Telomere Length in Pig Sperm Is Related to In Vitro Embryo Development Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Semen Samples and Ethics
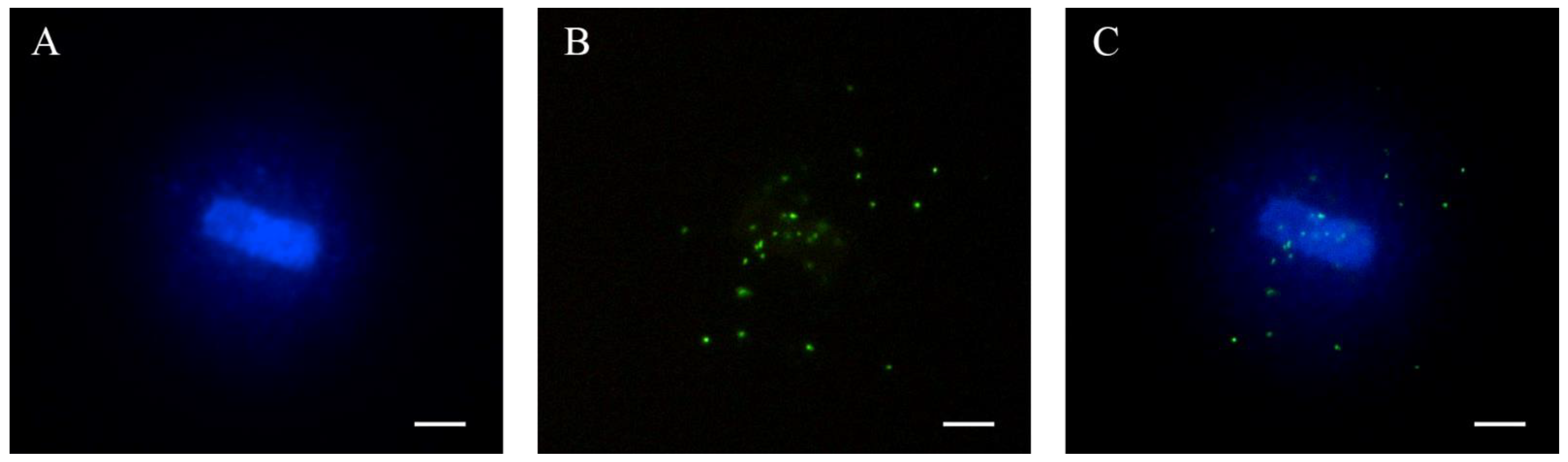
2.3. Telomere Length Analysis through Quantitative Fluorescent In Situ Hybridization (qFISH)
2.3.1. Sperm Chromatin Dispersion
2.3.2. Quantitative Fluorescent In Situ Hybridization (qFISH)
2.3.3. Image Captures and Analysis
2.3.4. Setting Up the Exposure Time for the Evaluation of Fluorescence Intensity
2.3.5. Absolute Telomere Length Estimation
2.4. Sperm Quality Parameters
2.4.1. Sperm Motility
2.4.2. Sperm Morphology
2.4.3. Sperm Viability
2.4.4. Sperm DNA Damage
2.5. In Vitro Fertilization (IVF)
2.6. Statistical Analysis
3. Results
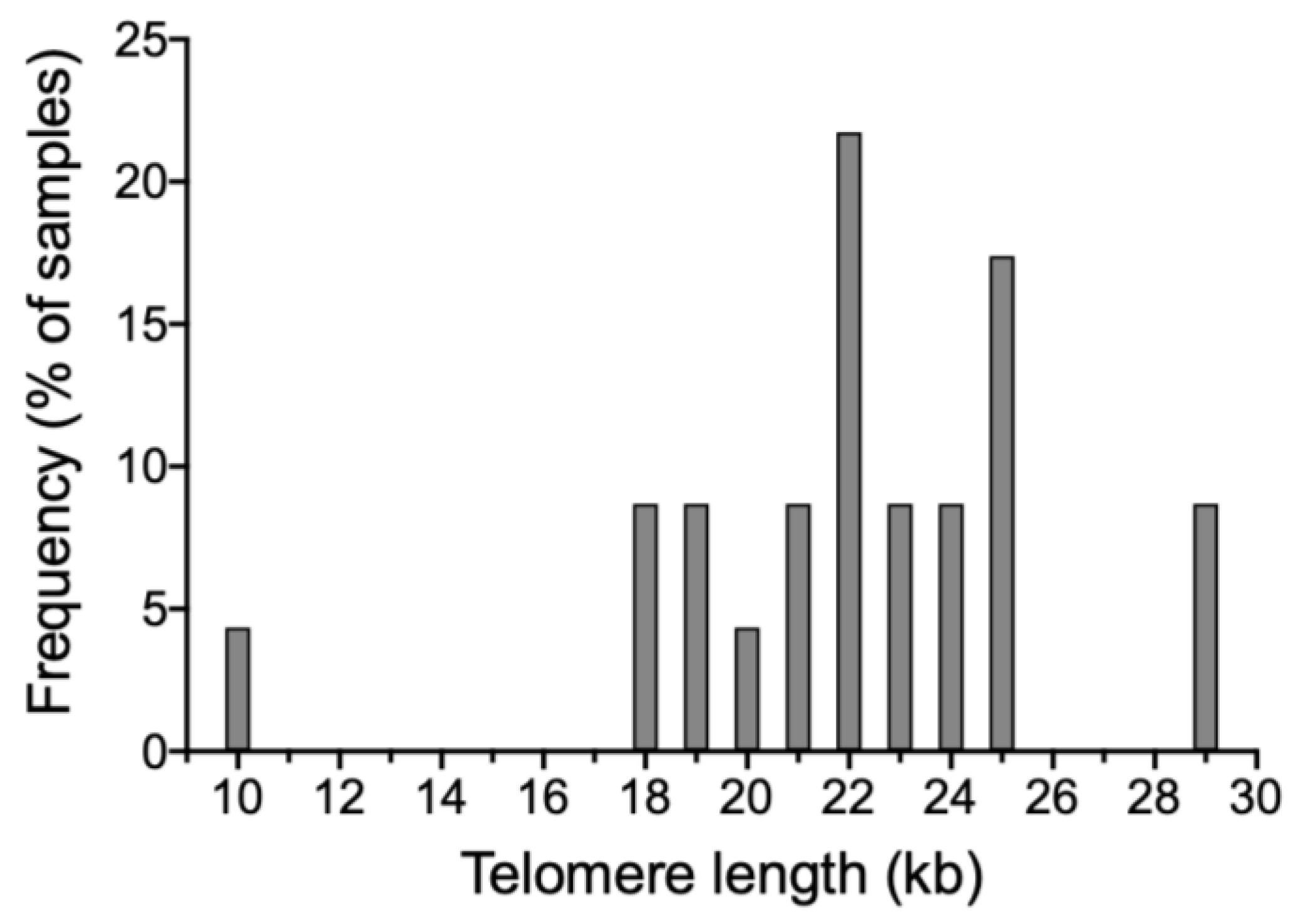
3.1. Setting Up the Evalution of Sperm Telomere Length
3.2. Relationship between Sperm Telomere Length and Quality Parameters
3.3. Relationship between Sperm Telomere Length and In Vitro Fertilization Outcomes
4. Discussion
4.1. Telomere Length in Pig Sperm
4.2. Relationship between Sperm Telomere Length and Quality Parameters
4.3. Relationship between Sperm Telomere Length and IVF Outcomes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Calado, R.T.; Dumitriu, B. Telomere dynamics in mice and humans. Semin. Hematol. 2013, 50, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsyth, N.R.; Wright, W.E.; Shay, J.W. Telomerase and differentiation in multicellular organisms: Turn it off, turn it on, and turn it off again. Differentiation 2002, 69, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.S.; Foresta, C.; Ferlin, A. Telomere length: Lights and shadows on their role in human reproduction. Biol. Reprod. 2019, 100, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Grollman, A.P.; Moriya, M. Mutagenesis by 8-oxoguanine: An enemy within. Trends Genet. 1993, 9, 246–249. [Google Scholar] [CrossRef]
- Coluzzi, E.; Colamartino, M.; Cozzi, R.; Leone, S.; Meneghini, C.; O’Callaghan, N.; Sgura, A. Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PLoS ONE 2014, 9, e110963. [Google Scholar] [CrossRef] [PubMed]
- Cherif, H.; Tarry, J.L.; Ozanne, S.E.; Hales, C.N. Ageing and telomeres: A study into organ- and gender-specific telomere shortening. Nucleic Acids Res. 2003, 31, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Samassekou, O.; Gadji, M.; Drouin, R.; Yan, J. Sizing the ends: Normal length of human telomeres. Ann. Anat. 2010, 192, 284–291. [Google Scholar] [CrossRef]
- Thilagavathi, J.; Kumar, M.; Mishra, S.S.; Venkatesh, S.; Kumar, R.; Dada, R. Analysis of sperm telomere length in men with idiopathic infertility. Arch. Gynecol. Obstet. 2013, 287, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Pech, M.F.; Garbuzov, A.; Hasegawa, K.; Sukhwani, M.; Zhang, R.J.; Benayoun, B.A.; Brockman, S.A.; Lin, S.; Brunet, A.; Orwig, K.E.; et al. High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev. 2015, 29, 2420–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keefe, D.L.; Liu, L. Telomeres and reproductive aging. Reprod. Fertil. Dev. 2009, 21, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Boniewska-Bernacka, E.; Pańczyszyn, A.; Cybulska, N. Telomeres as a molecular marker of male infertility. Hum. Fertil. 2019, 22, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Cariati, F.; Jaroudi, S.; Alfarawati, S.; Raberi, A.; Alviggi, C.; Pivonello, R.; Wells, D. Investigation of sperm telomere length as a potential marker of paternal genome integrity and semen quality. Reprod. Biomed. Online. 2016, 33, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Ferlin, A.; Rampazzo, E.; Rocca, M.S.; Keppel, S.; Frigo, A.C.; De Rossi, A.; Foresta, C. In young men sperm telomere length is related to sperm number and parental age. Hum. Reprod. 2013, 28, 3370–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocca, M.S.; Speltra, E.; Menegazzo, M.; Garolla, A.; Foresta, C.; Ferlin, A. Sperm telomere length as a parameter of sperm quality in normozoospermic men. Hum. Reprod. 2016, 31, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, F.; Dai, S.; Zhang, N.; Zhao, W.; Bai, R.; Sun, Y. Sperm telomere length is positively associated with the quality of early embryonic development. Hum. Reprod. 2015, 30, 1876–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torra-Massana, M.; Barragán, M.; Bellu, E.; Oliva, R.; Rodríguez, A.; Vassena, R. Sperm telomere length in donor samples is not related to ICSI outcome. J. Assist. Reprod. Genet. 2018, 35, 649–657. [Google Scholar] [CrossRef]
- Knox, R.V. The fertility of frozen boar sperm when used for artificial insemination. Reprod. Domest. Anim. 2015, 50, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanapiwat, P.; Olanratmanee, E.O.; Kaeoket, K.; Tummaruk, P. Conception rate and litter size in multiparous sows after intrauterine insemination using frozen-thawed boar semen in a commercial swine herd in Thailand. J. Vet. Med. Sci. 2014, 76, 1347–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fradiani, P.A.; Ascenzioni, F.; Lavitrano, M.; Donini, P. Telomeres and telomerase activity in pig tissues. Biochimie. 2004, 86, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kozik, A.; Bradbury, E.M.; Zalensky, A. Increased telomere size in sperm cells of mammals with long terminal (TTAGGG)n arrays. Mol. Reprod. Dev. 1998, 51, 98–104. [Google Scholar] [CrossRef]
- Lara-Cerrillo, S.; Gual-Frau, J.; Benet, J.; Abad, C.; Prats, J.; Amengual, M.J.; Ribas-Maynou, J.; García-Peiró, A. Microsurgical varicocelectomy effect on sperm telomere length, DNA fragmentation and seminal parameters. Hum. Fertil. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, R.; Bosch-Rue, E.; Ribas-Maynou, J.; Alvarez, J.; Brassesco, C.; Amengual, M.J.J.; Benet, J.; Garcia-Peiró, A.; Brassesco, M. Sperm telomere length in motile sperm selection techniques: A qFISH approach. Andrologia 2018, 50, e12840. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Garcia-Bonavila, E.; Hidalgo, C.O.; Catalán, J.; Miro, J.; Yeste, M. Species-specific differences in sperm chromatin decondensation between eutherian mammals underlie distinct lysis requirements. Front. Cell Dev. Biol. 2021, 9, 669182. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Delgado-Bermúdez, A.; Garcia-Bonavila, E.; Pinart, E.; Yeste, M.; Bonet, S. Complete Chromatin Decondensation of Pig Sperm Is Required to Analyze Sperm DNA Breaks With the Comet Assay. Front. Cell Dev. Biol. 2021, 9, 675973. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample size calculation in medical studies. Gastroenterol. Hepatol. Bed Bench 2013, 6, 14–17. [Google Scholar] [PubMed]
- Zeng, F.; Chen, Y.; Guo, C.; Li, C.; Wei, H.; Li, L.; Meng, L.; Zhang, S. Analysis of differentially abundant proteins related to boar fertility in seminal plasma using iTRAQ-based quantitative proteomics. J. Proteom. 2021, 236, 104120. [Google Scholar] [CrossRef]
- Kim, E.; Cai, L.; Hyun, S.-H. Effects of Stem Cell Factor/c-Kit Signaling on In Vitro Maturation of Porcine Oocytes and Subsequent Developmental Competence After Fertilization. Front. Vet. Sci. 2021, 8, 745488. [Google Scholar] [CrossRef]
- Abeydeera, L.; Day, B. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol. Reprod. 1997, 57, 729–734. [Google Scholar] [CrossRef] [Green Version]
- Balaban, B.; Gardner, D.K.D.K. Morphological assessment of Blastocyst Stage Embryos: Types of Grading Systems and Their Reported Outcomes. In Human Gametes and Preimplantation Embryos: Assessment and Diagnosis; Gardner, D.K., Seli, E., Sakkas, D., Wells, D., Eds.; Springer: New York, NY, USA, 2013; pp. 31–43. ISBN 9781461466512. [Google Scholar]
- Jørgensen, P.B.; Fedder, J.; Koelvraa, S.; Graakjaer, J. Age-dependence of relative telomere length profiles during spermatogenesis in man. Maturitas 2013, 75, 380–385. [Google Scholar] [CrossRef]
- Fice, H.E.; Robaire, B. Telomere dynamics throughout spermatogenesis. Genes 2019, 10, 525. [Google Scholar] [CrossRef] [Green Version]
- Fick, L.J.; Fick, G.H.; Li, Z.; Cao, E.; Bao, B.; Heffelfinger, D.; Parker, H.G.; Ostrander, E.A.; Riabowol, K. Telomere Length Correlates with Life Span of Dog Breeds. Cell Rep. 2012, 2, 1530–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, D.M.; Britt-Compton, B.; Rowson, J.; Amso, N.N.; Gregory, L.; Kipling, D. Telomere instability in the male germline. Hum. Mol. Genet. 2006, 15, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, S.; Hartshorne, G.M. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol. Hum. Reprod. 2013, 19, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, A.; Della Valle, G.; Russo, M.; Longobardi, V.; Albero, G.; De Canditiis, C.; Kosior, M.A.; Pistucci, R.; Gasparrini, B. Evaluation of bovine sperm telomere length and association with semen quality. Theriogenology 2020, 158, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Siderakis, M.; Tarsounas, M. Telomere regulation and function during meiosis. Chromosome Res. 2007, 15, 667–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safranski, T.J. Genetic selection of boars. Theriogenology 2008, 70, 1310–1316. [Google Scholar] [CrossRef]
- Gilchrist, G.C.; Kurjanowicz, P.; Mereilles, F.V.; King, W.A.; LaMarre, J. Telomere length and telomerase activity in bovine pre-implantation embryos in vitro. Reprod. Domest. Anim. 2015, 50, 58–67. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Mean ± SD | p-Value | ||
|---|---|---|---|---|
| All Samples | Group with Shorter Telomere Length | Group with Longer Telomere Length | ||
| n = 23 | n = 11 | n = 12 | ||
| Telomere length (kb) | 22.1 ± 3.6 | 19.4 ± 3.3 | 24.7 ± 2.3 | N/A |
| Progressive motility (%) | 73.2 ± 7.9 | 71.0 ± 8.5 | 75.1 ± 7.1 | >0.999 |
| Non-progressive motility (%) | 18.9 ± 8.6 | 19.4 ± 6.2 | 18.4 ± 10.5 | >0.999 |
| Fast sperm (%) | 67.2 ± 16.6 | 62.3 ± 16.1 | 71.7 ± 16.3 | 0.277 |
| Intermediate sperm (%) | 17.8 ± 12.5 | 20.2 ± 11.3 | 15.7 ± 13.6 | >0.999 |
| Slow sperm (%) | 7.0 ± 4.7 | 8.0 ± 4.8 | 6.0 ± 4.5 | >0.999 |
| Static sperm (%) | 8.0 ± 7.9 | 9.6 ± 9.4 | 6.6 ± 6.2 | >0.999 |
| VCL (µm/s) | 64 ± 13.6 | 61 ± 13.2 | 66.7 ± 13.9 | >0.999 |
| VSL (µm/s) | 37.5 ± 9.6 | 35.2 ± 6.5 | 39.7 ± 11.7 | >0.999 |
| VAP (µm/s) | 48.1 ± 9.5 | 45.7 ± 8.9 | 50.3 ± 9.8 | >0.999 |
| LIN (VSL/VCL) (%) | 59.2 ± 11.2 | 58.7 ± 8.1 | 59.9 ± 13.9 | >0.999 |
| STR (VSL/VAP) (%) | 77.8 ± 10.9 | 77.6 ± 9 | 78.1 ± 12.9 | >0.999 |
| WOB (VAP/VCL) (%) | 75.7 ± 6.2 | 75.4 ± 5.4 | 76 ± 7 | >0.999 |
| ALH (µm) | 2.3 ± 0.5 | 2.3 ± 0.4 | 2.5 ± 0.6 | >0.999 |
| BCF (Hz) | 7.8 ± 1.3 | 7.8 ± 1.2 | 7.8 ± 1.4 | >0.999 |
| Viability (%) | 87.4 ± 5.3 | 87.7 ± 4.7 | 87.2 ± 6.0 | >0.999 |
| Normal morphology (%) | 91.6 ± 6.9 | 89.1 ± 8.5 | 93.8 ± 4.2 | 0.164 |
| DNA damage (Olive Tail Moment) | 15.9 ± 3.0 | 16.6 ± 2.7 | 15.2 ± 3.2 | 0.211 |
| Parameter | Correlation Coefficient (95% C.I.) | p-Value |
|---|---|---|
| Progressive motility (%) | −0.018 (−0.429 to 0.395) | 0.934 |
| Non-progressive motility (%) | −0.029 (−0.380 to 0.443) | 0.896 |
| Fast sperm (%) | −0.326 (−0.812 to 0.335) | 0.129 |
| Intermediate sperm (%) | −0.229 (−0.673 to 0.582) | 0.292 |
| Slow sperm (%) | −0.311 (−0.663 to 0.078) | 0.149 |
| Static sperm (%) | −0.381 (−0.710 to −0.010) | 0.073 |
| VCL (µm/s) | 0.368 (−0.052 to 0.678) | 0.084 |
| VSL (µm/s) | 0.076 (−0.347 to 0.473) | 0.730 |
| VAP (µm/s) | 0.231 (−0.200 to 0.588) | 0.288 |
| LIN (VSL/VCL) (%) | −0.015 (−0.424 to 0.400) | 0.947 |
| STR (VSL/VAP) (%) | 0.226 (−0.205 to 0.584) | 0.299 |
| WOB (VAP/VCL) (%) | 0.110 (−0.317 to 0.499) | 0.618 |
| ALH (µm) | −0.068 (−0.467 to 0.354) | 0.757 |
| BCF (Hz) | −0.366 (−0.809 to 0.342) | 0.298 |
| Viability (%) | −0.246 (−0.598 to 0.185) | 0.257 |
| Normal morphology (%) | 0.139 (−0.502 to 0.730) | 0.528 |
| DNA damage (Olive Tail Moment) | −0.206 (−0.578 to 0.238) | 0.347 |
| Parameter | Mean ± SD | p-Value | ||
|---|---|---|---|---|
| All Samples | Group with Shorter Telomere Length | Group with Longer Telomere Length | ||
| Sperm samples = 13 | Sperm samples = 6 | Sperm samples = 7 | ||
| Oocytes used = 525 | Oocytes used = 240 | Oocytes used = 285 | ||
| Fertilization rate Day 2 (total n; %) | 177; 34.9% ± 12.3% | 72; 30.0% ± 6.9% | 105; 39.1% ± 15.5% | 0.465 |
| Morulae Day 6 (total n; % per sample) | 84; 17.0% ± 7.3% | 27; 11.5% ± 3.6% | 57; 21.8% ± 6.9% | 0.018 * |
| Early blastocysts + blastocysts Day 6 (total n; %) | 67; 13.1% ± 9.8% | 18; 7.6% ± 1.4% | 49; 17.9% ± 12.2% | 0.018 * |
| Hatched blastocysts Day 6 (total n; %) | 10; 1.9% ± 2.6% | 1; 0.4% ± 1.0% | 9; 3.2% ± 3.1% | >0.999 |
| Parameter | Correlation Coefficient (95% C.I.) | p-Value |
|---|---|---|
| Fertilization rate at Day 2 (%) | 0.056 (−0.524 to 0.601) | 0.856 |
| Morulae at Day 6 (%) | 0.559 (−0.007 to 0.854) | 0.047 * |
| Early blastocysts + blastocysts at Day 6 (%) | 0.410 (−0.200 to 0.791) | 0.164 |
| Hatched blastocysts at Day 6 (%) | 0.356 (−0.260 to 0.766) | 0.233 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribas-Maynou, J.; Mateo-Otero, Y.; Sanchez-Quijada, M.; Recuero, S.; Delgado-Bermúdez, A.; Llavanera, M.; Yeste, M. Telomere Length in Pig Sperm Is Related to In Vitro Embryo Development Outcomes. Animals 2022, 12, 204. https://doi.org/10.3390/ani12020204
Ribas-Maynou J, Mateo-Otero Y, Sanchez-Quijada M, Recuero S, Delgado-Bermúdez A, Llavanera M, Yeste M. Telomere Length in Pig Sperm Is Related to In Vitro Embryo Development Outcomes. Animals. 2022; 12(2):204. https://doi.org/10.3390/ani12020204
Chicago/Turabian StyleRibas-Maynou, Jordi, Yentel Mateo-Otero, Marina Sanchez-Quijada, Sandra Recuero, Ariadna Delgado-Bermúdez, Marc Llavanera, and Marc Yeste. 2022. "Telomere Length in Pig Sperm Is Related to In Vitro Embryo Development Outcomes" Animals 12, no. 2: 204. https://doi.org/10.3390/ani12020204









