Ultrasound, Dacryocystorhinography and Morphological Examination of Normal Eye and Lacrimal Apparatus of the Donkey (Equus asinus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals
2.3. Clinical Examination
2.4. Ultrasonographic Examination
2.5. Radiographic Examination (Dacryocystorhinography)
2.6. Anatomical Studies
3. Results
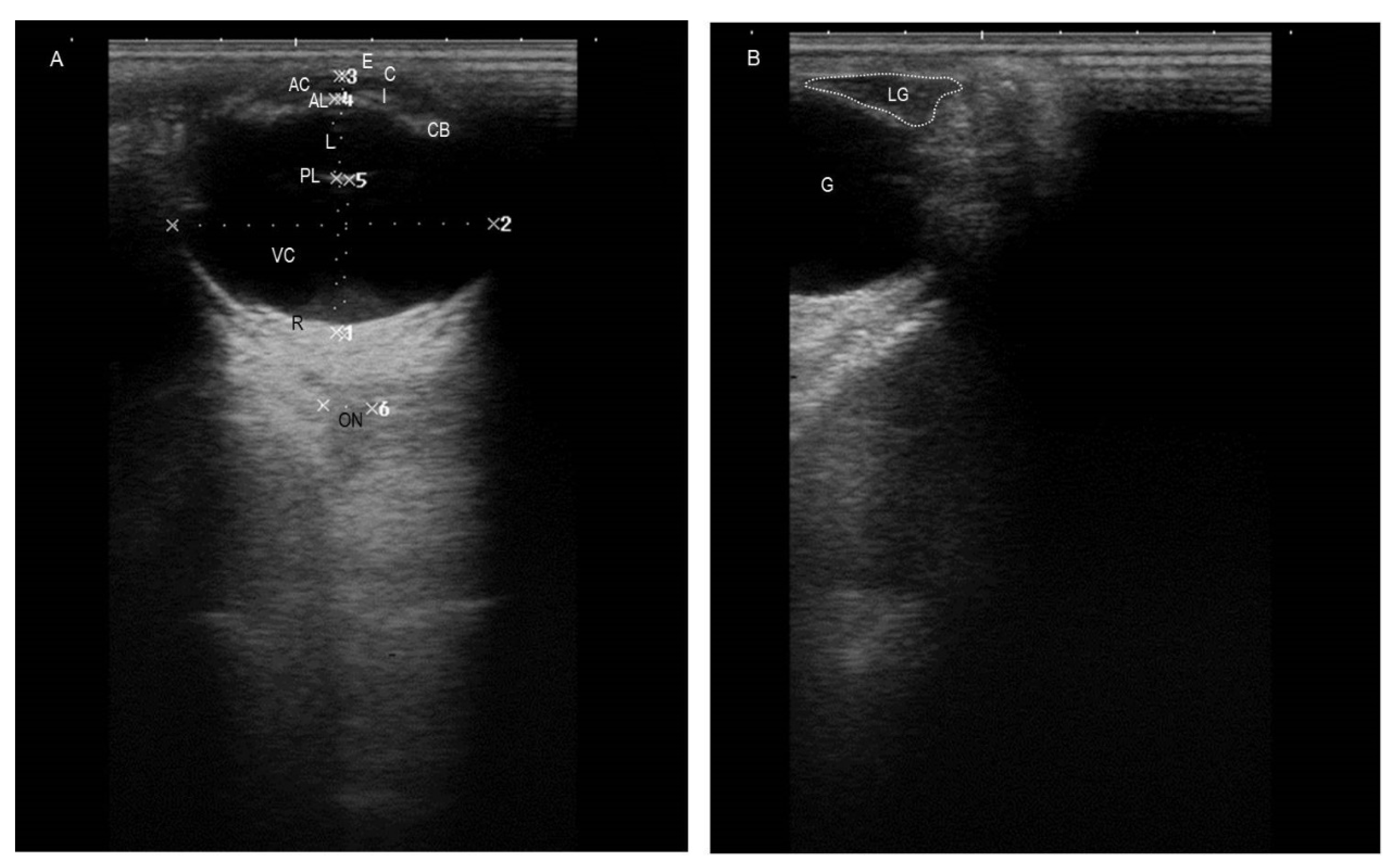
3.1. Ultrasonographic Findings
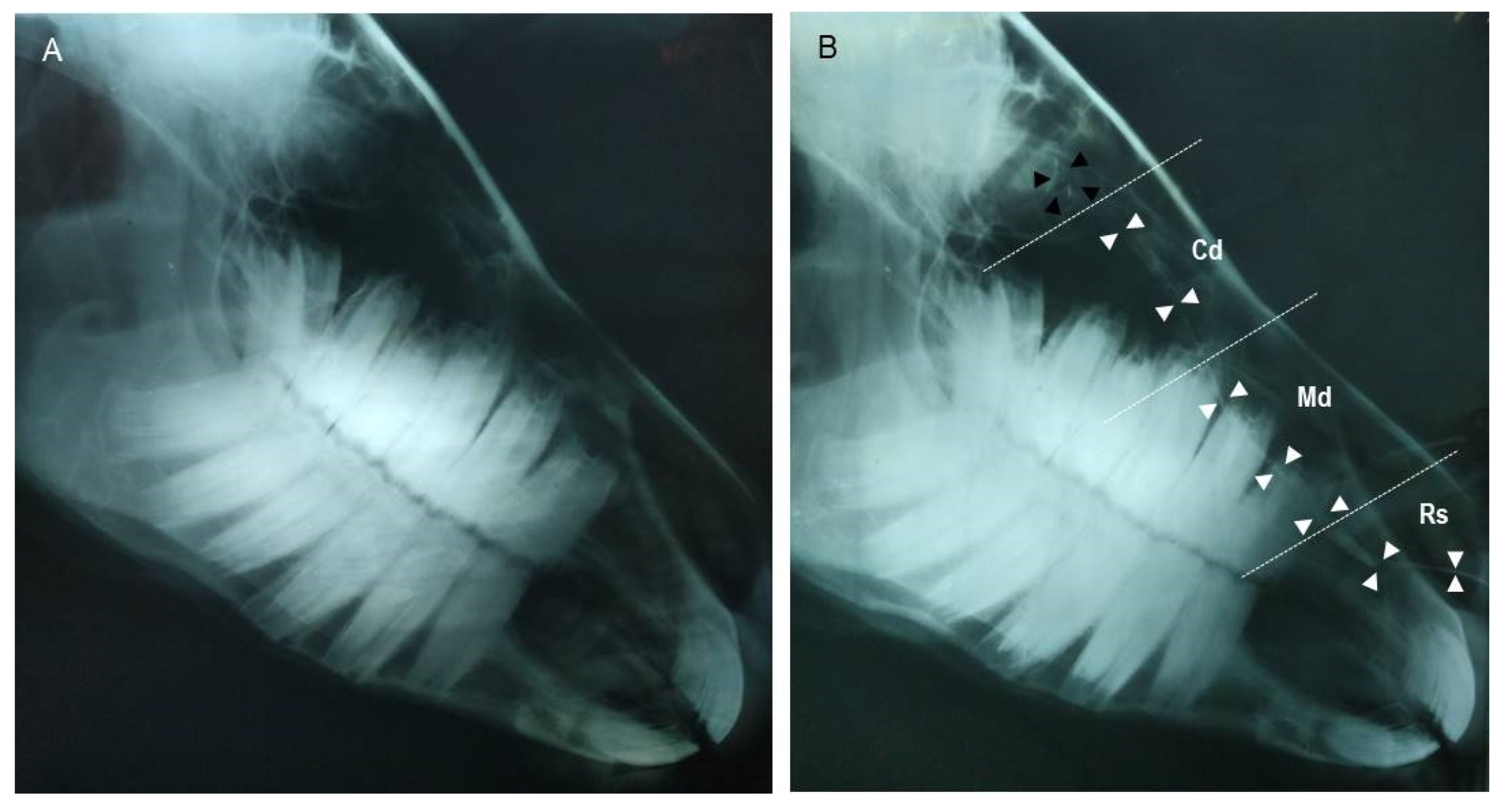
3.2. Dacryocystorhinography
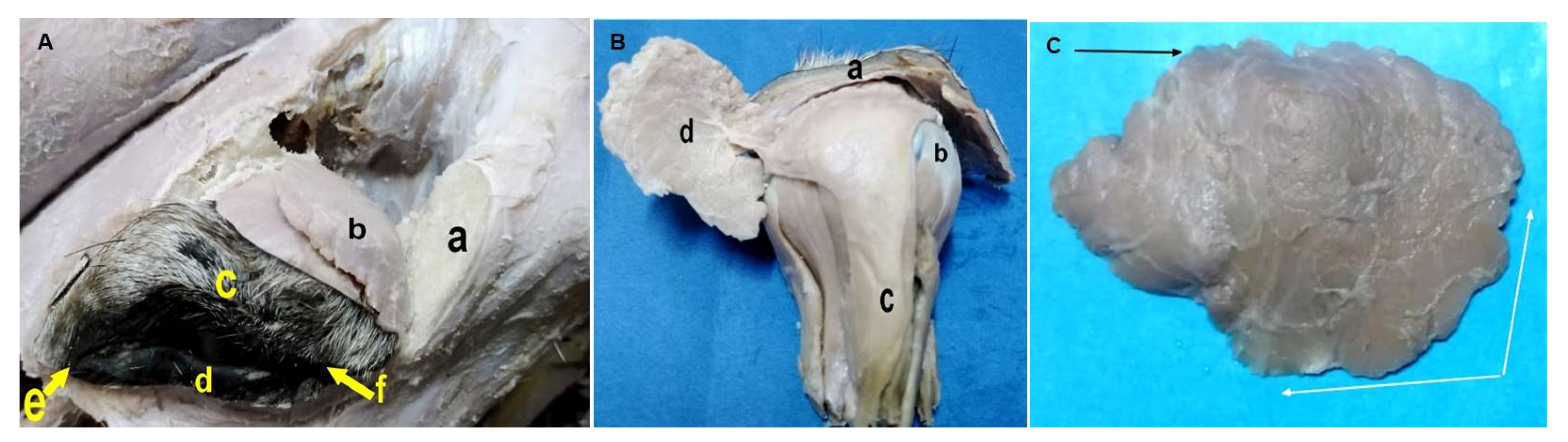
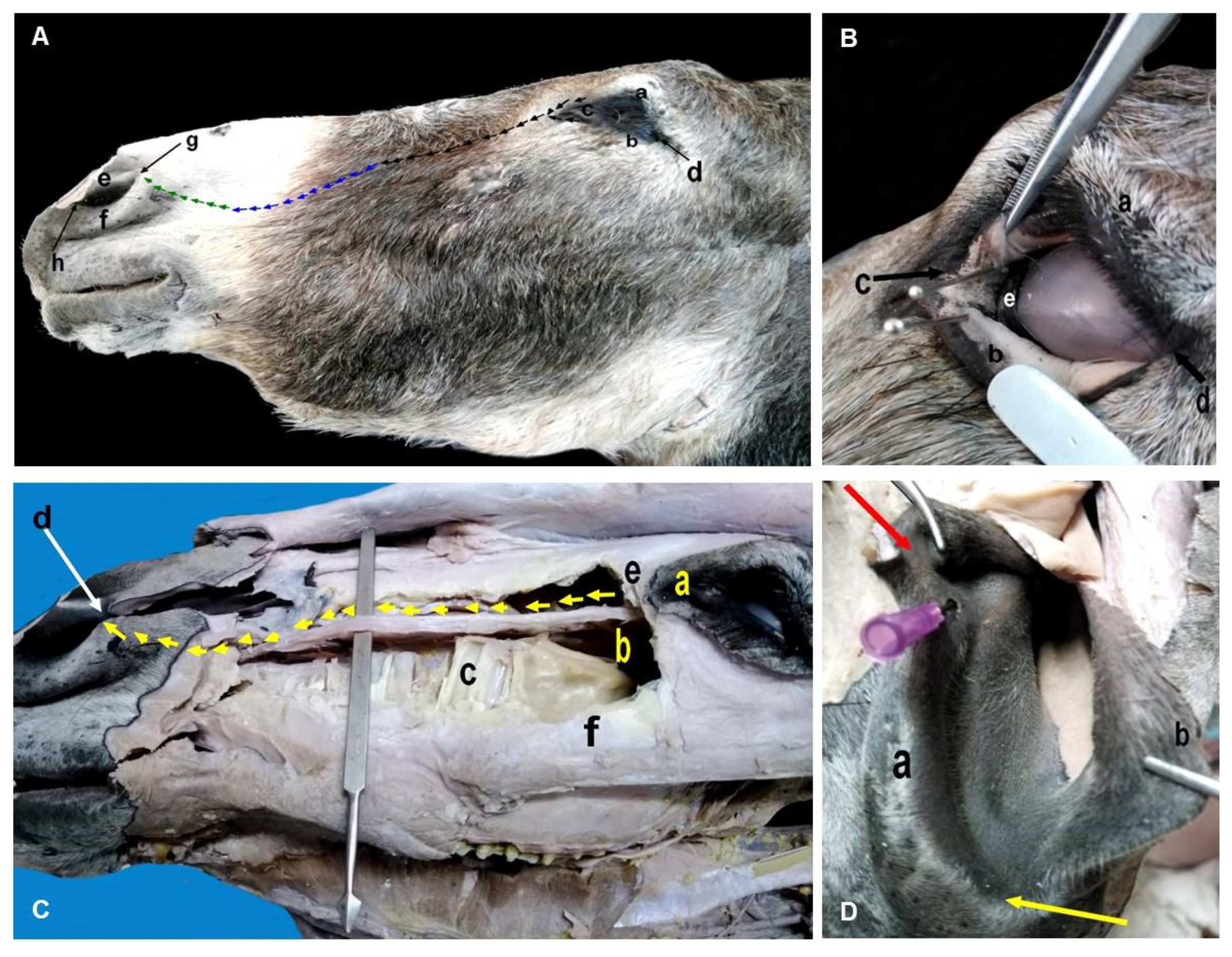
3.3. Macromorphological Findings for the Lacrimal Apparatus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dik, K.J.; Leitch, M. Soft tissue injuries of the tarsus. Vet. Clin. N. Am. Equine Pract. 1995, 11, 235–247. [Google Scholar] [CrossRef]
- Gallhoefer, N.S.; Bentley, E.; Ruetten, M.; Grest, P.; Haessig, M.; Kircher, P.R.; Dubielzig, R.R.; Spiess, B.M.; Pot, S.A. Comparison of ultrasonography and histologic examination for identification of ocular diseases of animals: 113 cases (2000–2010). J. Am. Vet. Med. Assoc. 2013, 243, 376–388. [Google Scholar] [CrossRef]
- Liuti, T.; Smith, S.; Dixon, P.M. A Comparison of Computed Tomographic, Radiographic, Gross and Histological, Dental, and Alveolar Findings in 30 Abnormal Cheek Teeth from Equine Cadavers. Front. Vet. Sci. 2017, 4, 236. [Google Scholar] [CrossRef]
- Aref, M.; Ismail, A.; Emam, H.; Dhama, K. Identification of Normal Horse Head Structures, with Special Reference to Paranasal Sinuses, by Anatomical Cross-section and Magnetic Resonance Imaging (MRI). Adv. Anim. Vet. Sci. 2018, 7, 200–204. [Google Scholar] [CrossRef]
- Quaresma, M.; Payan-Carreira, R.; Silva, S.R. Relationship between ultrasound measurements of body fat reserves and body condition score in female donkeys. Vet. J. 2013, 197, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Crisci, A.; Rota, A.; Panzani, D.; Sgorbini, M.; Ousey, J.C.; Camillo, F. Clinical, ultrasonographic, and endocrinological studies on donkey pregnancy. Theriogenology 2014, 81, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.L.; Dukes-McEwan, J. Assessment of cardiovascular disease in the donkey: Clinical, echocardiographic and pathological observations. Vet. Rec. 2016, 179, 384. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, M.B.; Vaughan, B.; Katzman, S.; Hersman, J. Ultrasound-guided Injections in Horses with Cranioventral Distension of the Coxofemoraljoint capsul: Feasibility for a carnioventral Approach. Vet. Radiol. Ultrasound 2016, 57, 199–206. [Google Scholar] [CrossRef]
- Fouad, K.-E.; Elzomor, S.; Farghali, H.A.M.; Emam, I.A. Ultrasonography guidance for total splenectomy in donkeys. Int. J. Vet. Sci. Med. 2018, 6, 233–238. [Google Scholar] [CrossRef]
- Carastro, S.M. Equine ocular anatomy and ophthalmic examination. Vet. Clin. N. Am. Equine Pract. 2004, 20, 285–299. [Google Scholar] [CrossRef]
- Liebich, H.; Konig, H. Eye (organum visus). In Veterinary Anatomy of Domestic Mammals Textbook and Colour Atlas; Schattauer Verlag: Stuttgart, Germany; NewYork, NY, USA, 2004. [Google Scholar]
- Pohlmeyer, K.; Wissdorf, H. Anatomical basis for the irrigation of the lacrimal ducts of donkeys, Equus africanus f. asinus Macedonian dwarf donkey. Dtsch. Tierarztl. Wochenschr. 1975, 82, 314–316. [Google Scholar]
- Ollivier, F. The precorneal tear film in horses: Its importance and disorders. Vet. Clin. N. Am. Equine Pract. 2004, 20, 301–318. [Google Scholar] [CrossRef]
- Grinninger, P.; Skalicky, M.; Nell, B. Evaluation of healthy equine eyes by use of retinoscopy, keratometry, and ultrasonographic biometry. Am. J. Vet. Res. 2010, 71, 677–681. [Google Scholar] [CrossRef]
- Latimer, C.A.; Wyman, M.; Diesem, C.D.; Burt, J.K. Radiographic and gross anatomy of the nasolacrimal duct of the horse. Am. J. Vet. Res. 1984, 45, 451–458. [Google Scholar] [PubMed]
- McIlnay, T.R.; Miller, S.M.; Dugan, S.J. Use of canaliculorhinostomy for repair of nasolacrimal duct obstruction in a horse. J. Am. Vet. Med. Assoc. 2001, 218, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Plummer, C.E.; Brooks, D.E.; Porter, M.; Farina, L.L.; Winter, M.D. Unilateral orbital lacrimal gland abscess in a horse. Vet. Ophthalmol. 2011, 14, 55–60. [Google Scholar] [CrossRef]
- Reimer, J.M.; Latimer, C.S. Ultrasound findings in horses with severe eyelid swelling, and recognition of acute dacryoadenitis: 10 cases (2004–2010). Vet. Ophthalmol. 2011, 14, 86–92. [Google Scholar] [CrossRef]
- Laus, F.; Paggi, E.; Faillace, V.; Marchegiani, A.; Spaziante, D.; Cerquetella, M.; Tesei, B. Ultrasonographic biometry and relationship with gender, age and weight in healthy donkeys. J. Anim. Vet. Adv. 2013, 12, 803–806. [Google Scholar]
- Laus, F.; Paggi, E.; Marchegiani, A.; Cerquetella, M.; Spaziante, D.; Faillace, V.; Tesei, B. Ultrasonographic biometry of the eyes of healthy adult donkeys. Vet. Rec. 2014, 174, 326. [Google Scholar] [CrossRef]
- Scotty, N.C.; Cutler, T.J.; Brooks, D.E.; Ferrell, E. Diagnostic ultrasonography of equine lens and posterior segment abnormalities. Vet. Ophthalmol. 2004, 7, 127–139. [Google Scholar] [CrossRef]
- Gialletti, R.; Marchegiani, A.; Valeriani, T.; Nannarone, S.; Beccati, F.; Fruganti, A.; Laus, F. A survey of ocular ultrasound abnormalities in horse: 145 cases. J. Ultrasound 2018, 21, 53–59. [Google Scholar] [CrossRef]
- Waibl, H.; Gasse, H.; Constantinescu, G.; Hashimoto, Y.; Simoens, P. Nomina Anatomica Veterinaria, 5th ed.; Revised Version; Van Den Broeck, W., Hashimoto, Y., Constantinescu, G.M., Budras, K.-D., Saber, A.S., Simoens, P., Bragulla, H., Sótonyi, P., Salazar, I., Augsburger, H., Eds.; Editorial Committee: Hannover, Germany; Columbia, MO, USA; Ghent, Belgium, 2012. [Google Scholar]
- Onwuama, K.T.; Salami, S.O.; Ali, M.; Nzalak, J.O. Effect of Different Methods of Bone Preparation on the Skeleton of the African Giant Pouched Rat (Cricetomys gambianus). Int. J. Morphol. 2012, 30, 425–427. [Google Scholar] [CrossRef]
- McMullen, R.J., Jr.; Gilger, B.C. Keratometry, biometry and prediction of intraocular lens power in the equine eye. Vet. Ophthalmol. 2006, 9, 357–360. [Google Scholar] [CrossRef]
- Valentini, S.; Castagnetti, C.; Musella, V.; Spinella, G. Assessment of Intraocular Measurements in Neonatal Foals and Association with Gender, Laterality, and Body Weight: A Clinical Study. PLoS ONE 2014, 9, e109491. [Google Scholar]
- Potter, T.J.; Hallowell, G.D.; Bowen, I.M. Ultrasonographic anatomy of the bovine eye. Vet. Radiol. Ultrasound 2008, 49, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Osuobeni, E.P.; Hamidzada, W.A. Ultrasonographic determination of the dimensions of ocular components in enucleated eyes of the one-humped camel (Camelus dromedarius). Res. Vet. Sci. 1999, 67, 125–129. [Google Scholar] [CrossRef]
- Kassab, A. Ultrasonographic and macroscopic anatomy of the enucleated eyes of the buffalo (Bos bubalis) and the one-humped camel (Camelus dromedarius) of different ages. Anat. Histol. Embryol. 2012, 41, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Fornazari, G.A.; Montiani-Ferreira, F.; de Barros Filho, I.R.; Somma, A.T.; Moore, B. The eye of the Barbary sheep or aoudad (Ammotragus lervia): Reference values for selected ophthalmic diagnostic tests, morphologic and biometric observations. Open Vet. J. 2016, 6, 102–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribeiro, A.P.; Silva, M.L.; Rosa, J.P.; Souza, S.F.; Teixeira, I.A.; Laus, J.L. Ultrasonographic and echobiometric findings in the eyes of Saanen goats of different ages. Vet. Ophthalmol. 2009, 12, 313–317. [Google Scholar] [CrossRef]
- Cottrill, N.B.; Banks, W.J.; Pechman, R.D. Ultrasonographic and biometric evaluation of the eye and orbit of dogs. Am. J. Vet. Res. 1989, 50, 898–903. [Google Scholar]
- Kovaļčuka, L.; Mūrniece, G. Normal reference ranges of ocular physiology and sonographic biometry of Latvian Hunting dogs. Vet. World 2019, 13, 807–811. [Google Scholar] [CrossRef]
- Gilger, B.C.; Davidson, M.G.; Howard, P.B. Keratometry, ultrasonic biometry, and prediction of intraocular lens power in the feline eye. Am. J. Vet. Res. 1998, 59, 131–134. [Google Scholar]
- Mirshahi, A.; Shafigh, S.H.; Azizzadeh, M. Ultrasonographic biometry of the normal eye of the Persian cat. Aust. Vet. J. 2014, 92, 246–249. [Google Scholar] [CrossRef]
- Bapodra, P.; Bouts, T.; Mahoney, P.; Turner, S.; Silva-Fletcher, A.; Waters, M. Ultrasonographic anatomy of the Asian elephant (Elephas maximus) eye. J. Zoo Wildl. Med. 2010, 41, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Apruzzese, A.; Rodríguez, A.; González, F.; López, I.; Suárez, L.; González-Alonso-Alegre, E. Ocular Ultrasonography and Biometry in the Cinereous Vulture (Aegypius monachus). J. Avian Med. Surg. 2018, 32, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Squarzoni, R.; Perlmann, E.; Antunes, A.; Milanelo, L.; de Moraes Barros, P.S. Ultrasonographic aspects and biometry of Striped owl’s eyes (Rhinoptynx clamator). Vet. Ophthalmol. 2010, 13, 86–90. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Hann, L.E.; Yee, L.; Allansmith, M.R. Age- and gender-related influence on the lacrimal gland and tears. Acta Ophthalmol. 1990, 68, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Gerhards, H. The examination of the equine lacrimal gland. Pferdeheilkunde 2011, 27, 31–38. [Google Scholar] [CrossRef][Green Version]
- Diesem, C. Equine sense organs and common integument. Ruminant sense organs and common integument. Ruminant osteology. In Sisson and Grossman’s: The Anatomy of the Domestic Animals, 5th ed.; Getty, R., Ed.; W.B. Saunders Co.: Philadelphia, PA, USA; London, UK, 1975. [Google Scholar]
- Budras, K.; Sack, W.; Rock, S. Anatomy of the Horse an Illustrated Text, 3rd ed.; Schlütersche GmbH and Co. KG; Verlag und Druckerei Hans-Böckler-Allee: Hannover, Germany, 2009. [Google Scholar]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy, 4th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 2010. [Google Scholar]
- Alsafy, M. Comparative Morphological Studies on the Lacrimal Apparatus of One Humped Camel, Goat and Donkey. J. Biol. Sci. 2010, 4, 49–53. [Google Scholar] [CrossRef]
- Bigham Sadegh, A.; Shadkhast, M. Lacrimal Apparatus System in One-humped Camel of Iran (Camelus dromedarius): Anatomical and Radiological Study. Iran. J. Vet. Surg. 2007, 2, 75–80. [Google Scholar]
- Sapienza, J.S.; Isaza, R.; Johnson, R.D.; Miller, T.R. Anatomic and radiographic study of the lacrimal apparatus of llamas. Am. J. Vet. Res. 1992, 53, 1007–1009. [Google Scholar] [PubMed]
- Gilanpour, H. Anatomic and radiographic studies of the lacrimal drainage system in sheep (Ovis aries). Am. J. Vet. Res. 1979, 40, 1177–1179. [Google Scholar]
- Gelatt, K.N.; Cure, T.H.; Guffy, M.M.; Jessen, C. Dacryocystorhinography in the dog and cat. J. Small Anim. Pract. 1972, 13, 381–397. [Google Scholar] [CrossRef]
- van der Woerdt, A.; Wilkie, D.A.; Gilger, B.C.; Smeak, D.D.; Kerpsack, S.J. Surgical treatment of dacryocystitis caused by cystic dilatation of the nasolacrimal system in three dogs. J. Am. Vet. Med. Assoc. 1997, 211, 445–447. [Google Scholar] [PubMed]
- Adams, M.F.; Castro, J.R.; Morandi, F.; Reese, R.E.; Reed, R.B. The nasolacrimal duct of the mule: Anatomy and clinical considerations. Equine Vet. Educ. 2013, 25, 636–642. [Google Scholar] [CrossRef]
- Hendrickson, D.A. Management of Deep and chronic wounds. In Equine Surgery, 3rd ed.; Auer, J.A., Stick, J.A., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2006; pp. 299–305. [Google Scholar]
| Variable (mm) | 95% Confidence Interval (CI) | Mean (Standard Deviation) | ||
|---|---|---|---|---|
| Lower 95% CI of Mean | Upper 95% CI of Mean | |||
| Ultrasonography | Axial globe length (AGL) | 30.4 | 36.1 | 33.7 (1.7) |
| Globe diameter (GD) | 35.7 | 43.1 | 39.8 (2.1) | |
| Lens thickness (LT) | 10.1 | 12.5 | 10.8 (0.7) | |
| Anterior chamber diameter (ACD) | 2.5 | 3.9 | 3.2 (0.5) | |
| Vitreous chamber diameter (VCD) | 16.3 | 21.9 | 19.3 (1.6) | |
| Lacrimal gland length (LGL) | 14.3 | 20.1 | 16.9 (1.6) | |
| Lacrimal gland diameter (LGD) | 5.1 | 6.4 | 6.1 (0.3) | |
| Radiography | Nasolacrimal duct rostral (NLDRs) diameter | 4.1 | 4.9 | 4.5 (0.2) |
| Nasolacrimal duct middle (NLDMd) diameter | 2.2 | 2.9 | 2.6 (0.2) | |
| Nasolacrimal duct caudal (NLDCd) diameter | 0.8 | 1.5 | 1.1 (0.2) | |
| Nasolacrimal duct (NLD) length | 172.0 | 209.0 | 193.0 (9.8) | |
| Nasolacrimal duct (NLD) rostral cartilaginous | 60.0 | 79.0 | 67.4 (4.9) | |
| Nasolacrimal duct (NLD) middle membranous | 60.0 | 81.5 | 67.9 (6.0) | |
| Nasolacrimal duct (NLD) caudal osseous | 60.0 | 79.0 | 67.2 (5.1) | |
| Variable | 95% Confidence Interval (CI) | Mean (Standard Deviation) | |
|---|---|---|---|
| Lower 95% CI of Mean | Upper 95% CI of Mean | ||
| Lacrimal gland weight (LGW) (gm) | 2.5 | 3.5 | 2.9 (0.2) |
| Lacrimal gland length (LGL) (mm) | 19.0 | 31.5 | 25.1 (3.9) |
| Lacrimal gland width (LGD) (mm) | 28.0 | 43.0 | 34.7 (4.9) |
| Nasolacrimal duct (NLD) length (mm) | 180.0 | 245.0 | 206.0 (20.4) |
| Nasolacrimal duct (NLD) rostral cartilaginous (mm) | 75.0 | 95.0 | 85.2 (8.2) |
| Nasolacrimal duct (NLD) middle membranous (mm) | 40.0 | 65.0 | 45.3 (6.9) |
| Nasolacrimal duct (NLD) caudal osseous (mm) | 60.0 | 85.0 | 74.8 (8.6) |
| Nasolacrimal (NL) orifice diameter (mm) | 3.0 | 5.0 | 3.9 (0.9) |
| Distance from dorsal commissure to NL orifice (mm) | 9.0 | 16.0 | 12.1 (2.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelbaset-Ismail, A.; Aref, M.; Ezzeldein, S.; Eisa, E.; Gugjoo, M.B.; Abdelaal, A.; Emam, H.; Al Syaad, K.; Ahmed, A.E.; Alshati, A.; et al. Ultrasound, Dacryocystorhinography and Morphological Examination of Normal Eye and Lacrimal Apparatus of the Donkey (Equus asinus). Animals 2022, 12, 132. https://doi.org/10.3390/ani12020132
Abdelbaset-Ismail A, Aref M, Ezzeldein S, Eisa E, Gugjoo MB, Abdelaal A, Emam H, Al Syaad K, Ahmed AE, Alshati A, et al. Ultrasound, Dacryocystorhinography and Morphological Examination of Normal Eye and Lacrimal Apparatus of the Donkey (Equus asinus). Animals. 2022; 12(2):132. https://doi.org/10.3390/ani12020132
Chicago/Turabian StyleAbdelbaset-Ismail, Ahmed, Mohamed Aref, Shimaa Ezzeldein, Eslam Eisa, Mudasir Bashir Gugjoo, Ahmed Abdelaal, Hassan Emam, Khalid Al Syaad, Ahmed Ezzat Ahmed, Ali Alshati, and et al. 2022. "Ultrasound, Dacryocystorhinography and Morphological Examination of Normal Eye and Lacrimal Apparatus of the Donkey (Equus asinus)" Animals 12, no. 2: 132. https://doi.org/10.3390/ani12020132
APA StyleAbdelbaset-Ismail, A., Aref, M., Ezzeldein, S., Eisa, E., Gugjoo, M. B., Abdelaal, A., Emam, H., Al Syaad, K., Ahmed, A. E., Alshati, A., & Abd El Raouf, M. (2022). Ultrasound, Dacryocystorhinography and Morphological Examination of Normal Eye and Lacrimal Apparatus of the Donkey (Equus asinus). Animals, 12(2), 132. https://doi.org/10.3390/ani12020132






