Non-Invasive Assessment of Mild Stress-Induced Hyperthermia by Infrared Thermography in Laboratory Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing Conditions
2.2. Study Design
2.3. Daily Handling (DH)
2.4. Voluntary Interaction Test (VIT)
2.5. Elevated Plus Maze (EPM)
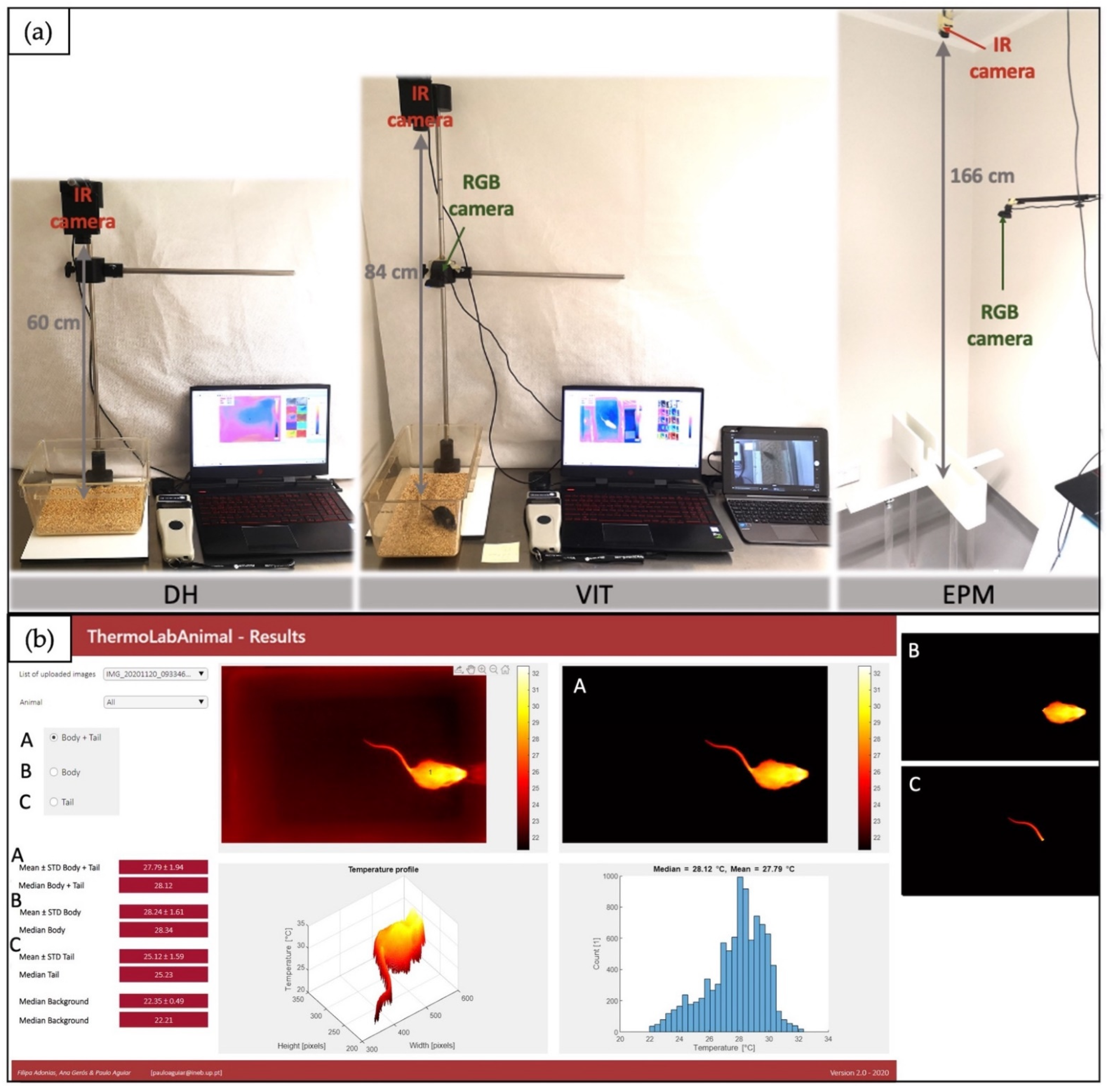
2.6. Equipment and Software
2.7. Analysis and Statistics
3. Results
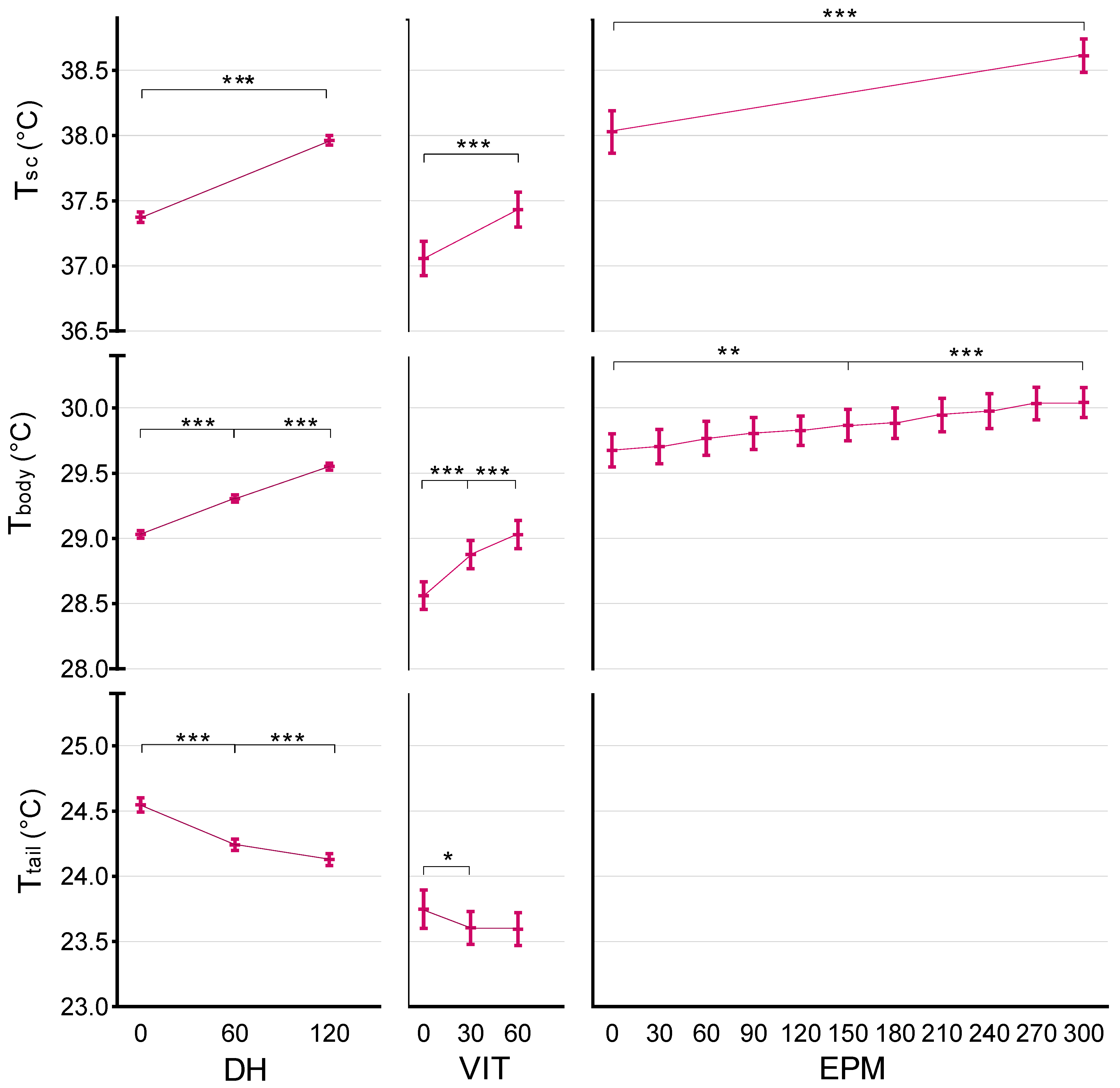
3.1. Daily Handling (DH) Effects on Temperature
3.2. Voluntary Interaction Test (VIT) Effects on Temperature
3.3. Elevated Plus Maze (EPM) Effects on Temperature
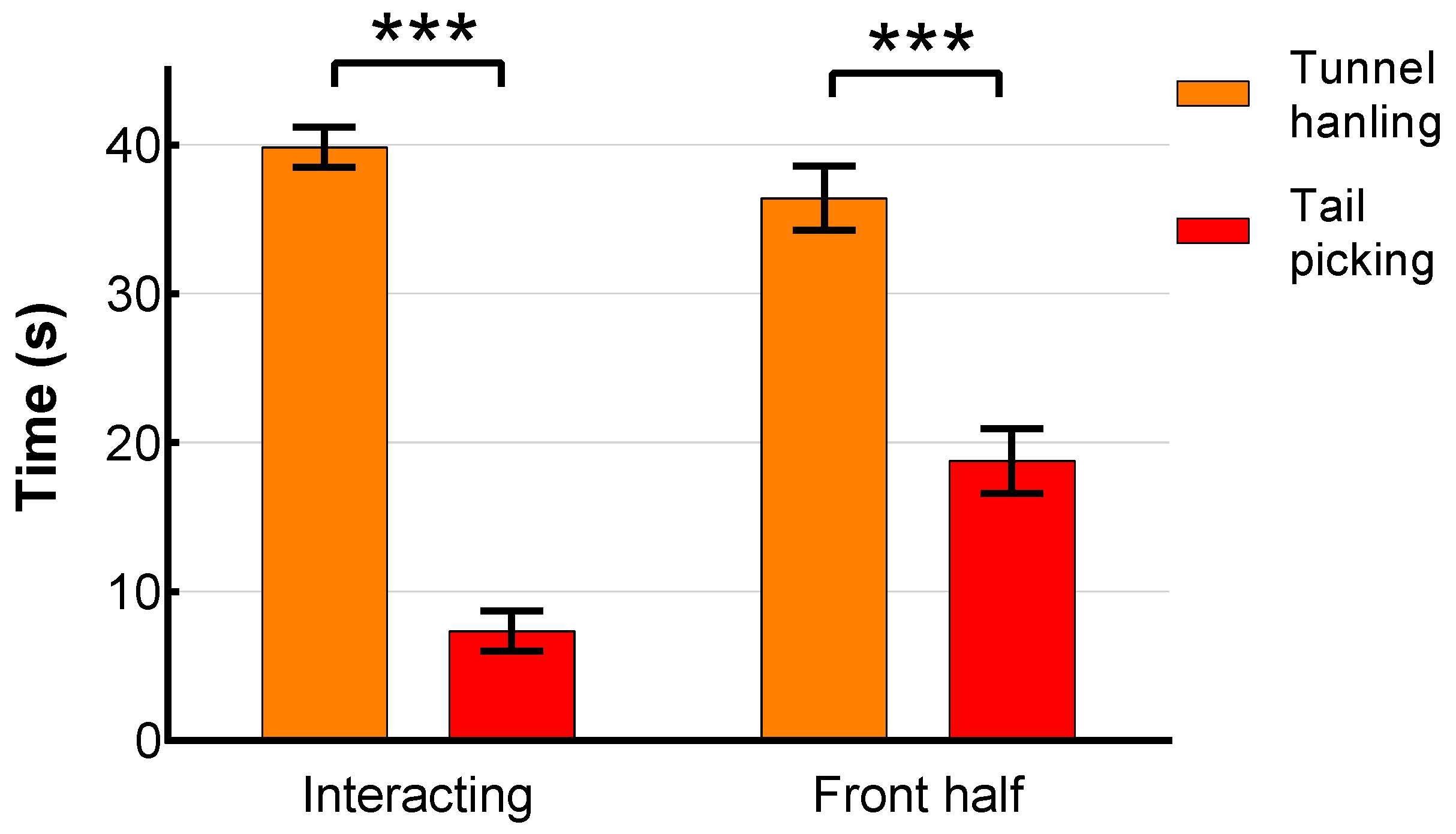
3.4. Behaviour in the Voluntary Interaction Test (VIT)
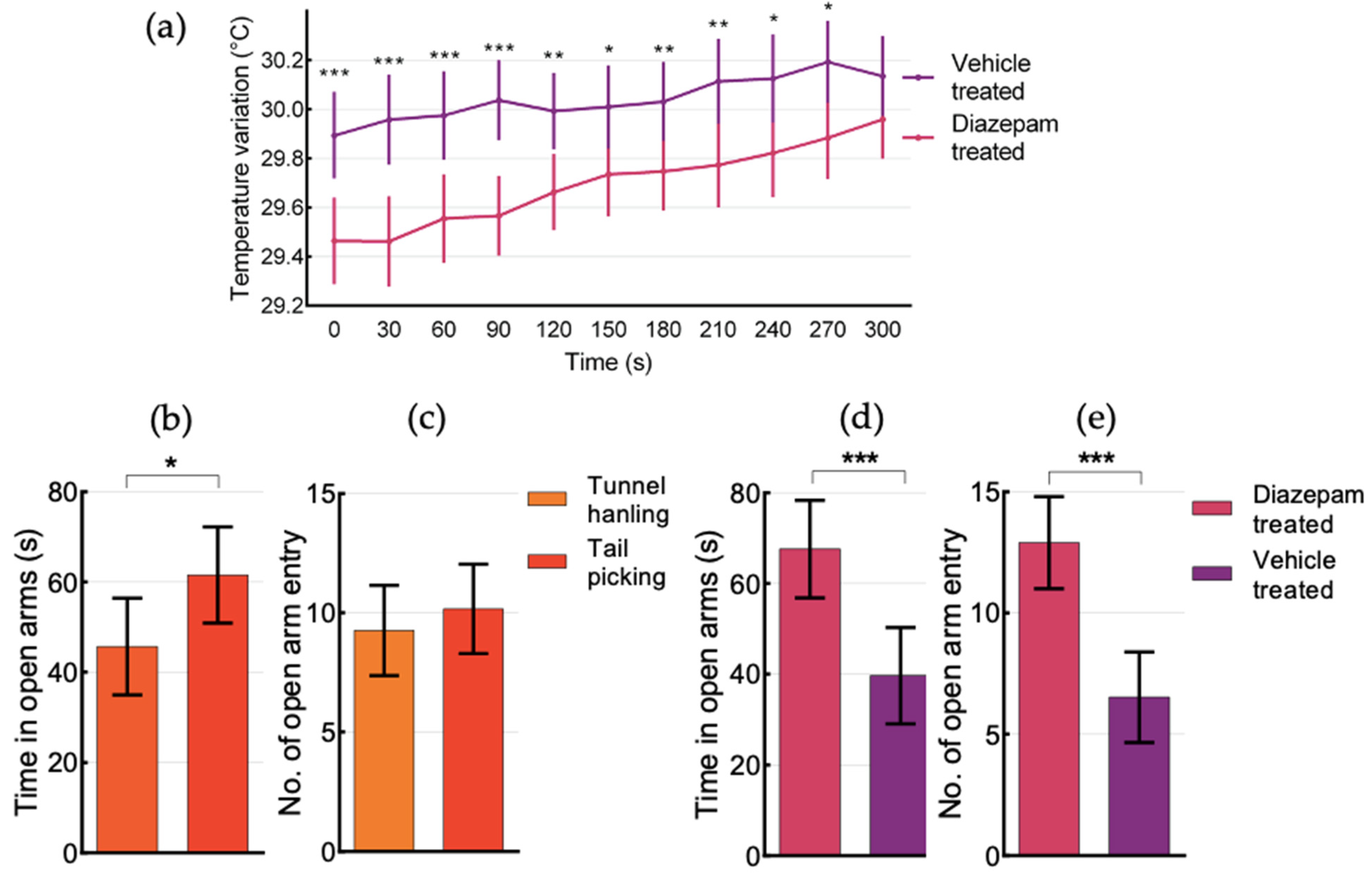
3.5. Behaviour in the Elevated Plus Maze (EPM)
3.6. Effect of Testing Order
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
Appendix B.1. Analysis and Statistics
Appendix B.2. Results

References
- Bouwknecht, A.J.; Olivier, B.; Paylor, R.E. The Stress-Induced Hyperthermia Paradigm as a Physiological Animal Model for Anxiety: A Review of Pharmacological and Genetic Studies in the Mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [Green Version]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Gordon, C.J. Thermal Physiology of Laboratory Mice: Defining Thermoneutrality. J. Therm. Biol. 2012, 37, 654–685. [Google Scholar] [CrossRef]
- Mei, J.; Riedel, N.; Grittner, U.; Endres, M.; Banneke, S.; Emmrich, J.V. Body Temperature Measurement in Mice during Acute Illness: Implantable Temperature Transponder versus Surface Infrared Thermometry. Sci. Rep. 2018, 8, 3526. [Google Scholar] [CrossRef]
- Zethof, T.J.J.; Van Der Heyden, J.A.M.; Tolboom, J.T.B.M.; Olivier, B. Stress-Induced Hyperthermia in Mice: A Methodological Study. Physiol. Behav. 1994, 55, 109–115. [Google Scholar] [CrossRef]
- Hartinger, J.; Külbs, D.; Volkers, P.; Cussler, K. Suitability of Temperature-Sensitive Transponders to Measure Body Temperature During Animal Experiments Required for Regulatory Tests. Altex 2003, 20, 65–70. [Google Scholar]
- Gordon, C.J.; Spencer, P.J.; Hotchkiss, J.; Miller, D.B.; Hinderliter, P.M.; Pauluhn, J. Thermoregulation and Its Influence on Toxicity Assessment. Toxicology 2008, 244, 87–97. [Google Scholar] [CrossRef]
- Wang, Y.; Vaddiraju, S.; Gu, B.; Papadimitrakopoulos, F.; Burgess, D.J. Foreign Body Reaction to Implantable Biosensors: Effects of Tissue Trauma and Implant Size. J. Diabetes Sci. Technol. 2015, 9, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Helwig, B.G.; Ward, J.A.; Blaha, M.D.; Leon, L.R. Effect of Intraperitoneal Radiotelemetry Instrumentation on Voluntary Wheel Running and Surgical Recovery in Mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 600–608. [Google Scholar]
- Tang, X.; Sanford, L.D. Telemetric Recording of Sleep and Home Cage Activity in Mice. Sleep 2002, 25, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.H.; White, C.E. Comparison of Rectal, Microchip Transponder, and Infrared Thermometry Techniques for Obtaining Body Temperature in the Laboratory Rabbit (Oryctolagus Cuniculus). J. Am. Assoc. Lab. Anim. Sci. 2006, 45, 7. [Google Scholar]
- Vadlejch, J.; Knížková, I.; Makovcová, K.; Kunc, P.; Jankovská, I.; Janda, K.; Borkovcová, M.; Langrová, I. Thermal Profile of Rabbits Infected with Eimeria Intestinalis. Vet. Parasitol. 2010, 171, 343–345. [Google Scholar] [CrossRef]
- David, J.M.; Chatziioannou, A.F.; Taschereau, R.; Wang, H.; Stout, D.B. The Hidden Cost of Housing Practices: Using Noninvasive Imaging to Quantify the Metabolic Demands of Chronic Cold Stress of Laboratory Mice. Comp. Med. 2013, 63, 6. [Google Scholar]
- Vianna, D.M.L.; Carrive, P. Stress-Induced Hyperthermia Is Not Mediated by Brown Adipose Tissue in Mice. J. Therm. Biol. 2012, 37, 125–129. [Google Scholar] [CrossRef]
- Ågren, G.; Lund, I.; Thiblin, I.; Lundeberg, T. Tail Skin Temperatures Reflect Coping Styles in Rats. Physiol. Behav. 2009, 96, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Lecorps, B.; Rödel, H.G.; Féron, C. Short-Term Thermal Responses after Exposure to Predator Odor (TMT) in the House Mouse. Mamm. Biol. 2019, 94, 25–29. [Google Scholar] [CrossRef]
- Vianna, D.M.L.; Carrive, P. Changes in Cutaneous and Body Temperature during and after Conditioned Fear to Context in the Rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef] [Green Version]
- Lecorps, B.; Rödel, H.G.; Féron, C. Assessment of Anxiety in Open Field and Elevated plus Maze Using Infrared Thermography. Physiol. Behav. 2016, 157, 209–216. [Google Scholar] [CrossRef]
- Miyazono, S.; Hasegawa, K.; Miyazaki, S.; Sakakima, H.; Konno, S.; Meguro, S.; Sasajima, H.; Noguchi, T.; Osada, K.; Kashiwayanagi, M. Etizolam Attenuates the Reduction in Cutaneous Temperature Induced in Mice by Exposure to Synthetic Predator Odor. Eur. J. Pharmacol. 2018, 824, 157–162. [Google Scholar] [CrossRef]
- Vejmělka, F.; Okrouhlík, J.; Lövy, M.; Šaffa, G.; Nevo, E.; Bennett, N.C.; Šumbera, R. Heat Dissipation in Subterranean Rodents: The Role of Body Region and Social Organisation. Sci. Rep. 2021, 11, 2029. [Google Scholar] [CrossRef]
- Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental Applications and Factors Involved in Validating Thermal Windows Using Infrared Thermography to Assess the Health and Thermostability of Laboratory Animals. Animals 2021, 11, 3448. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Lecona-Butrón, H.; Martínez-Burnes, J.; Mora-Medina, P.; Gómez-Prado, J.; Orihuela, A. Infrared Thermal Imaging Associated with Pain in Laboratory Animals. Exp. Anim. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Vogel, B.; Wagner, H.; Gmoser, J.; Wörner, A.; Löschberger, A.; Peters, L.; Frey, A.; Hofmann, U.; Frantz, S. Touch-Free Measurement of Body Temperature Using Close-up Thermography of the Ocular Surface. MethodsX 2016, 3, 407–416. [Google Scholar] [CrossRef]
- Gjendal, K.; Franco, N.H.; Ottesen, J.L.; Sørensen, D.B.; Olsson, I.A.S. Eye, Body or Tail? Thermography as a Measure of Stress in Mice. Physiol. Behav. 2018, 196, 135–143. [Google Scholar] [CrossRef]
- Gordon, C.J. Thermoregulatory Effector Responses. In Temperature Regulation in Laboratory Rodents; Cambridge University Press: Cambridge, UK, 1993; pp. 74–79. [Google Scholar]
- Hurst, J.L.; West, R.S. Taming Anxiety in Laboratory Mice. Nat. Methods 2010, 7, 825–826. [Google Scholar] [CrossRef]
- Gouveia, K.; Hurst, J.L. Optimising Reliability of Mouse Performance in Behavioural Testing: The Major Role of Non-Aversive Handling. Sci. Rep. 2017, 7, 44999. [Google Scholar] [CrossRef] [Green Version]
- Lepicard, E.M.; Joubert, C.; Hagneau, I.; Perez-Diaz, F.; Chapouthier, G. Differences in Anxiety-Related Behaviour and Response to Diazepam in BALB/CByJ and C57BL/6J Strains of Mice. Pharmacol. Biochem. Behav. 2000, 67, 739–748. [Google Scholar] [CrossRef]
- NC3Rs. Mouse Handling FAQs. Available online: https://www.nc3rs.org.uk/mouse-handling-faqs (accessed on 9 January 2020).
- Walker, M.; Fureix, C.; Palme, R.; Newman, J.A.; Ahloy Dallaire, J.; Mason, G. Mixed-Strain Housing for Female C57BL/6, DBA/2, and BALB/c Mice: Validating a Split-Plot Design That Promotes Refinement and Reduction. BMC Med. Res. Methodol. 2016, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Belzung, C.; Griebel, G. Measuring Normal and Pathological Anxiety-like Behaviour in Mice: A Review. Behav. Brain Res. 2001, 125, 141–149. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behaviour in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Wahlsten, D. Mouse Behavioural Testing: How to Use Mice in Behavioural Neuroscience; Elsevier: London, UK, 2011; ISBN 978-0-12-375674-9. [Google Scholar]
- Hankenson, F.C.; Marx, J.O.; Gordon, C.J.; David, J.M. Effects of Rodent Thermoregulation on Animal Models in the Research Environment. Comp. Med. 2018, 68, 425–438. [Google Scholar] [CrossRef]
- Marks, A.; Vianna, D.M.L.; Carrive, P. Nonshivering Thermogenesis without Interscapular Brown Adipose Tissue Involvement during Conditioned Fear in the Rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1239–R1247. [Google Scholar] [CrossRef] [Green Version]
- Duparcq, M.; Jean, O.; Verjat, A.; Jaravel, L.; Jacquet, D.; Robles Guerrero, F.; Féron, C.; Rödel, H.G. Differences between Fast and Slow Explorers in Short-Term Tail Temperature Responses to Handling in a Rodent of Wild Origin. Behav. Brain Res. 2019, 376, 112194. [Google Scholar] [CrossRef]
- Depino, A.; Gross, C. Simultaneous Assessment of Autonomic Function and Anxiety-Related Behaviour in BALB/c and C57BL/6 Mice. Behav. Brain Res. 2007, 177, 254–260. [Google Scholar] [CrossRef]
- Faraji, J.; Metz, G.A.S. Infrared Thermography Reveals Sex-Specific Responses to Stress in Mice. Front. Behav. Neurosci. 2020, 14, 79. [Google Scholar] [CrossRef]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory Routines Cause Animal Stress. Am. Assoc. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar]
- Castelhano-Carlos, M.J.; Baumans, V. The Impact of Light, Noise, Cage Cleaning and in-House Transport on Welfare and Stress of Laboratory Rats. Lab Anim. 2009, 43, 311–327. [Google Scholar] [CrossRef]
- Kerr, N.L. HARKing: Hypothesizing after the Results Are Known. Personal. Soc. Psychol. Rev. 1998, 2, 196–217. [Google Scholar] [CrossRef]
- Borsini, F.; Lecci, A.; Volterra, G.; Meli, A. A Model to Measure Anticipatory Anxiety in Mice? Psychopharmacology 1989, 98, 207–211. [Google Scholar] [CrossRef]
- Henderson, L.J.; Dani, B.; Serrano, E.M.N.; Smulders, T.V.; Roughan, J.V. Benefits of Tunnel Handling Persist after Repeated Restraint, Injection and Anaesthesia. Sci. Rep. 2020, 10, 14562. [Google Scholar] [CrossRef]
- Clarkson, J.M.; Dwyer, D.M.; Flecknell, P.A.; Leach, M.C.; Rowe, C. Handling Method Alters the Hedonic Value of Reward in Laboratory Mice. Sci. Rep. 2018, 8, 2448. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Suzuki, K. Tunnel Use Facilitates Handling of ICR Mice and Decreases Experimental Variation. J. Vet. Med. Sci. 2018, 80, 886–892. [Google Scholar] [CrossRef] [Green Version]
- Franco, N.H.; Gerós, A.; Oliveira, L.; Olsson, I.A.S.; Aguiar, P. ThermoLabAnimal—A High-Throughput Analysis Software for Non-Invasive Thermal Assessment of Laboratory Mice. Physiol. Behav. 2019, 207, 113–121. [Google Scholar] [CrossRef]



| Hypotheses | Confirmed | Evidence | |
|---|---|---|---|
| I IRT can detect hyperthermic stress response in mice | Yes | DH | Average Tbody rise of 0.5 °C (p < 0.001) and Ttail drop of 0.4 °C (p < 0.001). |
| VIT | Average Tbody rise of 0.5 °C (p < 0.001) and Ttail drop of 0.2 °C (p = 0.004). | ||
| EPM | Average Tbody rise of 0.4 °C (p < 0.001). | ||
| II Hyperthermic stress responses differ between tail-picked and tunnel-handled animals | No | DH | Tbody higher (p = 0.012) in tunnel-handled mice. No handling technique differences in Tsc or Ttail. |
| VIT | No handling technique differences in Tsc, Tbody or Ttail. | ||
| EPM | No handling technique differences in Tsc or Tbody. | ||
| III Findings will be consistent between the two sexes | Mostly | DH | Small yet significant sex differences in Tsc (p = 0.004), Tbody (p = 0.013) and Ttail (p = 0.004). |
| VIT | No sex differences in Tsc, Tbody or Ttail. | ||
| EPM | No sex differences in Tsc or Tbody. | ||
| Findings will be consistent between the two mouse strains | Yes (Ttail, Tbody) No (Tsc) | DH | BALB/cByJ show higher (p < 0.001) Tsc. No strain differences in Tbody and Ttail. |
| VIT | BALB/cByJ show higher (p = 0.013) Tsc. No strain differences in Tbody and Ttail. | ||
| EPM | BALB/cByJ show higher (p = 0.011) Tsc. No strain differences in Tbody and Ttail. | ||
| IV Tbody and Ttail will be consistent with Tsc | Mostly | Both Tsc and Tbody rose during all behavioural tests and were higher during the dark period. Effect of testing order were observed in both Tsc and Tbody. No consistent differences detected between the two strains of mice. | |
| V Magnitude of SIH indicates stress intensity, in accordance with behavioural test | Not clear | VIT was able to detect differences between the two handling techniques (p < 0.001). Handling technique differences were not observed during EPM or in thermal response (Tsc, Tbody and Ttail). Further research needed to test the magnitude intensity for which SIH can be detected. | |
| IV SIH in EPM will be less pronounced in animals treated with an anxiolytic drug | Yes | Diazepam-treated mice showed lower Tsc at 0 s (p = 0.001) and Tbody from 0–270 s (p < 0.001–0.05), spend more time in open arms and had higher number of open arms entries. Diazepam-treated mice showed no differences between animals tested first and second, while having lower Tsc and Tbody, despite being more active. Anxiolytic effects were consistent across sexes, strains and handling techniques. | |
| (added) Animals tested as second and third will show higher impact of SIH than animal tested first in the cage. | Yes | DH | Animals tested second showed higher Tsc (p < 0.001) Tbody (p < 0.001) and lower Ttail (p < 0.001), as well as animals tested third (Tsc (p < 0.001), Tbody (p < 0.001) and Ttail (p < 0.001)) |
| VIT | Animals tested second showed higher Tsc (p < 0.001) Tbody (p < 0.01) and Ttail (p < 0.001). | ||
| EPM | Animals tested second had higher Tsc (p < 0.001) at 0 s and higher Tbody (p < 0.01–0.05) from 0–60 s. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blenkuš, U.; Gerós, A.F.; Carpinteiro, C.; Aguiar, P.d.C.; Olsson, I.A.S.; Franco, N.H. Non-Invasive Assessment of Mild Stress-Induced Hyperthermia by Infrared Thermography in Laboratory Mice. Animals 2022, 12, 177. https://doi.org/10.3390/ani12020177
Blenkuš U, Gerós AF, Carpinteiro C, Aguiar PdC, Olsson IAS, Franco NH. Non-Invasive Assessment of Mild Stress-Induced Hyperthermia by Infrared Thermography in Laboratory Mice. Animals. 2022; 12(2):177. https://doi.org/10.3390/ani12020177
Chicago/Turabian StyleBlenkuš, Urša, Ana Filipa Gerós, Cristiana Carpinteiro, Paulo de Castro Aguiar, I. Anna S. Olsson, and Nuno Henrique Franco. 2022. "Non-Invasive Assessment of Mild Stress-Induced Hyperthermia by Infrared Thermography in Laboratory Mice" Animals 12, no. 2: 177. https://doi.org/10.3390/ani12020177






