Bioprocessing of Two Crop Residues for Animal Feeding into a High-Yield Lovastatin Feed Supplement
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Analysis of Crop Residues
2.2. Inoculum and Culture Propagation
2.3. Conditions of Solid-State Fermentation
2.4. Determination of Lovastatin
2.5. Identification of Organic Compounds from Post-Fermented Substrates by Gas Chromatography-Mass Spectrometry
2.6. Calculations and Statistical Analysis
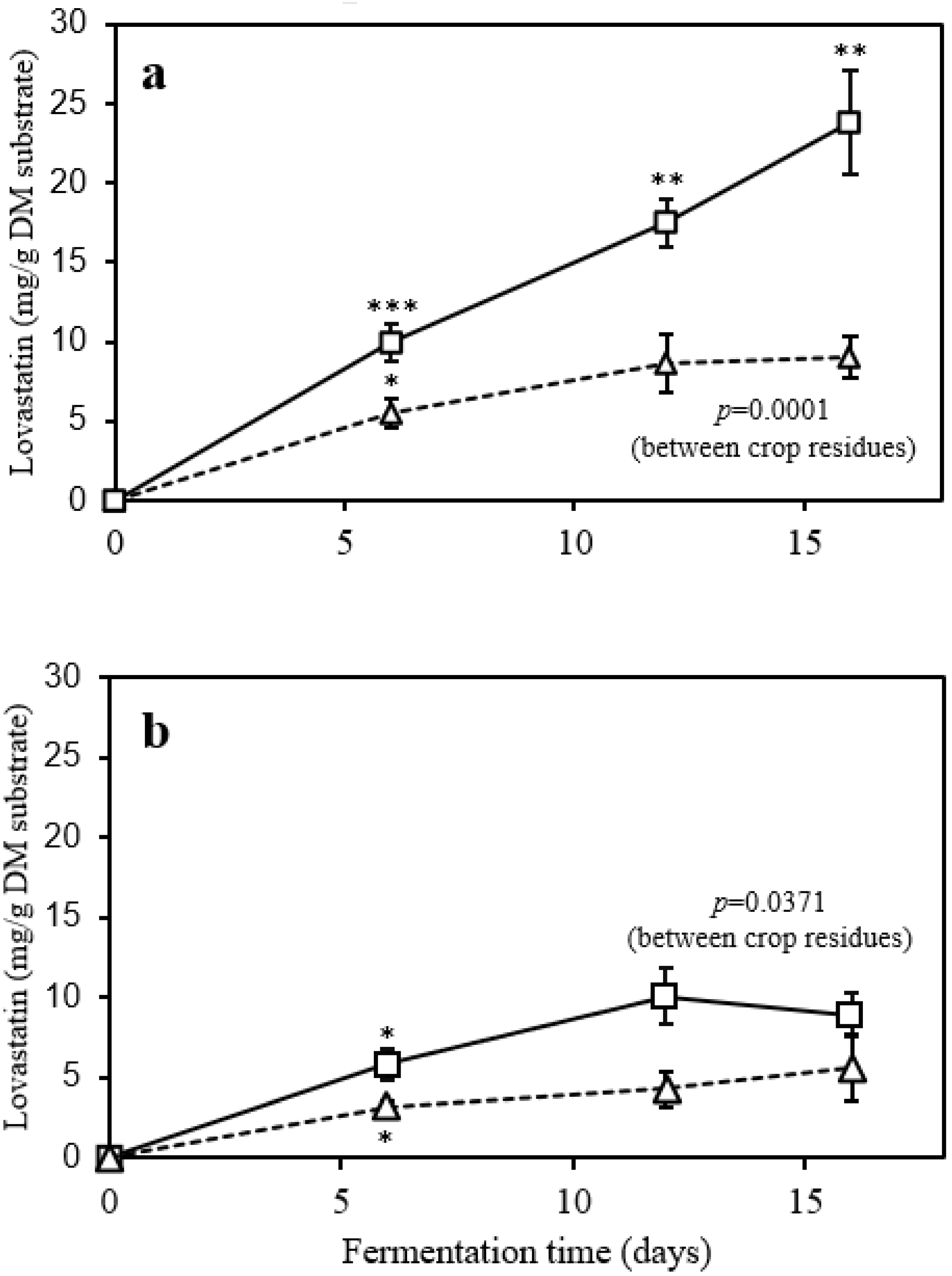
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Godoy, M.G.; Amorim, G.M.; Barreto, M.S.; Freire, D.M.G. Agricultural residues as animal feed: Protein enrichment and detoxification using solid-state fermentation. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C., Eds.; Elseiver: Amsterdam, The Netherlands, 2018; pp. 235–256. ISBN 978-0-444-63990-5. [Google Scholar]
- Van Kuijk, S.; Sonnenberg, A.; Baars, J.; Hendriks, W.; Cone, J. Fungal treated lignocellulosic biomass as ruminant feed ingredient: A review. Biotechnol. Adv. 2015, 33, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kothari, C. Critical review of statins: A bio-analytical perspective for therapeutic drug monitoring. Trends Anal. Chem. 2017, 86, 206–221. [Google Scholar] [CrossRef]
- Ábrego-Gacía, A.; Poggi-Varaldo, H.M.; Mendoza-Vargas, A.; Mercado-Valle, F.G.; Ríos-Leal, E.; Ponce-Noyola, T.; Calva-Calva, G. Effects of Fermented Oat Straw as a Lovastatin Carrier on in vitro Methane Production and Rumen Microbiota. Front. Energy Res. 2021, 9, 630701. [Google Scholar] [CrossRef]
- Ábrego-Gacía, A.; Poggi-Varaldo, H.M.; Robles-González, V.; Ponce-Noyola, T.; Calva-Calva, G.; Ríos-Leal, E.; Estrada-Bárcenas, D.; Mendoza-Vargas, A. Lovastatin as a supplement to mitigate rumen methanogenesis: An overview. J. Anim. Sci. Biotechnol. 2021, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Nkamga, V.D.; Armstrong, N.; Drancourt, M. In vitro susceptibility of cultured human methanogens to lovastatin. Int. J. Antimicrob. Agents 2017, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ravuri, M.; Shivakumar, S. Statistical optimization of production parameters for enhanced lovastatin production from endophytic Aspergillus terreus. Biocatal. Agric. Biotechnol. 2020, 29, 101787. [Google Scholar] [CrossRef]
- Patil, R.H.; Krishnan, P.; Maheshwari, V.L. Production of lovastatin by wild strains of Aspergillus terreus. Nat. Prod. Commun. 2011, 6, 183–186. [Google Scholar] [CrossRef]
- Syed, M.B.; Rajendran, A.; Seraman, S.; Thangavelu, V. Valorization of Agricultural Residues for Compactin Production by Aspergillus terreus MTCC 279 in Mixed Substrate Solid State Fermentation. Waste Biomass Valorization 2014, 5, 715–724. [Google Scholar] [CrossRef]
- Pansuriya, R.C.; Singhal, R.S. Response surface methodology for optimization of lovastatin production by solid state fermentation. Braz. J. Microbiol. 2010, 41, 164–172. [Google Scholar] [CrossRef]
- Gulyamova, T.; Ruzieva, D.; Nasmetova, S.; Sattarova, R.; Lobanova, K.; Abdulmyanova, L.; Rasulova, G. Lovastatin production by Aspergillus terreus in solid state and submerged fermentations. Int. J. Eng. Sci. Technol. 2013, 5, 19–24. [Google Scholar] [CrossRef]
- Azlan, P.M.; Jahromi, M.F.; Ariff, M.O.; Ebrahimi, M.; Candyrine, S.C.L.; Liang, J.B. Aspergillus terreus treated rice straw suppresses methane production and enhances feed digestibility in goats. Trop. Anim. Health Prod. 2018, 50, 565–571. [Google Scholar] [CrossRef]
- Bashir, T.; Asgher, M.; Hussain, F.; Bhatti, H.N. Optimization of process variables for hyper-production of lovastatin from wild type Aspergillus terreus and its efficacy studies. Rev. Mex. Ing. Quim. 2019, 9, 929–939. [Google Scholar] [CrossRef]
- Munir, N.; Asghar, M.; Murtaza, M.A.; Akhter, N.; Rasool, G.; Shah, S.M.A.; Tahir, I.M.; Khan, F.S.; Riaz, M.; Sultana, S.; et al. Enhanced production of lovastatin by filamentous fungi through solid state fermentation. Pak. J. Pharm. Sci. 2018, 31, 1583–1589. [Google Scholar]
- Ali, H.K.Q.; Zulkali, M.M.D. Design aspects of bioreactors for solid-state fermentation: A review. Chem. Biochem. Eng. Q. 2011, 25, 255–266. [Google Scholar] [CrossRef]
- Mulder, K.C.L.; Mulinari, F.; Franco, O.L.; Soares, M.S.F.; Magalhães, B.S.; Parachin, N.S. Lovastatin production: From molecular basis to industrial process optimization. Biotechnol. Adv. 2015, 33, 648–665. [Google Scholar] [CrossRef]
- Subhan, M.; Faryal, R.; Macreadie, I. Exploitation of Aspergillus terreus for the Production of Natural Statins. J. Fungi 2013, 2, 13. [Google Scholar] [CrossRef]
- Neto, R.N.M.; Gomes, E.D.B.; Weba-Soares, L.; Dias, L.R.; Da Silva, L.C.; Miranda, R.D.C.M.D.; Da Silva, L.C.N. Biotechnology Production of Statins: Metabolic Aspects and Genetic Approaches. Curr. Pharm. Biotechnol. 2019, 20, 1244–1259. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Song, J.; Luo, J.; Ma, Z.; Sun, Q.; Wu, C.; Li, X. Quality and Authenticity Control of Functional Red Yeast Rice—A Review. Molecules 2019, 24, 1944. [Google Scholar] [CrossRef]
- Wen, Q.; Cao, X.; Chen, Z.; Xiong, Z.; Liu, J.; Cheng, Z.; Zheng, Z.; Long, C.; Zheng, B.; Huang, Z. An overview of Monascus fermentation processes for monacolin K production. Open Chem. J. 2020, 18, 10–21. [Google Scholar] [CrossRef]
- Anthony, J.M.; Asthana, M.; Kumar, A. Mycotoxins and their consequences in livestock. In Fungal Diseases in Animals. Fungal Biology; Gupta, A., Pratap Singh, N., Eds.; Springer: Cham, Switzerland, 2021; pp. 15–34. ISBN 978-3-030-69507-1. [Google Scholar]
- Křížová, L.; Dadáková, K.; Dvořáčková, M.; Kašparovský, T. Feedborne Mycotoxins Beauvericin and Enniatins and Livestock Animals. Toxins 2021, 13, 32. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Martin, C.; Boudra, H. Fungal secondary metabolites from Monascus spp. reduce rumen methane production in vitro and in vivo. J. Anim. Sci. 2013, 91, 848–860. [Google Scholar] [CrossRef]
- Candyrine, S.C.L.; Mahadzir, M.F.; Garba, S.; Jahromi, M.F.; Ebrahimi, M.; Goh, Y.M.; Samsudin, A.A.; Sazili, A.Q.; Chen, W.L.; Ganesh, S.; et al. Effects of naturally-produced lovastatin on feed digestibility, rumen fermentation, microbiota and methane emissions in goats over a 12-week treatment period. PLoS ONE 2018, 13, e0199840. [Google Scholar] [CrossRef]
- Khonkhaeng, B.; Cherdthong, A. Improving nutritive value of purple field corn residue and rice straw by culturing with white-rot fungi. J. Fungi 2020, 6, 69. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2002; ISBN 093558467-6. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Mouafi, F.E.; Ibrahim, G.S.; Elsoud, M.M.A. Optimization of lovastatin production from Aspergillus fumigatus. J. Genet. Eng. Biotechnol. 2016, 14, 253–259. [Google Scholar] [CrossRef]
- Jirasatid, S.; Nopharatana, M.; Kitsubun, P.; Vichitsoonthonkul, T.; Tongta, A. Statistical Optimization for Monacolin K and Yellow Pigment Production and Citrinin Reduction by Monascus purpureus in Solid-State Fermentation. J. Microbiol. Biotechnol. 2013, 23, 364–374. [Google Scholar] [CrossRef]
- Xu, B.-J.; Wang, Q.-J.; Jia, X.-Q.; Sung, C.-K. Enhanced lovastatin production by solid state fermentation of Monascus ruber. Biotechnol. Bioprocess Eng. 2005, 10, 78–84. [Google Scholar] [CrossRef]
- Jaivel, N.; Marimuthu, P. Optimization of lovastatin production in solid state fermentation by Aspergillus terreus. Int. J. Eng. Sci. Technol. 2010, 2, 2730–2733. [Google Scholar]
- Yang, D.-J.; Hwang, L.S. Study on the conversion of three natural statins from lactone forms to their corresponding hydroxy acid forms and their determination in Pu-Erh tea. J. Chromatogr. A 2006, 1119, 277–284. [Google Scholar] [CrossRef]
- Nyilasi, I.; Kocsubé, S.; Krizsán, K.; Galgóczy, L.; Papp, T.; Pesti, M.; Nagy, K.; Vágvölgyi, C. Susceptibility of clinically important dermatophytes against statins and different statin-antifungal combinations. Med. Mycol. 2014, 52, 140148. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Fernandes, C.; Barros, L.; Ferreira, I.C.F.R.; Pereira, L.; Gonçalves, T. The antifungal activity of extracts of Osmundea pinnatifida, an edible seaweed, indicates its usage as a safe environmental fungicide or as food additive preventing post-harvest fungal food contamination. Food Funct. 2018, 9, 6187–6195. [Google Scholar] [CrossRef] [PubMed]
- Gezan, S.A.; Carvalho, M. Analysis of repeated measures for the biological and agricultural sciences. In Applied Statistics in Agricultural, Biological, and Environmental Sciences; Glaz, B., Yeater, K.M., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America Inc.: Madison, WI, USA, 2018; pp. 279–298. [Google Scholar]
- SAS Institute Inc. SAS® Studio 3.8: Administrator’s Guide; SAS Institute Inc.: Cary, NC, USA, 2018. Available online: https://go.documentation.sas.com/api/docsets/webeditorag/3.8/content/webeditorag.pdf (accessed on 9 February 2022).
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models; SAS Institute Inc.: Cary, NC, USA, 2006; 828p. [Google Scholar]
- Shrivastava, B.; Jain, K.K.; Kalra, A.; Kuhad, R.C. Bioprocessing of wheat straw into nutritionally rich and digested cattle feed. Sci. Rep. 2014, 4, 6360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Lu, L.P.; Xu, G.R. Why solid-state fermentation is more advantageous over submerged fermentation for converting high concentration of glycerol into Monacolin K by Monascus purpureus 9901: A mechanistic study. J. Biotechnol. 2015, 206, 60–65. [Google Scholar] [CrossRef]
- Hutchinson, C.R.; Kennedy, J.; Park, C.; Kendrew, S.; Auclair, K.; Vederas, J. Aspects of the biosynthesis of non-aromatic fungal polyketides by iterative polyketide synthases. Antonie Leeuwenhoek 2000, 78, 287–295. [Google Scholar] [CrossRef]
- Rollini, M.; Manzoni, M. Influence of medium design on lovastatin and mevastatin production by Aspergillus terreus strains. Ann. Microbiol. 2006, 56, 47–51. [Google Scholar] [CrossRef]
- Ryngajłło, M.; Boruta, T.; Bizukojć, M. Complete genome sequence of lovastatin producer Aspergillus terreus ATCC 20542 and evaluation of genomic diversity among A. terreus strains. Appl. Microbiol. Biotechnol. 2021, 105, 1615–1627. [Google Scholar] [CrossRef]
- Praveen, V.K.; Bhargavi, S.D.; Savitha, J. Endophytic Fungi: A Poor Candidate for the Production of Lovastatin. Br. Microbiol. Res. J. 2014, 4, 1511–1520. [Google Scholar] [CrossRef]
- Bhargavi, S.D.; Praveen, V.K.; Savitha, J. Bioinformatic Comparative Analysis of Lovastatin Gene Cluster in Endophytic Fungi and a Soil Fungus, Aspergillus terreus. MOJ Proteom. Bioinform. 2014, 1, 114–117. [Google Scholar] [CrossRef]
- Walsh, T.J.; Petraitis, V.; Petraitiene, R.; Field-Ridley, A.; Sutton, D.; Ghannoum, M.; Sein, T.; Schaufele, R.; Peter, J.; Bacher, J.; et al. Experimental Pulmonary Aspergillosis Due to Aspergillus terreus: Pathogenesis and Treatment of an Emerging Fungal Pathogen Resistant to Amphotericin B. J. Infect. Dis. 2003, 188, 305–319. [Google Scholar] [CrossRef]
- Bhargavi, S.D.; Praveen, V.K.; Kumar, M.A.; Savitha, J. Comparative Study on Whole Genome Sequences of Aspergillus terreus (Soil Fungus) and Diaporthe ampelina (Endophytic Fungus) with Reference to Lovastatin Production. Curr. Microbiol. 2017, 75, 84–91. [Google Scholar] [CrossRef]
- Levenspiel, O. Ingeniería de las Reacciones Químicas, 3rd ed.; Limusa Wiley: Mexico City, Mexico, 2004; p. 669. [Google Scholar]
- Darwish, G.A.M.A.; Bakr, A.A.; Abdallah, M.M.F. Nutritional value upgrading of maize stalk by using Pleurotus ostreatus and Saccharomyces cerevisiae in solid state fermentation. Ann. Agric. Sci. 2012, 57, 47–51. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, P.; Zhang, X.; Zhu, X.; van Lier, J.B.; Spanjers, H. White rot fungi pretreatment to advance volatile fatty acid production from solid-state fermentation of solid digestate: Efficiency and mechanisms. Energy 2018, 162, 534–541. [Google Scholar] [CrossRef]
- Costa-Silva, V.; Anunciação, M.; Andrade, E.; Fernandes, L.; Costa, A.; Fraga, I.; Barros, A.; Marques, G.; Ferreira, L.; Rodrigues, M. Biovalorization of Grape Stalks as Animal Feed by Solid State Fermentation Using White-Rot Fungi. Appl. Sci. 2022, 12, 6800. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S. Cellulose-degrading enzymes from Aspergillus terreus D34 and enzymatic saccharification of mild-alkali and dilute-acid pretreated lignocellulosic biomass residues. Bioresour. Bioprocess. 2015, 2, 85. [Google Scholar] [CrossRef][Green Version]
- Joch, M.; Vadroňová, M.; Výborná, A.; Jochová, K. Inhibition of in vitro rumen methane production by three statins. Ann. Anim. Sci. 2021, 22, 271–282. [Google Scholar] [CrossRef]
- Del Río, J.C.; Prinsen, P.; Gutiérrez, A. A Comprehensive Characterization of Lipids in Wheat Straw. J. Agric. Food Chem. 2013, 61, 1904–1913. [Google Scholar] [CrossRef]
- Bharose, A.A.; Gajera, H.P. Antifungal Activity and Metabolites Study of Bacillus Strain Against Aflatoxin Producing Aspergillus. J. Appl. Microbiol. Biochem. 2018, 2, 8. [Google Scholar] [CrossRef]
- Jubie, S.; Ramesh, P.N.; Dhanabal, P.; Kalirajan, R.; Muruganantham, N.; Shanish, A. Synthesis, antidepressant and antimicrobial activities of some novel stearic acid analogues. Eur. J. Med. Chem. 2012, 54, 931–935. [Google Scholar] [CrossRef]
- Alger, B.E. Defense of the Scientific Hypothesis: From Reproducibility Crisis to Big Data; Oxford University Press: New York, NY, USA, 2020; pp. 161–195. [Google Scholar]
- Gastel, B.; Day, R.A. How to Write and Publish a Scientific Paper, 8th ed.; Greenwood, ABC-CLIO LLC: Santa Barbara, CA, USA, 2016; pp. 66–71. [Google Scholar]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Review: Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12, s295–s309. [Google Scholar] [CrossRef]
- Diggle, P.J.; Chetwynd, A.G. Statistics and Scientific Method. An Introduction for Students and Researchers; Oxford University Press: New York, NY, USA, 2011; 172p. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 10th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2019; pp. 152–188. [Google Scholar]

| SSF Process | Performance and Results | Remarks | Ref. |
|---|---|---|---|
| Strain: A. terreus PM3 Spore concentration: 108 spores/mL Substrate: wheat bran | T = 25 °C, M0 = 60%, Fermentation time = 10 d, pH = 7.1. [Lv] = 12.5 mg/g DM substrate | Sa: No/0; Bcs: No/0; Des: No/0; Sar: No/0; Klp: No/0; Coc: No/0 Score 0. | [8] |
| Strain: A. terreus MTCC 279 Spore concentration: 106 spores/mL Substrate: wheat bran. | T = 25 °C, M0 = 66%, Fermentation time = 3 d, pH = 6, [Lv] = 13.4 mg/g DM substrate | Sa: Yes/1; Pas: No/0; Des: No/0, Sar: Yes/1; Klp: No/0; Coc: No/0 Score 2. | [9] |
| Strain: A. terreus UV 1718 Spore concentration: 108 spores/mL Substrate: wheat bran | T = 28 °C, M0 = 70%, Fermentation time = 10 d, pH = 6, [Lv] = 3.7 mg/g DM substrate | Sa: No/0; Pas: No/0; Des: No/0; Sar: Yes/1; Klp: No/0; Coc: No/0 Score 1. | [10] |
| Strain: A. terreus 20 Spore concentration: 107 spores/mL Substrate: Oat bran | T = 28 °C, M0 = 55–65%, Fermentation time = 14 d, pH = 7.5, [Lv] = 9.5 mg/g DM substrate | Sa: No/0; Pas: No/0; Des: No/0; Sar: No/0; Klp: No/0; Coc: No/0 Score 0. | [11] |
| Strain: A. terreus ATCC 74135 Spore concentration: 107 spores/mL Substrate: rice straw | T = 25 °C, M0 = 50%, Fermentation time = 14 d, pH = 6, [Lv] = 0.69 mg/g DM substrate | Sa: Yes/1; Pas: Yes/1; Des: No/0; Sar: Yes/1; Klp: No/0; Coc: No/0 Score 3. | [12] |
| Strain: A. terreus Spore concentration: 108 spores/mL Substrate: wheat straw | T = 30 °C, M0 = Not reported, Fermentation time = 8 d, pH = 7.3, [Lv] = 60 mg/g DM substrate | Sa: No/0; Pas: No/0; Des: No/0; Sar: Yes/1; Klp: No. Coc: No/0 Score 1. | [13] |
| Strain: A. terreus FFCBP-1053 Substrate: rice straw Spore concentration: 5 × 107–5 × 108 spores/mL | T = 35 °C, M0 = 70%, Fermentation time = 10 d, pH = 4.5, [Lv] = 2.1 mg/g DM substrate | Sa: Yes/1; Pas: No/0; Des: No/0; Sar: Yes/1; Klp: No/0; Coc: No/0 Score 2. | [14] |
| Strain: A terreus CDBB H-194 Substrate: Oat straw Spore concentration: 107 spores/mL | T = 26 °C, M0 = 70%, Fermentation time = 16 d, pH = 5.5, [Lv] = 23.8 mg/g DM substrate | Sa: Yes/1; Pas: Yes/1; Des: Yes/1; Sar: Yes/1; Klp: Yes/1; Coc: Yes/1 Score 6. | This work |
| Item | Oat Straw | Wheat Bran |
|---|---|---|
| Dry Matter (g/kg) | 945.2 ± 25.1 | 932.6 ± 18.7 |
| Chemical composition parameter (g/kg DM): | ||
| CP a | 43.3 ± 6.1 | 151.8 ± 12.0 |
| EE b | 31.1 ± 4.8 | 47.5 ± 5.2 |
| Ash | 62.6 ± 5.5 | 77.5 ± 6.7 |
| NDF c | 680.2 ± 52.6 | 568.0 ± 47.3 |
| ADF d | 415.8 ± 27.3 | 174.2 ± 15.8 |
| Oat Straw | Wheat Bran | |||
|---|---|---|---|---|
| Item | Unfermented | Fermented | Unfermented | Fermented |
| Hemicellulose (g/kg DM) | 267.5 ± 12.6 | 232.1 ± 15.5 | 393.9 ± 18.3 | 340.2 ± 16.0 * e |
| Cellulose (g/kg DM) | 324.2 ±11.2 | 291.7 ± 12.0 | 118.0 ± 8.3 | 82.2 ± 7.4 * f |
| Lignin (g/kg DM) | 83.8 ± 10.2 | 74.2 ± 5.18 | 56.0 ± 3.0 | 47.6 ± 4.2 |
| η(c + h) a (%) | – | 24.10 | – | 30.24 |
| ηlig b (%) | – | 14.92 | – | 15.93 |
| ε (-) c | – | 0.6191 | – | 0.5268 |
| ESSF (%) d | – | 14.14 | – | 9.38 |
| PN a | RT b (Min) | Compound Name | # CAS | Area (%) |
|---|---|---|---|---|
| 1 | 3.319 | 3,6-Dioxa-2,7-disilaoctane, 2,2,4,7,7-pentamethyl- | 17887-27-3 | 0.509 |
| 2 | 9.891 | Trimethylsilyl ether of glycerol | 6787-10-6 | 6.020 |
| 3 | 13.593 | Silane, [(11-bromoundecyl)oxy]trimethyl- | 26305-83-9 | 0.631 |
| 4 | 15.303 | Butanoic acid, 3-methyl-2-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 55124-92-0 | 0.621 |
| 5 | 15.979 | Gulonic acid, 2,3,5,6-tetrakis-O-(trimethylsilyl)-, lactone | 55528-75-1 | 1.648 |
| 6 | 18.915 | bis [2-Trimethylsiloxy]ethyl sulfone | 97916-04-6 | 11.670 |
| 7 | 20.861 | Xylitol, 1,2,3,4,5-pentakis-O-(trimethylsilyl)- | 14199-72-5 | 1.136 |
| 8 | 21.811 | Propanoic acid, 2-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 17596-96-2 | 0.458 |
| 9 | 24.982 | Hexacosanoic acid, methyl ester | 5802-82-4 | 1.787 |
| 10 | 26.575 | Heptadecanoic acid, trimethylsilyl ester | 55517-58-3 | 3.270 |
| 11 | 27.313 | 9-Octadecenoic acid (Z)-, methyl ester | 112-62-9 | 14.416 |
| 12 | 27.987 | Linoleic acid ethyl ester | 544-35-4 | 0.867 |
| 13 | 28.062 | Ethyl 9-hexadecenoate | 54546-22-4 | 1.271 |
| 14 | 28.447 | 9,12-Octadecadienoic acid (Z,Z)-, trimethylsilyl ester | 56259-07-5 | 12.062 |
| 15 | 28.747 | Oleic acid, trimethylsilyl ester | 21556-26-3 | 2.250 |
| 16 | 32.134 | Dodecanedioic acid, bis(trimethylsilyl) ester | 22396-19-6 | 0.601 |
| 17 | 32.959 | 1-Monooleoylglycerol trimethylsilyl ether | 54284-47-8 | 1.492 |
| 18 | 33.224 | 1-Monooleoylglycerol trimethylsilyl ether | 54284-47-8 | 2.152 |
| 19 | 33.344 | 3á,4á-Bis(trimethylsiloxy)cholest-5-ene | 33287-25-1 | 0.492 |
| 20 | 33.419 | 1-Monooleoylglycerol trimethylsilyl ether | 54284-47-8 | 0.743 |
| 21 | 33.634 | Dodecanedioic acid, bis(trimethylsilyl) ester | 22396-19-6 | 0.630 |
| 22 | 35.180 | Cholesterol trimethylsilyl ether | 1856-05-9 | 2.149 |
| 23 | 35.415 | 9,12-Octadecadienoic acid (Z,Z)-, trimethylsilyl ester | 56259-07-5 | 1.560 |
| 24 | 36.666 | 3Beta-hydroxy-5-cholen-24-oic acid | 5255-17-4 | 1.243 |
| 25 | 37.196 | Simvastatin | 79902-63-9 | 4.141 |
| 26 | 38.156 | Cystathionine, bis(triemthylsilyl) ester | 73090-79-6 | 0.533 |
| 27 | 39.522 | Dodecanedioic acid, bis(trimethylsilyl) ester | 22396-19-6 | 0.470 |
| 28 | 40.722 | Cholesterol trimethylsilyl ether | 1856-05-9 | 1.822 |
| 29 | 41.027 | Dodecanedioic acid, bis(trimethylsilyl) ester | 22396-19-6 | 0.645 |
| 30 | 42.358 | 1,3-Dipalmitin trimethylsilyl ether | 53212-95-6 | 1.148 |
| PN a | RT b (Min) | Compound Name | # CAS | Area (%) |
|---|---|---|---|---|
| 1 | 9.741 | Trimethylsilyl ether of glycerol | 6787-10-6 | 1.753 |
| 2 | 10.901 | Silane, [(1-methyl-1,3-propanediyl)bis(oxy)]bis[trimethyl- | 56771-47-2 | 2.963 |
| 3 | 11.492 | Carbonic dihydrazide | 497-18-7 | 0.686 |
| 4 | 13.508 | Stearic acid hydrazide | 4130-54-5 | 13.508 |
| 5 | 15.233 | Carbonic dihydrazide | 497-18-7 | 0.66 |
| 6 | 20.81 | Xylitol, 1,2,3,4,5-pentakis-O-(trimethylsilyl)- | 14199-72-5 | 1.635 |
| 7 | 22.166 | Gulonic acid, 2,3,5,6-tetrakis-O-(trimethylsilyl)-, lactone | 55528-75-1 | 3.484 |
| 8 | 23.421 | Dodecanedioic acid, bis(trimethylsilyl) ester | 22396-19-6 | 1.005 |
| 9 | 24.819 | D-Mannitol, 1,2,3,4,5,6-hexakis-O-(trimethylsilyl)- | 14317-07-8 | 5.914 |
| 10 | 25.339 | Gulonic acid, 2,3,5,6-tetrakis-O-(trimethylsilyl)-, lactone | 55528-75-1 | 2.513 |
| 11 | 25.784 | Xylitol, 1,2,3,4,5-pentakis-O-(trimethylsilyl)- | 14199-72-5 | 5.427 |
| 12 | 26.329 | Dodecanedioic acid, bis(trimethylsilyl) ester | 22396-19-6 | 1.443 |
| 13 | 26.655 | Gulonic acid, 2,3,5,6-tetrakis-O-(trimethylsilyl)-, lactone | 55528-75-1 | 2.171 |
| 14 | 27.211 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 112-63-0 | 10.538 |
| 15 | 27.952 | 9-Octadecynoic acid, methyl ester | 1120-32-7 | 0.99 |
| 16 | 28.132 | 8,11,14-Eicosatrienoic acid, (Z,Z,Z)- | 1783-84-2 | 10.041 |
| 17 | 31.493 | Guanosine | 118-00-3 | 0.632 |
| 18 | 32.859 | 1-Monooleoylglycerol trimethylsilyl ether | 54284-47-8 | 0.662 |
| 19 | 33.124 | 1-Monooleoylglycerol trimethylsilyl ether | 54284-47-8 | 1.333 |
| 20 | 33.424 | 9,12-Octadecadienoic acid (Z,Z)-, trimethylsilyl ester | 56259-07-5 | 1.011 |
| 21 | 33.649 | 8,11,14-Eicosatrienoic acid, (Z,Z,Z)- | 1783-84-2 | 1.541 |
| 22 | 35.335 | á Carotene | 7235-40-7 | 2.235 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ábrego-García, A.; Poggi-Varaldo, H.M.; Ponce-Noyola, M.T.; Calva-Calva, G.; Galíndez-Mayer, C.J.J.; Medina-Mendoza, G.G.; Rinderknecht-Seijas, N.F. Bioprocessing of Two Crop Residues for Animal Feeding into a High-Yield Lovastatin Feed Supplement. Animals 2022, 12, 2697. https://doi.org/10.3390/ani12192697
Ábrego-García A, Poggi-Varaldo HM, Ponce-Noyola MT, Calva-Calva G, Galíndez-Mayer CJJ, Medina-Mendoza GG, Rinderknecht-Seijas NF. Bioprocessing of Two Crop Residues for Animal Feeding into a High-Yield Lovastatin Feed Supplement. Animals. 2022; 12(19):2697. https://doi.org/10.3390/ani12192697
Chicago/Turabian StyleÁbrego-García, Amaury, Héctor M. Poggi-Varaldo, M. Teresa Ponce-Noyola, Graciano Calva-Calva, Cutberto José Juvencio Galíndez-Mayer, Gustavo G. Medina-Mendoza, and Noemí F. Rinderknecht-Seijas. 2022. "Bioprocessing of Two Crop Residues for Animal Feeding into a High-Yield Lovastatin Feed Supplement" Animals 12, no. 19: 2697. https://doi.org/10.3390/ani12192697
APA StyleÁbrego-García, A., Poggi-Varaldo, H. M., Ponce-Noyola, M. T., Calva-Calva, G., Galíndez-Mayer, C. J. J., Medina-Mendoza, G. G., & Rinderknecht-Seijas, N. F. (2022). Bioprocessing of Two Crop Residues for Animal Feeding into a High-Yield Lovastatin Feed Supplement. Animals, 12(19), 2697. https://doi.org/10.3390/ani12192697





