Dietary Fatty Acid Composition Impacts the Fatty Acid Profiles of Different Regions of the Bovine Brain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Brain Dissection
2.3. Fatty Acid Analysis
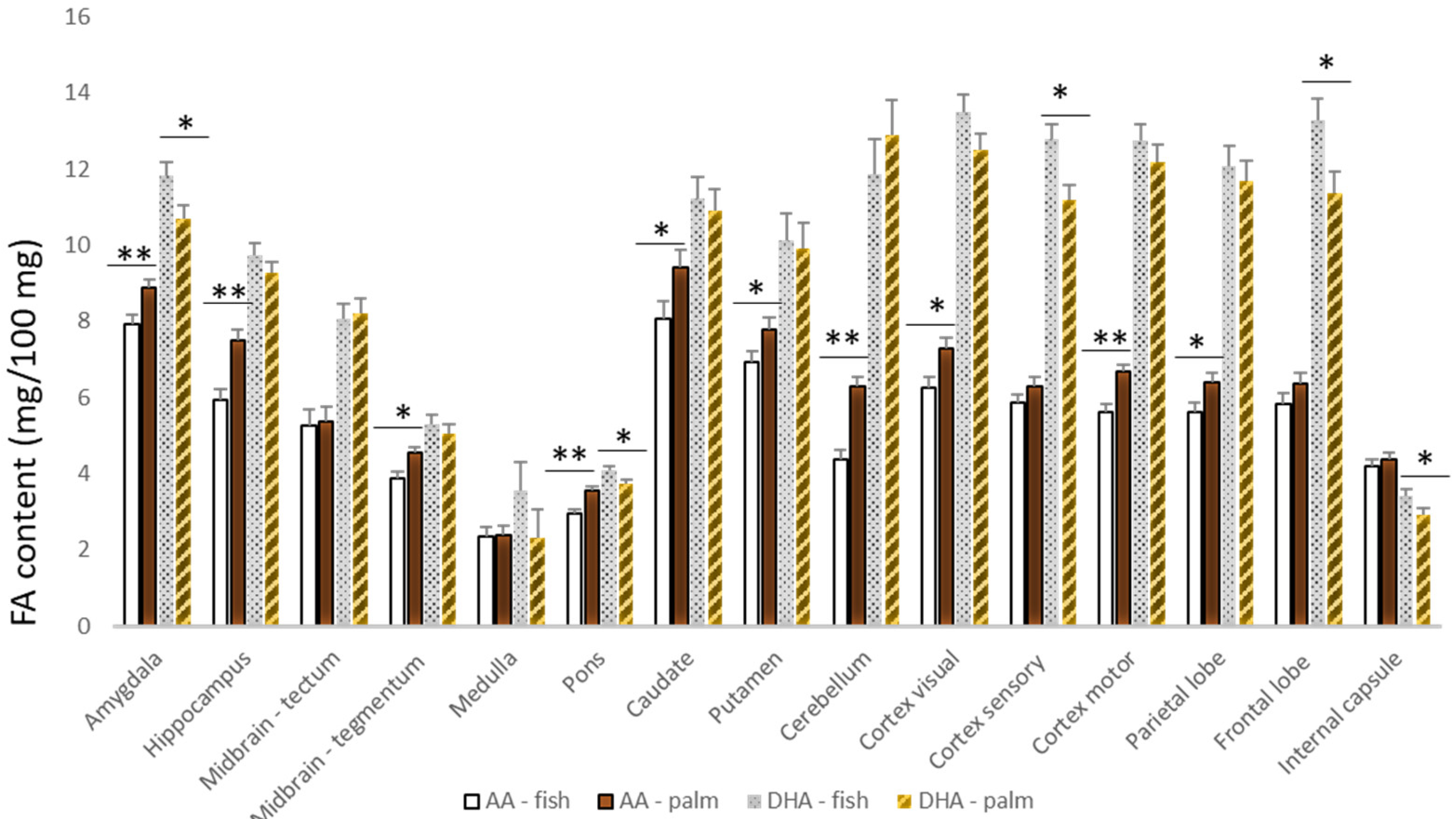
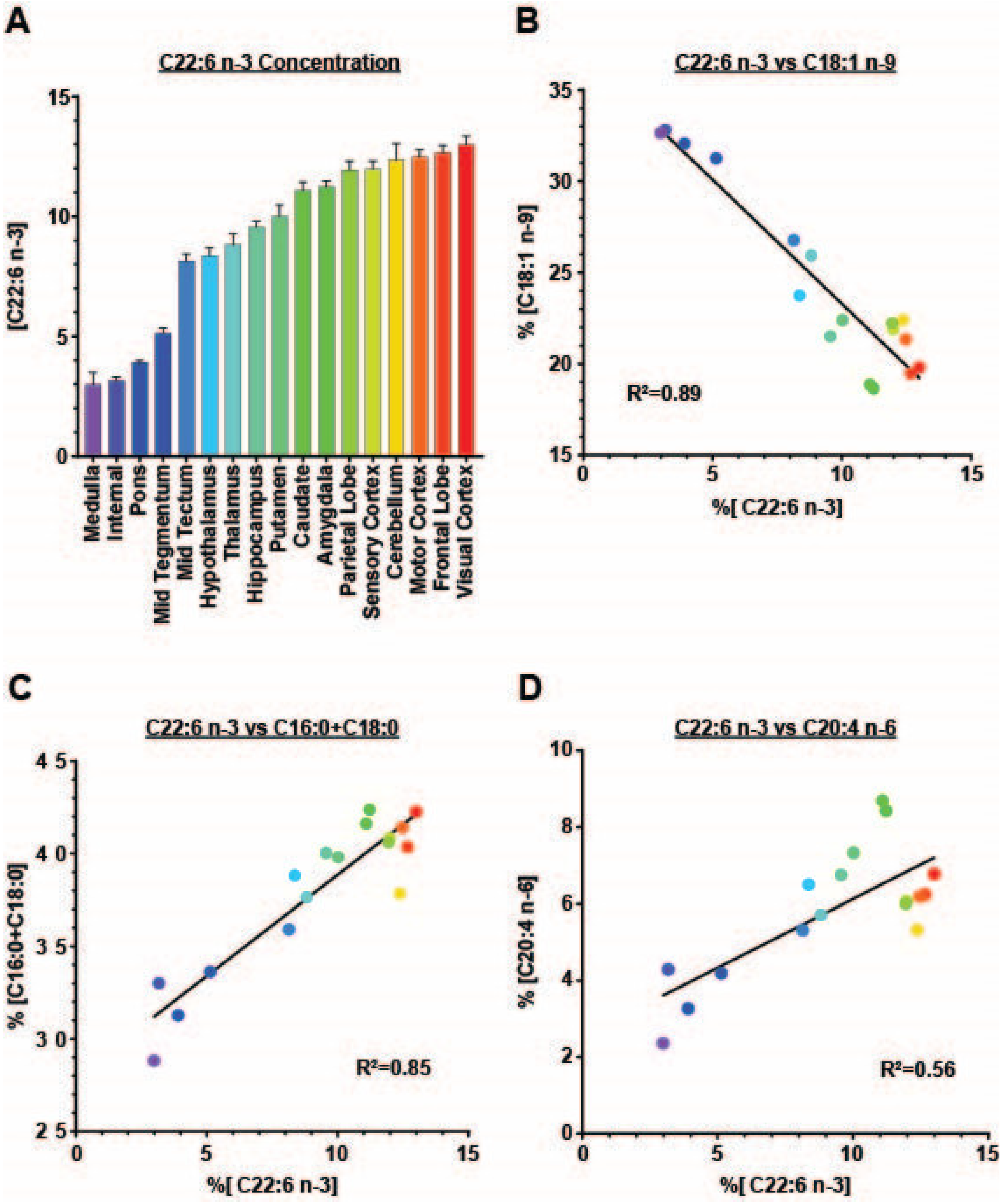
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrié, I.; Clément, M.; de Javel, D.; Francès, H.; Bourre, J.-M. Specific phospholipid fatty acid composition of brain regions in mice: Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000, 41, 465–472. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty acid transporting proteins: Roles in brain development, aging, and stroke. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Levant, B.; Ozias, M.K.; Carlson, S.E. Diet (n-3) polyunsaturated fatty acid content and parity interact to alter maternal rat brain phospholipid fatty acid composition. J. Nutr. 2006, 136, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Diau, G.-Y. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 247–250. [Google Scholar] [CrossRef]
- McNamara, R.K.; Carlson, S.E. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Y.; Chen, Z.-Y. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br. J. Nutr. 2005, 94, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Lamaziere, A.; Richard, D.; Barbe, U.; Kefi, K.; Bausero, P.; Wolf, C.; Visioli, F. Differential distribution of DHA-phospholipids in rat brain after feeding: A lipidomic approach. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.I.; Rao, J.S.; Igarashi, M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 251–261. [Google Scholar] [CrossRef] [PubMed]
- DeMar, J.C.; Lee, H.-J.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I.; Bazinet, R.P. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2006, 1761, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Green, J.T.; Orr, S.K.; Bazinet, R.P. Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Liu, Z.; Ouellet, M.; Calon, F.; Bazinet, R.P. Rapid β-oxidation of eicosapentaenoic acid in mouse brain: An in situ study. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Bazinet, R.P. β-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.; Emond, V.; Chen, C.T.; Julien, C.; Bourasset, F.; Oddo, S.; LaFerla, F.; Bazinet, R.P.; Calon, F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochem. Int. 2009, 55, 476–482. [Google Scholar] [CrossRef]
- Mitchell, R.W.; Hatch, G.M. Fatty acid transport into the brain: Of fatty acid fables and lipid tails. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Pélerin, H.; Jouin, M.; Lallemand, M.-S.; Alessandri, J.-M.; Cunnane, S.C.; Langelier, B.; Guesnet, P. Gene expression of fatty acid transport and binding proteins in the blood-brain barrier and the cerebral cortex of the rat: Differences across development and with different DHA brain status. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 213–220. [Google Scholar] [CrossRef]
- Levant, B.; Ozias, M.K.; Carlson, S.E. Sex-specific effects of brain LC-PUFA composition on locomotor activity in rats. Physiol. Behav. 2006, 89, 196–204. [Google Scholar] [CrossRef]
- Levant, B.; Ozias, M.K.; Carlson, S.E. Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J. Nutr. 2007, 137, 130–134. [Google Scholar] [CrossRef]
- Extier, A.; Langelier, B.; Perruchot, M.-H.; Guesnet, P.; Van Veldhoven, P.P.; Lavialle, M.; Alessandri, J.-M. Gender affects liver desaturase expression in a rat model of n-3 fatty acid repletion. J. Nutr. Biochem. 2010, 21, 180–187. [Google Scholar] [CrossRef]
- McNamara, R.K.; Able, J.; Jandacek, R.; Rider, T.; Tso, P. Gender differences in rat erythrocyte and brain docosahexaenoic acid composition: Role of ovarian hormones and dietary omega-3 fatty acid composition. Psychoneuroendocrinology 2009, 34, 532–539. [Google Scholar] [CrossRef]
- Diau, G.-Y.; Hsieh, A.T.; Sarkadi-Nagy, E.A.; Wijendran, V.; Nathanielsz, P.W.; Brenna, J.T. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 2005, 3, 1–12. [Google Scholar] [CrossRef]
- Poureslami, R.; Raes, K.; Huyghebaert, G.; De Smet, S. Effects of diet, age and gender on the polyunsaturated fatty acid composition of broiler anatomical compartments. Br. Poult. Sci. 2010, 51, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Watkins, B.; Florant, G.; Kelher, M.; Rogers, L.; Li, Y. Fatty acid analysis of wild ruminant tissues: Evolutionary implications for reducing diet-related chronic disease. Eur. J. Clin. Nutr. 2002, 56, 181–191. [Google Scholar] [CrossRef]
- Rule, D.C.; Beitz, D.C. Fatty-Acids of Adipose-Tissue, Plasma, Muscle and Duodenal Ingesta of Steers Fed Extruded Soybeans. J. Am. Oil Chem. Soc. 1986, 63, 1429–1436. [Google Scholar] [CrossRef]
- Harfoot, C.G. Lipid Metabolism in the Rumen. In Lipid Metabolism in Ruminant Animals; Christie, W.W., Ed.; Pergamon: Oxford, UK, 1981; pp. 21–55. [Google Scholar]
- Ponnampalam, E.; Sinclair, A.; Holman, B. The sources, synthesis and biological actions of omega-3 and omega-6 fatty acids in red meat: An overview. Foods 2021, 10, 1358. [Google Scholar] [CrossRef]
- Christie, W.W. The composition, structure and function of lipids in the tissues of ruminant animals. Lipid Metab. Rumin. Anim. 1981, 95–191. [Google Scholar] [CrossRef]
- Vanderwolf, C.H.; Cooley, R.K. The Sheep Brain: A Photographic Series; AJ Kirby Company: London, ON, Canada, 1990. [Google Scholar]
- Murrieta, C.; Hess, B.; Rule, D. Comparison of acidic and alkaline catalysts for preparation of fatty acid methyl esters from ovine muscle with emphasis on conjugated linoleic acid. Meat Sci. 2003, 65, 523–529. [Google Scholar] [CrossRef]
- Weston, T.R.; Derner, J.D.; Murrieta, C.M.; Rule, D.C.; Hess, B.W. Comparison of catalysts for direct transesterification of fatty acids in freeze-dried forage samples. Crop Sci. 2008, 48, 1636–1641. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Martin, R.E.; Wickham, J.Q.; Om, A.-S.; Sanders, J.; Ceballos, N. Uptake and incorporation of docosahexaenoic acid (DHA) into neuronal cell body and neurite/nerve growth cone lipids: Evidence of compartmental DHA metabolism in nerve growth factor-differentiated PC12 cells. Neurochem. Res. 2000, 25, 715–723. [Google Scholar] [CrossRef]
- Gajos, J.M.; Beaver, K.M. The effect of omega-3 fatty acids on aggression: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 69, 147–158. [Google Scholar] [CrossRef]
- Jumpsen, J.; Lien, E.L.; Goh, Y.K.; Clandinin, M.T. Small changes of dietary (n-6) and (n-3) fatty acid content ratio alter phosphatidylethanolamine and phosphatidylcholine fatty acid composition during development of neuronal and glial cells in rats. J. Nutr. 1997, 127, 724–731. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.J.; Greenwood, C.E. Dietary saturated fatty acids and brain function. Neurochem. Res. 1998, 23, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.I.; Ramadan, E.; Basselin, M. Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an in vivo biomarker of brain DHA metabolism and neurotransmission. Prostaglandins Other Lipid Mediat. 2011, 96, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Polozova, A.; Salem, N. Role of liver and plasma lipoproteins in selective transport of n-3 fatty acids to tissues: A comparative study of C-14-DHA and H-3-oleic acid tracers. J. Mol. Neurosci. 2007, 33, 56–66. [Google Scholar] [CrossRef]
- Rapoport, S.I. In vivo fatty acid incorporation into brain phosholipids in relation to plasma availability, signal transduction and membrane remodeling. J. Mol. Neurosci. 2001, 16, 243–262. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | Forage | Fish Oil Calcium Salt | Palm Oil Calcium Salt |
|---|---|---|---|
| C14:0 | 0.90 | 6.08 | 0.81 |
| C16:0 | 24.20 | 21.08 | 48.76 |
| C16:1 | 2.13 | 6.39 | 1.92 |
| C18:0 | 3.29 | 6.89 | 3.74 |
| C18:1 n-9 | 2.22 | 14.99 | 34.11 |
| C18:2 n-6 | 18.88 | 6.25 | 10.15 |
| C18:3 n-3 | 48.38 | 1.06 | 0.51 |
| C20:4 n-6 | -- | 1.13 | -- |
| C20:5 n-3 (EPA) | -- | 11.23 | -- |
| C22:5 n-3 | -- | 1.80 | -- |
| C22:6 n-3 (DHA) | -- | 7.73 | -- |
| Serum Fatty Acids | Liver Fatty Acids | |||||
|---|---|---|---|---|---|---|
| Fatty Acid a | Fish Oil | Palm Oil | SEM b | Fish Oil | Palm Oil | SEM b |
| C16:0 | 13.39 | 14.01 | 0.31 | 15.00 | 16.00 | 0.59 |
| C18:0 | 15.93 | 17.70 | 0.32 ** | 28.00 | 27.40 | 0.50 |
| C18:1 n-9 | 6.24 | 9.18 | 0.20 ** | 7.94 | 10.87 | 0.45 ** |
| C18:2 n-6 | 24.39 | 27.87 | 1.30 | 5.69 | 6.61 | 0.19 ** |
| C18:3 n-3 | 10.96 | 9.69 | 0.47 | 1.98 | 1.66 | 0.07 ** |
| C20:4 n-6 (AA) | 3.01 | 3.92 | 0.20 ** | 4.98 | 7.80 | 0.13 ** |
| C20:5 n-3 | 8.86 | 2.06 | 0.46 ** | 6.30 | 2.52 | 0.25 ** |
| C22:6 n-3 (DHA) | 2.27 | 1.08 | 0.20 ** | 6.55 | 2.72 | 0.22 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rule, D.C.; Melson, E.A.; Alexander, B.M.; Brown, T.E. Dietary Fatty Acid Composition Impacts the Fatty Acid Profiles of Different Regions of the Bovine Brain. Animals 2022, 12, 2696. https://doi.org/10.3390/ani12192696
Rule DC, Melson EA, Alexander BM, Brown TE. Dietary Fatty Acid Composition Impacts the Fatty Acid Profiles of Different Regions of the Bovine Brain. Animals. 2022; 12(19):2696. https://doi.org/10.3390/ani12192696
Chicago/Turabian StyleRule, Daniel C., Emily A. Melson, Brenda M. Alexander, and Travis E. Brown. 2022. "Dietary Fatty Acid Composition Impacts the Fatty Acid Profiles of Different Regions of the Bovine Brain" Animals 12, no. 19: 2696. https://doi.org/10.3390/ani12192696
APA StyleRule, D. C., Melson, E. A., Alexander, B. M., & Brown, T. E. (2022). Dietary Fatty Acid Composition Impacts the Fatty Acid Profiles of Different Regions of the Bovine Brain. Animals, 12(19), 2696. https://doi.org/10.3390/ani12192696





