosis in Fresh Cows Diagnosed with Subclinical Ketosis. J. Dairy Sci. 2011, 94, 6011–6020. [Google Scholar] [CrossRef]
- Mann, S.; Leal Yepes, F.A.; Wakshlag, J.J.; Behling-Kelly, E.; McArt, J.A.A. The Effect of Different Treatments for Early-Lactation Hyperketonemia on Liver Triglycerides, Glycogen, and Expression of Key Metabolic Enzymes in Dairy Cattle. J. Dairy Sci. 2018, 101, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
| Variable 3 | DIM 4 | n | Mean | SD | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|---|---|
| Parity | 64 | 3.08 | 1.25 | 2.00 | 2.00 | 3.00 | 4.00 | 7.00 | |
| Liver TG, % DM | 1 | 63 | 9.15 | 5.74 | 2.64 | 5.36 | 7.97 | 10.75 | 37.95 |
| 14 | 62 | 17.78 | 10.61 | 2.21 | 9.39 | 15.34 | 24.40 | 45.85 | |
| Max | 62 | 18.95 | 10.32 | 5.28 | 10.97 | 17.10 | 25.83 | 45.85 | |
| ln(Liver TG, % DM) | Max | 62 | 2.79 | 0.57 | 1.66 | 2.40 | 2.80 | 3.25 | 3.83 |
| Blood Biomarkers | |||||||||
| Glucose, mg/dL | 1 | 64 | 67.19 | 18.78 | 43.36 | 57.77 | 61.27 | 70.02 | 143.54 |
| 3 | 64 | 55.86 | 7.05 | 40.85 | 51.18 | 55.65 | 60.02 | 81.06 | |
| 4 | 62 | 54.23 | 7.40 | 33.06 | 50.12 | 54.90 | 59.25 | 70.12 | |
| 5 | 63 | 53.53 | 7.17 | 29.43 | 49.41 | 53.87 | 59.04 | 66.26 | |
| 14 | 64 | 53.64 | 6.17 | 38.93 | 49.62 | 54.32 | 58.62 | 64.79 | |
| tAUC | 62 | 341.67 | 38.18 | 252.96 | 311.38 | 346.08 | 368.43 | 430.08 | |
| Fatty acids, mEq/L | 1 | 38 | 0.49 | 0.24 | 0.20 | 0.34 | 0.43 | 0.58 | 1.32 |
| 3 | 63 | 0.50 | 0.23 | 0.09 | 0.36 | 0.45 | 0.65 | 1.05 | |
| 5 | 63 | 0.47 | 0.24 | 0.12 | 0.30 | 0.45 | 0.57 | 1.40 | |
| 7 | 60 | 0.42 | 0.22 | 0.09 | 0.28 | 0.35 | 0.54 | 1.14 | |
| 14 | 62 | 0.40 | 0.21 | 0.13 | 0.24 | 0.37 | 0.52 | 1.24 | |
| tAUC | 33 | 2.97 | 1.13 | 1.51 | 2.11 | 2.62 | 3.70 | 6.19 | |
| BHB, mM | 1 | 63 | 0.60 | 0.18 | 0.30 | 0.49 | 0.58 | 0.68 | 1.16 |
| 3 | 64 | 0.83 | 0.37 | 0.35 | 0.60 | 0.76 | 0.91 | 2.17 | |
| 5 | 63 | 0.91 | 0.66 | 0.41 | 0.62 | 0.76 | 0.94 | 4.03 | |
| 7 | 64 | 0.95 | 0.71 | 0.36 | 0.63 | 0.74 | 0.97 | 5.42 | |
| 14 | 64 | 0.90 | 0.44 | 0.37 | 0.66 | 0.78 | 0.94 | 2.47 | |
| tAUC | 63 | 5.03 | 2.53 | 2.57 | 3.73 | 4.34 | 5.45 | 17.54 | |
| Albumin, g/dL | 1 | 36 | 3.85 | 0.23 | 3.44 | 3.71 | 3.81 | 3.99 | 4.48 |
| 3 | 64 | 3.74 | 0.23 | 3.08 | 3.61 | 3.76 | 3.88 | 4.24 | |
| 14 | 57 | 3.89 | 0.31 | 3.27 | 3.61 | 3.95 | 4.14 | 4.48 | |
| ALT, U/L | 1 | 37 | 18.92 | 6.46 | 6.87 | 14.50 | 18.62 | 22.35 | 37.82 |
| 3 | 64 | 14.29 | 4.56 | 5.70 | 10.51 | 14.16 | 18.02 | 25.26 | |
| 14 | 62 | 15.98 | 5.48 | 7.51 | 11.70 | 15.41 | 19.88 | 34.11 | |
| AST, U/L | 1 | 40 | 73.74 | 22.09 | 40.28 | 52.76 | 74.29 | 89.73 | 119.04 |
| 3 | 64 | 82.97 | 29.77 | 38.31 | 62.49 | 78.77 | 93.94 | 209.51 | |
| 14 | 63 | 93.73 | 39.46 | 44.08 | 73.05 | 83.93 | 103.67 | 308.58 | |
| AST:ALT | 1 | 37 | 4.12 | 1.20 | 2.39 | 3.16 | 4.12 | 4.96 | 7.08 |
| 3 | 64 | 6.36 | 3.01 | 3.50 | 4.15 | 5.21 | 7.73 | 17.42 | |
| 14 | 62 | 6.47 | 3.19 | 2.52 | 3.98 | 5.67 | 8.09 | 15.83 | |
| BUN, mg/dL | 1 | 39 | 11.38 | 2.90 | 5.98 | 8.88 | 11.67 | 13.35 | 18.61 |
| 3 | 64 | 11.22 | 2.93 | 6.50 | 8.96 | 10.86 | 12.98 | 20.50 | |
| 14 | 63 | 13.33 | 2.87 | 7.19 | 11.12 | 13.36 | 15.00 | 20.33 | |
| Hp, mg/dL | 1 | 38 | 0.76 | 0.51 | 0.11 | 0.36 | 0.65 | 0.96 | 2.69 |
| 3 | 63 | 2.04 | 1.39 | 0.22 | 0.87 | 1.74 | 2.85 | 6.22 | |
| 14 | 62 | 0.51 | 0.49 | 0.06 | 0.23 | 0.32 | 0.56 | 2.83 | |
| Ca, mg/dL | 1 | 50 | 6.76 | 1.00 | 4.50 | 6.31 | 6.78 | 7.38 | 9.20 |
| 3 | 52 | 7.99 | 1.02 | 4.35 | 7.56 | 8.15 | 8.70 | 9.70 | |
| 5 | 52 | 8.41 | 1.07 | 5.15 | 7.61 | 8.35 | 9.35 | 10.85 | |
| 7 | 53 | 8.21 | 1.16 | 4.85 | 7.45 | 8.45 | 8.88 | 11.20 | |
| 14 | 52 | 8.65 | 0.82 | 6.45 | 8.28 | 8.73 | 9.25 | 10.05 | |
| Mg, mg/dL | 1 | 51 | 2.15 | 0.38 | 1.39 | 1.83 | 2.15 | 2.37 | 3.22 |
| 3 | 53 | 2.18 | 0.30 | 1.10 | 2.04 | 2.19 | 2.35 | 2.94 | |
| 5 | 53 | 1.89 | 0.33 | 1.01 | 1.67 | 1.88 | 2.12 | 2.52 | |
| 7 | 53 | 1.89 | 0.30 | 1.17 | 1.69 | 1.88 | 2.09 | 2.50 | |
| 14 | 52 | 2.20 | 0.38 | 1.26 | 2.02 | 2.24 | 2.53 | 2.87 | |
| Phos, mg/dL | 1 | 51 | 3.79 | 1.05 | 1.63 | 3.11 | 3.59 | 4.46 | 6.06 |
| 3 | 53 | 4.48 | 1.07 | 1.95 | 3.85 | 4.40 | 4.97 | 8.15 | |
| 5 | 53 | 4.49 | 0.88 | 2.16 | 3.93 | 4.46 | 5.16 | 6.01 | |
| 7 | 53 | 4.07 | 0.65 | 2.76 | 3.55 | 4.04 | 4.60 | 5.46 | |
| 14 | 52 | 4.28 | 0.80 | 2.66 | 3.70 | 4.18 | 4.80 | 5.89 | |
| Cholesterol, mg/dL | 1 | 51 | 59.30 | 11.87 | 38.46 | 49.75 | 58.92 | 65.27 | 92.08 |
| 3 | 53 | 63.81 | 12.28 | 34.68 | 54.55 | 64.30 | 71.71 | 96.81 | |
| 5 | 53 | 68.86 | 12.81 | 41.28 | 59.79 | 69.00 | 74.79 | 97.54 | |
| 7 | 53 | 77.05 | 13.51 | 45.88 | 67.37 | 75.86 | 85.80 | 105.48 | |
| 14 | 52 | 112.89 | 20.72 | 79.47 | 97.90 | 108.92 | 128.87 | 169.06 |
| Variables 3 | Random Split CV 4 | Block CV 8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSE 5 | MAE 6 | R2 | |||||||||
| Mean | SE 7 | Mean | SE | Mean | SE | RMSE | MAE | R2 | CCC 9 | Mean Bias | |
| ST3 | 0.81 | 0.07 | 1.40 | 0.06 | 0.20 | 0.05 | 0.47 | 0.39 | 0.25 | 0.28 | −0.01 |
| ST7 | 0.70 | 0.05 | 1.20 | 0.04 | 0.29 | 0.04 | 0.45 | 0.35 | 0.34 | 0.36 | <0.01 |
| ST14 | 0.89 | 0.04 | 1.40 | 0.04 | 0.11 | 0.03 | 0.52 | 0.43 | 0.12 | 0.17 | −0.05 |
| LT | 0.66 | 0.05 | 1.20 | 0.05 | 0.33 | 0.05 | 0.44 | 0.35 | 0.38 | 0.43 | −0.01 |
| Model | Random Split CV 3 | Block CV 7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BER 4 | rAUC 5 | ||||||||||
| Response 1 | Explanatory 2 | Mean | SE 6 | Mean | SE | BER | Accuracy | Sensitivity | Specificity | PPV 8 | NPV 9 |
| Low TG | ST3 | 35.7 | 5.2 | 69.5 | 5.1 | 43.1 | 53.9 | 45.5 | 68.5 | 28.6 | 74.2 |
| ST7 | 32.2 | 2.1 | 75.7 | 1.2 | 28.5 | 68.0 | 58.9 | 84.3 | 87.0 | 53.4 | |
| ST14 | 42.3 | 3.7 | 63.0 | 4.1 | 40.8 | 59.7 | 60.7 | 57.9 | 71.5 | 45.9 | |
| LT | 33.9 | 6.4 | 69.2 | 6.0 | 15.7 | 80.9 | 92.4 | 76.5 | 60.0 | 96.3 | |
| Median TG | ST3 | 33.8 | 4.3 | 69.9 | 3.9 | 33.0 | 67.4 | 65.3 | 69.0 | 37.5 | 82.2 |
| ST7 | 19.6 | 2.4 | 80.9 | 1.2 | 18.4 | 83.1 | 66.7 | 96.6 | 94.2 | 77.8 | |
| ST14 | 37.9 | 5.0 | 61.8 | 4.8 | 48.9 | 52.0 | 41.7 | 60.8 | 47.7 | 54.9 | |
| LT | 24.0 | 4.4 | 80.2 | 4.5 | 25.3 | 76.6 | 57.2 | 92.4 | 85.8 | 72.8 | |
| High TG | ST3 | 35.4 | 3.5 | 68.2 | 3.8 | 46.7 | 61.6 | 35.8 | 71.1 | 31.3 | 75.0 |
| ST7 | 13.9 | 2.5 | 95.0 | 0.8 | 17.3 | 86.8 | 73.4 | 92.2 | 78.6 | 89.8 | |
| ST14 | 15.3 | 3.0 | 90.5 | 1.9 | 15.5 | 86.6 | 80.0 | 89.2 | 75.0 | 91.7 | |
| LT | 15.4 | 3.9 | 90.8 | 3.7 | 11.6 | 93.7 | 77.0 | 100.0 | 100.0 | 91.9 | |
| Model | Random Split CV 3 | Block CV 7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BER 4 | ROC AUC 5 | ||||||||||
| Sampling | Explanatory 2 | Mean | SE 6 | Mean | SE | BER | Accuracy | Sensitivity | Specificity | PPV 8 | NPV 9 |
| ST14 | noHp | 15.3 | 3.1 | 90.2 | 1.8 | 13.4 | 86.5 | 86.7 | 86.5 | 72.2 | 94.1 |
| limit1 | 26.6 | 4.5 | 80.5 | 3.5 | 27.1 | 76.9 | 73.3 | 78.4 | 57.9 | 87.9 | |
| limit2 | 24.3 | 2.8 | 84.6 | 2.1 | 20.7 | 86.2 | 61.1 | 97.5 | 91.7 | 84.8 | |
| LT | no1DIM | 13.3 | 3.2 | 94.3 | 2.7 | 7.2 | 96.0 | 85.7 | 100.0 | 100.0 | 94.7 |
| limit1 | 15.3 | 5.0 | 91.5 | 4.1 | 15.4 | 91.5 | 69.2 | 100.0 | 100.0 | 89.5 | |
| limit2 | 17.6 | 3.6 | 86.5 | 2.7 | 18.3 | 86.8 | 68.8 | 94.6 | 84.6 | 87.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pralle, R.S.; Holdorf, H.T.; Caputo Oliveira, R.; Seely, C.R.; Kendall, S.J.; White, H.M. Prediction of Liver Triglyceride Content in Early Lactation Multiparous Holstein Cows Using Blood Metabolite, Mineral, and Protein Biomarker Concentrations. Animals 2022, 12, 2556. https://doi.org/10.3390/ani12192556
Pralle RS, Holdorf HT, Caputo Oliveira R, Seely CR, Kendall SJ, White HM. Prediction of Liver Triglyceride Content in Early Lactation Multiparous Holstein Cows Using Blood Metabolite, Mineral, and Protein Biomarker Concentrations. Animals. 2022; 12(19):2556. https://doi.org/10.3390/ani12192556
Chicago/Turabian StylePralle, Ryan S., Henry T. Holdorf, Rafael Caputo Oliveira, Claira R. Seely, Sophia J. Kendall, and Heather M. White. 2022. "Prediction of Liver Triglyceride Content in Early Lactation Multiparous Holstein Cows Using Blood Metabolite, Mineral, and Protein Biomarker Concentrations" Animals 12, no. 19: 2556. https://doi.org/10.3390/ani12192556
APA StylePralle, R. S., Holdorf, H. T., Caputo Oliveira, R., Seely, C. R., Kendall, S. J., & White, H. M. (2022). Prediction of Liver Triglyceride Content in Early Lactation Multiparous Holstein Cows Using Blood Metabolite, Mineral, and Protein Biomarker Concentrations. Animals, 12(19), 2556. https://doi.org/10.3390/ani12192556





