Effect of Music on Stress Parameters in Dogs during a Mock Veterinary Visit
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Veterinary Clinic
2.3. Study Design
2.4. Data Collection and Analysis
2.4.1. Eye, Nose, and Ear Temperature
2.4.2. Salivary Cortisol and IgA
2.4.3. Temperature, Pulse, and Respiration (TPR)
2.4.4. Dog Behavior
2.5. Statistical Analysis
3. Results
3.1. Physiological and Behavioral Measures
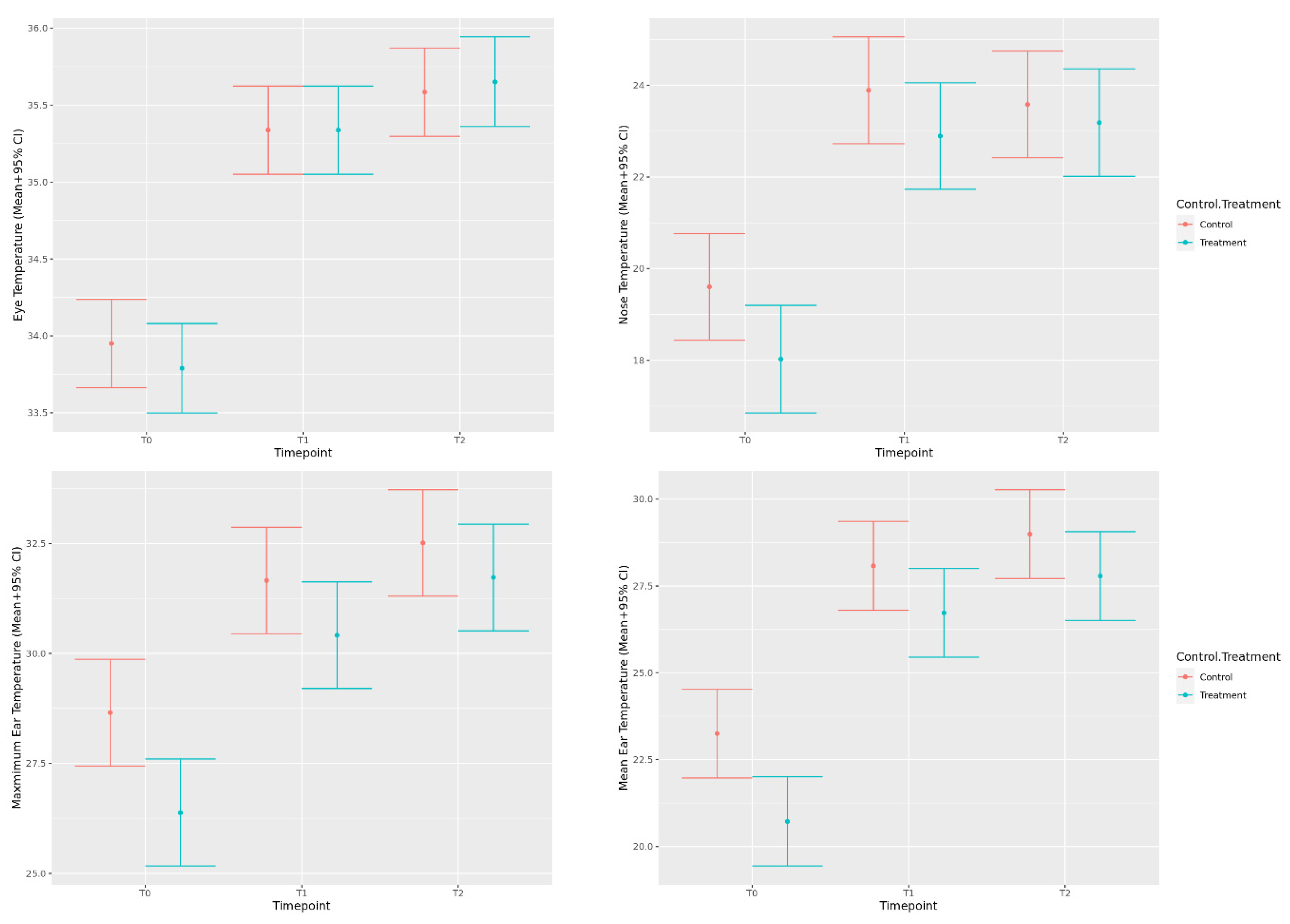
3.1.1. Eye, Nose, and Ear Temperature
3.1.2. Salivary Cortisol and IgA
3.1.3. Temperature, Pulse and Respiration (TPR)
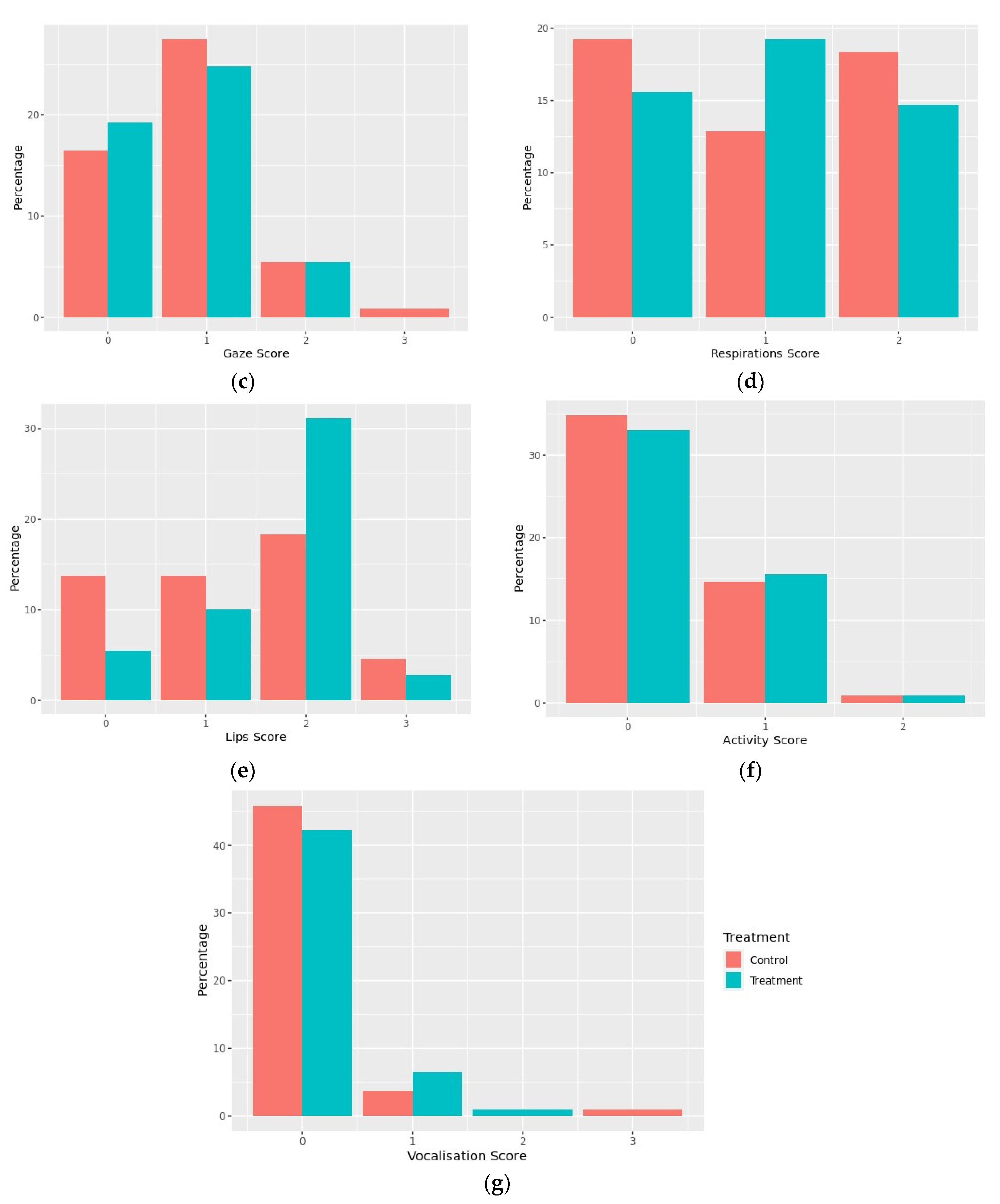
3.1.4. Dog Behavior
3.1.5. Behavioral and Physiological Measures Combined
4. Discussion
4.1. Dog Physiology during a Veterinary Visit
4.2. Dog Behavior during a Veterinary Visit
4.3. Combining Behavioral and Phsyiological Measures
4.4. The Future of Canine Stress Evaluation and Management in a Clinicial Setting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratsch, L.; Arhant, C.; Windschnurer, I.; Affenzeller, N.; Riemer, S. Fear-reducing strategies for small animal practice Part 1—Low-stress methods for the interaction with dogs and cats. Kleintierpraxis 2020, 65, 548–566. [Google Scholar] [CrossRef]
- Mariti, C.; Bowen, J.E.; Campa, S.; Grebe, G.; Sighieri, C.; Gazzano, A. Guardians’ Perceptions of Cats’ Welfare and Behavior Regarding Visiting Veterinary Clinics. J. Appl. Anim. Welf. Sci. 2016, 19, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Mariti, C.; Pierantoni, L.; Sighieri, C.; Gazzano, A. Guardians’ Perceptions of Dogs’ Welfare and Behaviors Related to Visiting the Veterinary Clinic. J. Appl. Anim. Welf. Sci. 2017, 20, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Csoltova, E.; Martineau, M.; Boissy, A.; Gilbert, C. Behavioral and physiological reactions in dogs to a veterinary examination: Owner-dog interactions improve canine well-being. Physiol. Behav. 2017, 177, 270–281. [Google Scholar] [CrossRef]
- Döring, D.; Roscher, A.; Scheipl, F.; Küchenhoff, H.; Erhard, M.H. Fear-related behaviour of dogs in veterinary practice. Vet. J. 2009, 182, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.T.; Hazel, S.J.; Browne, M.; Serpell, J.A.; McArthur, M.L.; Smith, B.P. Investigating risk factors that predict a dog’s fear during veterinary consultations. PLoS ONE 2019, 14, e0215416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitznagel, M.B.; Jacobson, D.M.; Cox, M.D.; Carlson, M.D. Caregiver burden in owners of a sick companion animal: A cross-sectional observational study. Vet. Rec. 2017, 181, 321. [Google Scholar] [CrossRef]
- Lloyd, J.K.F. Minimising stress for patients in the veterinary hospital: Why it is important and what can be done about it. Vet. Sci. 2017, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wielebnowski, N. Stress and distress: Evaluating their impact for the well-being of zoo animals. J. Am. Vet. Med. Assoc. 2003, 223, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Lind, A.K.; Hydbring-Sandberg, E.; Forkman, B.; Keeling, L.J. Assessing stress in dogs during a visit to the veterinary clinic: Correlations between dog behavior in standardized tests and assessments by veterinary staff and owners. J. Vet. Behav. Clin. Appl. Res. 2017, 17, 24–31. [Google Scholar] [CrossRef]
- Mariti, C.; Raspanti, E.; Zilocchi, M.; Carlone, B.; Gazzano, A. The assessment of dog welfare in the waiting room of a veterinary clinic. Anim. Welf. 2015, 24, 299–305. [Google Scholar] [CrossRef]
- Hekman, J.P.; Karas, A.Z.; Sharp, C.R. Psychogenic stress in hospitalized dogs: Cross species comparisons, implications for health care, and the challenges of evaluation. Animals 2014, 4, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Overall, K.L. Manual of Behavioral Medicine for Dogs and Cats; Elseiver: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Fleming, P.A.; Clarke, T.; Wickham, S.L.; Stockman, C.A.; Barnes, A.L.; Collins, T.; Miller, D.W. The contribution of qualitative behavioural assessment to appraisal of livestock welfare. Anim. Prod. Sci. 2016, 56, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Arena, L.; Wemelsfelder, F.; Messori, S.; Ferri, N.; Barnard, S. Development of a fixed list of terms for the Qualitative Behavioural Assessment of shelter dogs. PLoS ONE 2019, 14, e0212652. [Google Scholar] [CrossRef]
- Reisner, I.R. Differential diagnosis and management of human-directed aggression in dogs. Vet. Clin. North Am.-Small Anim. Pract. 2003, 33, 303–320. [Google Scholar] [CrossRef]
- Yin, S. Calm pets, happy vets. reducing stress and preventing and managing fear aggression in veterinary clinics. Spec. Issue Anim. Behav. 2014, 24, 28–36. [Google Scholar]
- Riemer, S.; Heritier, C.; Windschnurer, I.; Pratsch, L.; Arhant, C.; Affenzeller, N. A review on mitigating fear and aggression in dogs and cats in a veterinary setting. Animals 2021, 11, 158. [Google Scholar] [CrossRef]
- Lindig, A.M.; McGreevy, P.D.; Crean, A.J. Musical dogs: A review of the influence of auditory enrichment on canine health and behavior. Animals 2020, 10, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, U. The Anxiety- and Pain-Reducing Effects of Music Interventions: A Systematic Review. AORN J. 2008, 87, 780–807. [Google Scholar] [CrossRef] [PubMed]
- Bernatzky, G.; Presch, M.; Anderson, M.; Panksepp, J. Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neurosci. Biobehav. Rev. 2011, 35, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.; Chaboyer, W.; Schluter, P.; Hiratos, M. The effect of music on preoperative anxiety in day surgery. J. Adv. Nurs. 2005, 52, 47–55. [Google Scholar] [CrossRef]
- Ploukou, S.; Panagopoulou, E. Playing music improves well-being of oncology nurses. Appl. Nurs. Res. 2018, 39, 77–80. [Google Scholar] [CrossRef]
- McDonald, C.I.; Zaki, S. A role for classical music in veterinary practice: Does exposure to classical music reduce stress in hospitalised dogs? Aust. Vet. J. 2020, 98, 31–36. [Google Scholar] [CrossRef]
- Mira, F.; Costa, A.; Mendes, E.; Azevedo, P.; Carreira, L.M. Influence of music and its genres on respiratory rate and pupil diameter variations in cats under general anaesthesia: Contribution to promoting patient safety. J. Feline Med. Surg. 2015, 18, 150–159. [Google Scholar] [CrossRef]
- Engler, W.J.; Bain, M. Effect of different types of classical music played at a veterinary hospital on dog behavior and owner satisfaction. J. Am. Vet. Med. Assoc. 2017, 251, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Alworth, L.C.; Buerkle, S.C. The effects of music on animal physiology, behavior and welfare. Lab Anim. 2013, 42, 54–61. [Google Scholar] [CrossRef]
- Bowman, A.; Scottish, S.P.C.A.; Dowell, F.J.; Evans, N.P. “Four Seasons” in an animal rescue centre; classical music reduces environmental stress in kennelled dogs. Physiol. Behav. 2015, 143, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L.; Graham, L.; Hepper, P.G. The influence of auditory stimulation on the behaviour of dogs housed in a rescue shelter. Anim. Welf. 2002, 11, 385–393. [Google Scholar]
- Brayley, C.; Montrose, V.T. The effects of audiobooks on the behaviour of dogs at a rehoming kennels. Appl. Anim. Behav. Sci. 2016, 174, 111–115. [Google Scholar] [CrossRef]
- Kogan, L.R.; Schoenfeld-Tacher, R.; Simon, A.A. Behavioral effects of auditory stimulation on kenneled dogs. J. Vet. Behav. Clin. Appl. Res. 2012, 7, 268–275. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Lopes, B.; Jorge, P. Effect of Auditory Stimulation During Early Development in Puppy Testing of Future Police Working Dogs. Top. Companion Anim. Med. 2018, 33, 100–104. [Google Scholar] [CrossRef]
- Bowman, A.; Dowell, F.J.; Evans, N.P. The effect of different genres of music on the stress levels of kennelled dogs. Physiol. Behav. 2017, 171, 207–215. [Google Scholar] [CrossRef] [Green Version]
- McConnell, P. New Research on Dogs and Music. Available online: http://www.patriciamcconnell.com/theotherendoftheleash/new-research-on-dogs-and-music (accessed on 10 January 2022).
- Snowdon, C.T.; Teie, D.; Savage, M. Cats prefer species-appropriate music. Appl. Anim. Behav. Sci. 2015, 166, 106–111. [Google Scholar] [CrossRef]
- Hampton, A.; Ford, A.; Cox, R.E.; Liu, C.C.; Koh, R. Effects of music on behavior and physiological stress response of domestic cats in a veterinary clinic. J. Feline Med. Surg. 2020, 22, 122–128. [Google Scholar] [CrossRef]
- Panksepp, J.; Bernatzky, G. Emotional sounds and the brain: The neuro-affective foundations of musical appreciation. Behav. Processes 2002, 60, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, L.; Porta, C.; Sleight, P. Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non-musicians: The importance of silence. Heart 2006, 92, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Clayton, M.; Sager, R.; Will, U. In time with the music: The concept of entrainment and its significance for ethnomusicology. ESEM Counterpoint 2004, 1, 1–45. [Google Scholar]
- Trappe, H.J. Music and medicine: The effects of music on the human being. Appl. Cardiopulm. Pathophysiol. 2012, 16, 133–142. [Google Scholar]
- Thaut, M.H.; McIntosh, G.C.; Hoemberg, V. Neurobiological foundations of neurologic music therapy: Rhythmic entrainment and the motor system. Front. Psychol. 2015, 5, 1185. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 11 January 2022).
- Riemer, S.; Assis, L.; Pike, T.W.; Mills, D.S. Dynamic changes in ear temperature in relation to separation distress in dogs. Physiol. Behav. 2016, 167, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lush, J.; Ijichi, C. A preliminary investigation into personality and pain in dogs. J. Vet. Behav. 2018, 24, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Prato Previde, E.; Valsecchi, P. Hot dogs: Thermography in the assessment of stress in dogs (Canis familiaris)—A pilot study. J. Vet. Behav. Clin. Appl. Res. 2015, 10, 17–23. [Google Scholar] [CrossRef]
- Kikkawa, A.; Uchida, Y.; Nakade, T.; Taguchi, K. Salivary secretory IgA concentrations in beagle dogs. J. Vet. Med. Sci. 2003, 65, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Kikkawa, A.; Uchida, Y.; Suwa, Y.; Taguchi, K. A novel method for estimating the adaptive ability of guide dogs using salivary sIgA. J. Vet. Med. Sci. 2005, 67, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Skandakumar, S.; Stodulski, G.; Hau, J. Salivary IgA: A possible stress marker in dogs. Anim. Welf. 1995, 4, 339–350. [Google Scholar]
- Lensen, R.C.M.M.; Moons, C.P.H.; Diederich, C. Physiological stress reactivity and recovery related to behavioral traits in dogs (Canis familiaris). PLoS ONE 2019, 14, e0222581. [Google Scholar] [CrossRef] [Green Version]
- Kartashova, I.A.; Ganina, K.K.; Karelina, E.A.; Tarasov, S.A. How to evaluate and manage stress in dogs—A guide for veterinary specialist. Appl. Anim. Behav. Sci. 2021, 243, 105458. [Google Scholar] [CrossRef]
- Svobodová, I.; Chaloupková, H.; Končel, R.; Bartoš, L.; Hradecká, L.; Jebavy, L. Cortisol and Secretory Immunoglobulin A Response to Stress in German Shepherd Dogs. PLoS ONE 2014, 9, e90820. [Google Scholar] [CrossRef]
- Cobb, M.L.; Iskandarani, K.; Chinchilli, V.M.; Dreschel, N.A. A systematic review and meta-analysis of salivary cortisol measurement in domestic canines. Domest. Anim. Endocrinol. 2016, 57, 31–42. [Google Scholar] [CrossRef]
- Hekman, J.P.; Karas, A.Z.; Dreschel, N.A. Salivary cortisol concentrations and behavior in a population of healthy dogs hospitalized for elective procedures. Appl. Anim. Behav. Sci. 2012, 141, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Coppola, C.L.; Grandin, T.; Enns, R.M. Human interaction and cortisol: Can human contact reduce stress for shelter dogs? Physiol. Behav. 2006, 87, 537–541. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.H.; Janssen, N.S.C.R.M.; Mol, J.A. The Use of Saliva Cortisol, Urinary Cortisol, and Catecholamine Measurements for a Noninvasive Assessment of Stress Responses in Dogs. Horm. Behav. 1996, 30, 272–279. [Google Scholar] [CrossRef]
- Chmelíková, E.; Bolechová, P.; Chaloupková, H.; Svobodová, I.; Jovičić, M.; Sedmíková, M. Salivary cortisol as a marker of acute stress in dogs: A review. Domest. Anim. Endocrinol. 2020, 72, 106428. [Google Scholar] [CrossRef]
- Siracusa, C.; Manteca, X.; Cerón, J.; Martínez-Subiela, S.; Cuenca, R.; Lavin, S.; Garcia, F.; Pastor, J. Perioperative stress response in dogs undergoing elective surgery: Variations in behavioural, neuroendocrine, immune and acute phase responses. Anim. Welf. 2008, 17, 259–273. [Google Scholar]
- Vincent, I.C.; Michell, A.R. Comparison of cortisol concentrations in saliva and plasma of dogs. Res. Vet. Sci. 1992, 53, 342–345. [Google Scholar] [CrossRef]
- Rooney, N.J.; Gaines, S.A.; Bradshaw, J.W.S. Behavioural and glucocorticoid responses of dogs (Canis familiaris) to kennelling: Investigating mitigation of stress by prior habituation. Physiol. Behav. 2007, 92, 847–854. [Google Scholar] [CrossRef]
- Haverbeke, A.; Diederich, C.; Depiereux, E.; Giffroy, J.M. Cortisol and behavioral responses of working dogs to environmental challenges. Physiol. Behav. 2008, 93, 59–67. [Google Scholar] [CrossRef]
- Olivier, B.; Zethof, T.; Pattij, T.; Van Boogaert, M.; Van Oorschot, R.; Leahy, C.; Oosting, R.; Bouwknecht, A.; Veening, J.; Van Der Gugten, J.; et al. Stress-induced hyperthermia and anxiety: Pharmacological validation. Eur. J. Pharmacol. 2003, 463, 117–132. [Google Scholar] [CrossRef]
- Gerdin, A.; Igosheva, N.; Roberson, L.; Ismail, O.; Karp, N.; Sanderson, M.; Cambridge, E.; Shannon, C.; Sunter, D.; Ramirez-solis, R.; et al. Physiology & Behavior Experimental and husbandry procedures as potential modifiers of the results of phenotyping tests. Physiol. Behav. 2012, 106, 602–611. [Google Scholar] [CrossRef] [Green Version]
- Hart, L.A.; Ohta, M.; Wakshlag, J.; Baumans, V.; Zanghi, B.M. Eye and Ear Temperature Using Infrared Thermography Are Related to Rectal Temperature in Dogs at Rest or with Exercise. Front. Vet. Sci. 2016, 3, 111. [Google Scholar] [CrossRef] [Green Version]
- Travnik, I.C.; Sant’Anna, A.C. Do you see the same cat that I see? Relationships between Qualitative Behaviour Assessment and indicators traditionally used to assess temperament in domestic cats. Anim. Welf. 2021, 30, 211–223. [Google Scholar] [CrossRef]
- Stanford, T.L. Behavior of dogs entering a veterinary clinic. Appl. Anim. Ethol. 1981, 7, 271–279. [Google Scholar] [CrossRef]
- Everest, F.A.; Pohlmann, K.C. Master Handbook of Acoustics, 5th ed.; McGraw Hill: Chicago, IL, USA, 2015. [Google Scholar]
- Ogata, N.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Objective measurement of fear-associated learning in dogs. J. Vet. Behav. 2006, 1, 55–61. [Google Scholar] [CrossRef]
- Neethirajan, S. Transforming the adaptation physiology of farm animals through sensors. Animals 2020, 10, 1512. [Google Scholar] [CrossRef]
- Chambers, R.D.; Yoder, N.C.; Carson, A.B.; Junge, C.; Allen, D.E.; Prescott, L.M.; Bradley, S.; Wymore, G.; Lloyd, K.; Lyle, S. Deep learning classification of canine behavior using a single collar-mounted accelerometer: Real-world validation. Animals 2021, 11, 1549. [Google Scholar] [CrossRef]
- Köster, L.S.; Sithole, F.; Gilbert, G.E.; Artemiou, E. The potential beneficial effect of classical music on heart rate variability in dogs used in veterinary training. J. Vet. Behav. 2019, 30, 103–109. [Google Scholar] [CrossRef]
- Stegemann, T.; Geretsegger, M.; Phan Quoc, E.; Riedl, H.; Smetana, M. Music Therapy and Other Music-Based Interventions in Pediatric Health Care: An Overview. Medicines 2019, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Standley, J.M. A discussion of evidence-based music therapy to facilitate feeding skills of premature infants: The power of contingent music. Arts Psychother. 2012, 39, 379–382. [Google Scholar] [CrossRef]
- Stellato, A.C.; Flint, H.E.; Dewey, C.E.; Widowski, T.M.; Niel, L. Risk-factors associated with veterinary-related fear and aggression in owned domestic dogs. Appl. Anim. Behav. Sci. 2021, 241, 105374. [Google Scholar] [CrossRef]







| Term | Definition |
|---|---|
| Aggressive | Impetuous, shows signs and posture of defensive or offensive aggression |
| Alert | Vigilant, inquisitive, on guard |
| Anxious | Worried, unable to settle or cope with its environment, apprehensive |
| Attention Seeking | Interactive, looking for contact/interaction, vying for people’s attention, affectionate |
| Bored | Disinterested, passive, showing sub-optimal arousal levels/drowsiness signs |
| Comfortable | Without worries, settled in its environment, peaceful with other dogs, people, and external stimuli |
| Curious | Actively interested in people or things, explorative, inquiring, in a positive, relaxed manner |
| Depressed | Dull, sad demeanor, disengaged from and unresponsive to the environment, quiet, apathetic |
| Excited | Positively agitated in response to external stimuli, euphoric, exuberant, thrilled |
| Explorative | Confident in exploring the environment or net stimuli, investigative |
| Fearful | Timid, scared, timorous, does not approach people or moves away, shows postures typical of fear |
| Hesitant | Unsure, doubtful, shows conflicting behavior, uncertain whether to approach or trust a stimulus, other dog, or person |
| Interested | Attentive, attracted to stimuli and attempting to approach them |
| Nervous | Uneasy, agitated, shows fast arousal, unsettled, restless, hyperactive |
| Playful | Cheerful, high spirits, fun, showing play-related behavior, inviting others to play |
| Reactive | Responsive to external stimuli |
| Relaxed | Easy going, calm, or acting in a calm way, does not show tension |
| Sociable | Confident, friendly toward humans and other dogs, appreciates human attentions, shows greeting behavior |
| Stressed | Tense, shows signs of distress |
| Wary | Cautious, prudent, suspicious, circumspect |
| Calm | Tranquil and quiet. Not showing any signs of aggression, chaotic behaviors, worry or excitement |
| Stress Level | Body Posture | Ear Posture | Gaze | Respirations | Lips | Activity | Vocalization |
|---|---|---|---|---|---|---|---|
| 0 | Relaxed and moves on own | High and softly forward | Will look steadily at vet | Normal—jaw relaxed | Relaxed | Flexible | None |
| 1 | Tense—can manipulate | Moving back a bit | Looks only intermittently at vet | Normal—jaw tensed | Firm | Inactive | Whine, cry |
| 2 | Rigid–hard to manipulate and a bit lower | Fully back | Will not look at vet but scans room | Panting—dry | Licking lips | Paws flexed, may tremble | Whimper |
| 3 | Hunched—hard to see or examine belly and low posture | Ears back and down | Not scanning, looking steadily at distance or owner | Panting—dripping | Yawning and licking | Periodic trembling | Snarl, snap |
| 4 | Curled—completely withdrawn and belly maximally tucked | As low and back as is possible | Staring fixedly and steadily at immediate fore-distance | Profound panting, salivating, gasping | - | Uncontrollable trembling | Bite |
| Item | PC1—Stressed/Anxious | PC2—Interacting/Engaged | PC3—Afraid |
|---|---|---|---|
| Stressed | 0.915 | 0.031 | −0.140 |
| Anxious | 0.879 | 0.070 | −0.106 |
| Nervous | 0.789 | 0.224 | −0.206 |
| Wary | 0.670 | 0.126 | 0.428 |
| Comfortable | −0.892 | −0.126 | 0.054 |
| Relaxed | −0.865 | −0.167 | 0.028 |
| Calm | −0.771 | −0.373 | 0.140 |
| Bored | −0.478 | −0.463 | 0.053 |
| Reactive | 0.316 | 0.464 | −0.177 |
| Curious | −0.366 | 0.756 | 0.057 |
| Excited | −0.270 | 0.747 | 0.143 |
| Interested | −0.421 | 0.746 | 0.015 |
| Explorative | −0.211 | 0.698 | −0.060 |
| Sociable | −0.317 | 0.666 | 0.248 |
| Playful | −0.282 | 0.665 | 0.187 |
| Fearful | 0.463 | −0.033 | 0.780 |
| Hesitant | 0.483 | 0.139 | 0.725 |
| Depressed | 0.181 | −0.408 | 0.538 |
| Alert | 0.447 | 0.186 | −0.617 |
| Aggressive | 0.074 | 0.188 | 0.136 |
| Explained Variance (%) | 32.2% | 20.0% | 11.3% |
| Item | PC1—Temperature | PC2—Consult Stress | PC3—Kennel Confidence |
|---|---|---|---|
| IRT Nose Temp (T1) | 0.871 | 0.008 | −0.089 |
| IRT Max Ear Temp (T1) | 0.793 | −0.034 | 0.321 |
| IRT Mean Ear Temp (T1) | 0.782 | −0.026 | 0.416 |
| IRT Nose Temp (T2) | 0.768 | −0.094 | −0.125 |
| IRT Nose Temp (T0) | 0.749 | −0.156 | −0.025 |
| IRT Mean Ear Temp (T0) | 0.704 | −0.189 | 0.359 |
| IRT Max Ear Temp (T2) | 0.653 | 0.029 | 0.250 |
| IRT Mean Ear Temp (T2) | 0.642 | −0.070 | 0.423 |
| TPR Rectal Temp (Consult) | 0.636 | 0.013 | −0.196 |
| IRT Max Ear Temp (T0) | 0.586 | −0.184 | 0.293 |
| IgA (T1) | −0.527 | −0.150 | 0.549 |
| IgA (T2) | −0.500 | −0.049 | 0.460 |
| QBA PC3 Afraid (Consult) | −0.157 | 0.760 | 0.276 |
| CDSS Activity (Consult) | −0.068 | 0.732 | 0.181 |
| CDSS Body Posture (Consult) | 0.056 | 0.728 | 0.112 |
| CDSS Gaze (Consult) | −0.070 | 0.701 | 0.299 |
| QBA PC1 Stressed/Anxious (Consult) | 0.199 | 0.583 | −0.032 |
| Cortisol (T2) | 0.112 | 0.533 | −0.397 |
| QBA PC2 Interacting/Engaged (Consult) | 0.078 | −0.618 | −0.440 |
| QBA PC1 Stressed/Anxious (Kennel) | 0.280 | 0.097 | −0.538 |
| TPR Respiration Rate (Consult) | 0.466 | 0.410 | −0.003 |
| IRT Eye Temp (T1) | 0.447 | 0.046 | 0.490 |
| TPR Heart Rate (Consult) | 0.444 | 0.134 | −0.319 |
| QBA PC2 Interacting/Engaged (Kennel) | 0.331 | 0.150 | −0.402 |
| CDSS Respirations | 0.321 | 0.245 | 0.026 |
| Cortisol (T1) | 0.273 | 0.492 | −0.366 |
| IRT Eye Temp (T0) | 0.147 | −0.379 | 0.394 |
| IRT Eye Temp (T1) | 0.125 | −0.166 | 0.124 |
| CDSS Lips (Consult) | 0.075 | 0.059 | 0.059 |
| Cortisol (T0) | 0.057 | 0.176 | −0.281 |
| CDSS Vocalization (Consult) | 0.045 | −0.240 | −0.233 |
| CDSS Ear Posture (Consult) | −0.087 | 0.125 | 0.081 |
| QBA PC3 Afraid (Kennel) | −0.225 | 0.156 | 0.223 |
| IgA (T0) | −0.401 | 0.017 | 0.354 |
| Explained Variance (%) | 20.9% | 12.1% | 9.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, T.; Flint, H.E.; Hunt, A.B.G.; Werzowa, W.T.; Logan, D.W. Effect of Music on Stress Parameters in Dogs during a Mock Veterinary Visit. Animals 2022, 12, 187. https://doi.org/10.3390/ani12020187
King T, Flint HE, Hunt ABG, Werzowa WT, Logan DW. Effect of Music on Stress Parameters in Dogs during a Mock Veterinary Visit. Animals. 2022; 12(2):187. https://doi.org/10.3390/ani12020187
Chicago/Turabian StyleKing, Tammie, Hannah E. Flint, Alysia B. G. Hunt, Walter T. Werzowa, and Darren W. Logan. 2022. "Effect of Music on Stress Parameters in Dogs during a Mock Veterinary Visit" Animals 12, no. 2: 187. https://doi.org/10.3390/ani12020187






