Linking Animal Welfare and Antibiotic Use in Pig Farming—A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Stress as a Trigger for Disease
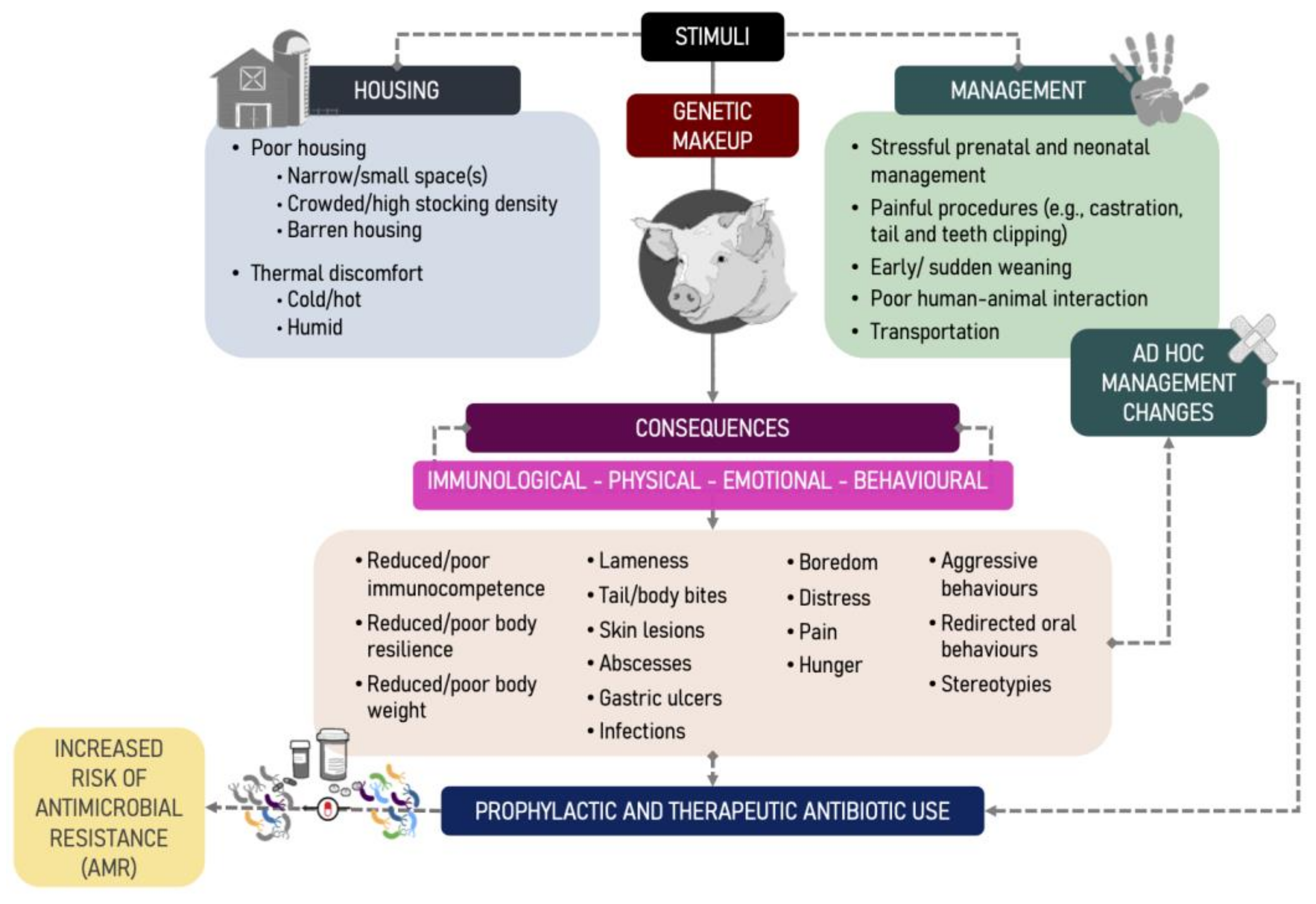
3. Sources of Stress in Pig Farm Management
3.1. Housing Stressors
3.1.1. Housing That Limits the Ability of Pigs to Move and Express Natural Behaviours
3.1.2. Housing That Causes Thermal Stress
3.2. Common Management Practices as Stressors
3.2.1. Feeding Strategies as a Source of Stress
3.2.2. Early Life Management
Prenatal Stress
Neonatal Management
Cross-Fostering and Artificial Rearing
Weaning Stress
Transportation of Young Pigs
3.2.3. Painful Procedures and Parturition as Sources of Pain in Pigs
3.2.4. Mixing Unfamiliar Animals
3.2.5. Human–Animal Interactions and Fear
4. So, Is There a Relationship between Animal Welfare and Use of Antibiotics in Pig Farming?
5. Improving Housing Environment and Management Practices to Reduce Stress in Pig Farming
5.1. Improved Housing and Environmental Enrichment
5.2. Group Housing-Increasing Space, Reducing Stocking Density or Both
5.3. Reducing Pre-Natal, Neonatal and Weaning Stress and Promoting Positive Human–Animal Interactions
5.4. Making a 180 Degree Turn into Genetic Selection to Improve Animal Welfare
5.5. The Intrinsic Value of Pigs—Re-Centring Pig Industry Values
6. Implications and Closing Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Burlingame, B.; Dawkins, M.; Dolan, L.; Fraser, D.; et al. Sustainable Intensification in Agriculture: Premises and Policies. Science 2013, 341, 33–34. [Google Scholar] [CrossRef] [PubMed]
- OIE. Animal Welfare—Introduction to the recommendations for animal welfare. In Terrestrial Animal Health Code; World Organisation for Animal Health: Paris, France, 2019; Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access (accessed on 13 October 2021).
- Mellor, D.J. Updating Animal Welfare Thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.M.; Genter, C.I.; Heinemann, C.; Steinhoff-Wagner, J. Impact of tearing spermatic cords during castration in live and dead piglets and consequences on welfare. Porc. Health Manag. 2021, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Herskin, M.S.; Di Giminiani, P. Pain in pigs: Characterisation, mechanisms and indicators. In Advances in Pig Welfare; Špinka, M., Camerlink, I., Eds.; Elsevier Ltd.: Cambridge, UK, 2017; pp. 325–355. [Google Scholar]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress—Basic Principles and Implications for Animal Welfare, 1st ed.; CAB International: Wallingford, UK, 2000. [Google Scholar]
- Cuong, N.V.; Padungtod, P.; Thwaites, G.; Carrique-Mas, J.J. Antimicrobial Usage in Animal Production: A Review of the Literature with a Focus on Low- and Middle-Income Countries. Antibiotics 2018, 7, 75. [Google Scholar] [CrossRef]
- Waluszewski, A.; Cinti, A.; Perna, A. Antibiotics in pig meat production: Restrictions as the odd case and overuse as normality? Experiences from Sweden and Italy. Humanit. Soc. Sci. Commun. 2021, 8, 172. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Wisener, L.V.; Sargeant, J.M.; O’Sullivan, T.L.; O’Connor, A.M.; McEwen, S.A.; Reist, M.; Churchill, K.J. Non-antibiotic Approaches for Disease Prevention and Control in Nursery Pigs: A Scoping Review. Front. Veter. Sci. 2021, 8, 620347. [Google Scholar] [CrossRef]
- Centner, T.J. Recent government regulations in the United States seek to ensure the effectiveness of antibiotics by limiting their agricultural use. Environ. Int. 2016, 94, 1–7. [Google Scholar] [CrossRef]
- Raasch, S.; Postma, M.; Dewulf, J.; Stärk, K.D.C.; Beilage, E.G. Association between antimicrobial usage, biosecurity measures as well as farm performance in German farrow-to-finish farms. Porc. Health Manag. 2018, 4, 30. [Google Scholar] [CrossRef]
- Dutra, M.; Moreno, L.; Dias, R.; Moreno, A. Antimicrobial Use in Brazilian Swine Herds: Assessment of Use and Reduction Examples. Microorganism 2021, 9, 881. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Minssen, T.; Outterson, K.; Van Katwyk, S.R.; Batista, P.H.D.; Chandler, C.I.R.; Ciabuschi, F.; Harbarth, S.; Kesselheim, A.S.; Laxminarayan, R.; Liddell, K.; et al. Social, cultural and economic aspects of antimicrobial resistance. Bull. World Health Organ. 2020, 98, 823A. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.; Ward, M.; Van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial Resistance in the Context of the Sustainable Development Goals: A Brief Review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic resistance in the food chain: A developing country-perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef]
- Ledingham, K.; Hinchliffe, S.; Jackson, M.; Thomas, F.; Tomson, G. Antibiotic Resistance: Using a Cultural Contexts of Health Approach to Address a Global Health Challenge Antibiotic Resistance: Using a Cultural Contexts. Policy brief. Copenhagen: World Health Organization. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/330029/9789289053945-eng.pdf?sequence=2&isAllowed=y (accessed on 13 October 2021).
- Herrero, M.; Hugas, M.; Lele, U.; Wira, A.; Torero, M. Shift to healthy and sustainable consumption patterns—A paper on Action Track 2. In United Nations Food Systems Summit; Braun, J., von Afsana, K., Fresco, L.O., Hassan, M., Eds.; UN Food Systems Summit: New York, NY, USA, 2021; pp. 1–25. [Google Scholar]
- van Bruggen, A.H.; Goss, E.M.; Havelaar, A.; van Diepeningen, A.D.; Finckh, M.; Morris, J.G. One Health—Cycling of diverse microbial communities as a connecting force for soil, plant, animal, human and ecosystem health. Sci. Total Environ. 2019, 664, 927–937. [Google Scholar] [CrossRef]
- Garcia Pinillos, R.; Appleby, M.C.; Manteca, X.; Scott-Park, F.; Smith, C.; Velarde, A. One Welfare—A platform for improving human and animal welfare. Vet. Rec. 2016, 179, 412–413. [Google Scholar] [CrossRef]
- Romero, L.M.; Dickens, M.J.; Cyr, N.E. The reactive scope model—A new model integrating homeostasis, allostasis, and stress. Horm. Behav. 2009, 55, 375–389. [Google Scholar] [CrossRef]
- Veissier, I.; Boissy, A. Stress and welfare: Two complementary concepts that are intrinsically related to the animal’s point of view. Physiol. Behav. 2007, 92, 429–433. [Google Scholar] [CrossRef]
- Broom, D.M. Animal welfare concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef]
- Boissy, A. Fear and fearfulness in animals. Q. Rev. Biol. 1995, 70, 165–191. [Google Scholar] [CrossRef]
- Rushen, J.; Taylor, A.A.; De Passillé, A.M. Domestic animals’ fear of humans and its effect on their welfare. Appl. Anim. Behav. Sci. 1999, 65, 285–303. [Google Scholar] [CrossRef]
- Hötzel, M.J.; Mota, S.M.; Ludtke, C.B.; Poletto, R. Knowledge and attitudes of official inspectors at slaughterhouses in southern Brazil regarding animal welfare. Rev. Bras. de Zootec. 2018, 47. [Google Scholar] [CrossRef]
- Hemsworth, P.; Coleman, G.J. Human-Livestock Interactions: The Stockperson and the Productivity and Welfare of Intensively Farmed Animals, 2nd ed.; CAB International: Melbourne, Australia, 2011. [Google Scholar]
- Beilharz, R.G.; Luxford, B.G.; Wilkinson, J.L. Quantitative genetics and evolution: Is our understanding of genetics sufficient to explain evolution? J. Anim. Breed Genet. 1993, 110, 161–170. [Google Scholar] [CrossRef]
- Degroot, J.; Ruis, M.; Scholten, J.; Koolhaas, J.; Boersma, W. Long-term effects of social stress on antiviral immunity in pigs. Physiol. Behav. 2001, 73, 145–158. [Google Scholar] [CrossRef]
- Rauw, W.M. Immune response from a resource allocation perspective. Front. Genet. 2012, 3, 267. [Google Scholar] [CrossRef]
- Burn, C.C. Bestial boredom: A biological perspective on animal boredom and suggestions for its scientific investigation. Anim. Behav. 2017, 130, 141–151. [Google Scholar] [CrossRef]
- Bracke, M.B.M.; Hopster, H. Assessing the importance of natural behavior for animal welfare. J. Agric. Environ. Ethics 2006, 19, 77–89. [Google Scholar] [CrossRef]
- Prunier, A.; Averos, X.; Dimitrov, I.; Edwards, S.A.; Hillmann, E.; Holinger, M.; Ilieski, V.; Leming, R.; Tallet, C.; Turner, S.; et al. Review: Early life predisposing factors for biting in pigs. Animals 2020, 14, 570–587. [Google Scholar] [CrossRef]
- Fertner, M.; Denwood, M.; Birkegård, A.C.; Stege, H.; Boklund, A. Associations between Antibacterial Treatment and the Prevalence of Tail-Biting-Related Sequelae in Danish Finishers at Slaughter. Front. Veter. Sci. 2017, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- Valros, A. Tail biting. In Advances in Pig Welfare; Špinka, M., Camerlink, I., Eds.; Elsevier Ltd.: Cambridge, UK, 2017; pp. 137–166. [Google Scholar]
- Stygar, A.H.; Chantziaras, I.; Toppari, I.; Maes, D.; Niemi, J.K. High biosecurity and welfare standards in fattening pig farms are associated with reduced antimicrobial use. Animal 2020, 14, 2178–2186. [Google Scholar] [CrossRef]
- EFSA. The risks associated with tail biting in pigs and possible means to reduce the need for tail docking considering the different housing and husbandry systems—Scientific Opinion of the Panel on Animal Health and Welfare. EFSA J. 2007, 5, 611. [Google Scholar] [CrossRef]
- Flowers, W.L. Factors Affecting the Efficient Production of Boar Sperm. Reprod. Domest. Anim. 2015, 50, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.J. Overview of commercial pig production systems and their main welfare challenges. In Advances in Pig Welfare; Špinka, M., Camerlink, I., Eds.; Elsevier Ltd.: Cambridge, UK, 2017; pp. 3–25. [Google Scholar]
- von Keyserlingk, M.; Hötzel, M. The Ticking Clock: Addressing Farm Animal Welfare in Emerging Countries. J. Agric. Environ. Ethics 2015, 28, 179–195. [Google Scholar] [CrossRef]
- Vandresen, B.; Hötzel, M.J. “Mothers Should Have Freedom of Movement”—Citizens’ Attitudes Regarding Farrowing Housing Systems for Sows and Their Piglets. Animals 2021, 11, 3439. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.; Jensen, P.; de Jonge, F.H.; Illmann, G.; Spinka, M. Maternal behaviour of domestic sows and crosses between domestic sows and wild boar. Appl. Anim. Behav. Sci. 1999, 65, 29–42. [Google Scholar] [CrossRef]
- Hötzel, M.J.; Machado Filho, L.C.P.; Dalla Costa, O.A. Behaviour of pre-parturient sows housed in intensive outdoor or indoor systems. Pesqui Agropecu Bras. 2005, 40, 169–174. [Google Scholar] [CrossRef]
- Verdon, M.; Hansen, C.F.; Rault, J.-L.; Jongman, E.; Hansen, L.U.; Plush, K.; Hemsworth, P.H. Effects of group housing on sow welfare: A review. J. Anim. Sci. 2015, 93, 1999–2017. [Google Scholar] [CrossRef]
- Marchant, J.N.; Broom, D.M. Factors affecting posture-changing in loose-housed and confined gestating sows. Anim. Sci. 1996, 63, 477–485. [Google Scholar] [CrossRef]
- Heinonen, M.; Peltoniemi, O.; Valros, A. Impact of lameness and claw lesions in sows on welfare, health and production. Livest. Sci. 2013, 156, 64–70. [Google Scholar] [CrossRef]
- Drolet, R. Urinary System. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 408–424. [Google Scholar]
- Albernaz-Gonçalves, R.; Olmos, G.; Hötzel, M.J. Exploring Farmers’ Reasons for Antibiotic Use and Misuse in Pig Farms in Brazil. Antibiotics 2021, 10, 331. [Google Scholar] [CrossRef]
- Kemper, N. Update on postpartum dysgalactia syndrome in sows. J. Anim. Sci. 2020, 98, S117–S125. [Google Scholar] [CrossRef] [PubMed]
- Brown-Brandl, T.; Eigenberg, R.; Nienaber, J.; Kachman, S. Thermoregulatory profile of a newer genetic line of pigs. Livest. Prod. Sci. 2001, 71, 253–260. [Google Scholar] [CrossRef]
- Ross, J.W.; Hale, B.J.; Gabler, N.K.; Rhoads, R.P.; Keating, A.F.; Baumgard, L.H. Physiological consequences of heat stress in pigs. Anim. Prod. Sci. 2015, 55, 1381–1390. [Google Scholar] [CrossRef]
- Yong, Y.; Zhao, Y.; Gooneratne, R.; Liao, M.; Ju, X. T regulatory and T helper 17 populations with transcription factors in pig tissues in response to chronic heat stress. Int. J. Agric. Biol. 2019, 21, 3–10. [Google Scholar]
- Chen, S.; Yong, Y.; Ju, X. Effect of heat stress on growth and production performance of livestock and poultry: Mechanism to prevention. J. Therm. Biol. 2021, 99, 103019. [Google Scholar] [CrossRef]
- Mayorga, E.; Ross, J.; Keating, A.; Rhoads, R.; Baumgard, L. Biology of heat stress; the nexus between intestinal hyperpermeability and swine reproduction. Theriogenology 2020, 154, 73–83. [Google Scholar] [CrossRef]
- Michiels, A.; Piepers, S.; Ulens, T.; Van Ransbeeck, N.; Sacristán, R.D.P.; Sierens, A.; Haesebrouck, F.; Demeyer, P.; Maes, D. Impact of particulate matter and ammonia on average daily weight gain, mortality and lung lesions in pigs. Prev. Veter. Med. 2015, 121, 99–107. [Google Scholar] [CrossRef]
- Santiago, P.R.; Martínez-Burnes, J.; Mayagoitia, A.L.; Ramírez-Necoechea, R.; Mota-Rojas, D. Relationship of vitality and weight with the temperature of newborn piglets born to sows of different parity. Livest. Sci. 2019, 220, 26–31. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Backus, B.L.; McGlone, J.J. Effects of transport at weaning on the behavior, physiology and performance of pigs. Animals 2014, 4, 657–669. [Google Scholar] [CrossRef]
- D’Eath, R.B.; Jarvis, S.; Baxter, E.M.; Houdijk, J. Mitigating hunger in pregnant sows. In Herd and Flock Welfare, Advances in Pig Welfare; Špinka, M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 199–234. [Google Scholar]
- Bench, C.; Rioja-Lang, F.; Hayne, S.; Gonyou, H. Group gestation housing with individual feeding—I: How feeding regime, resource allocation, and genetic factors affect sow welfare. Livest. Sci. 2013, 152, 208–217. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, P.; Feng, D.; Zhu, Y.; Shi, Q.; Wang, J.; Zhu, W. The role of liver metabolism in compensatory-growth piglets induced by protein restriction and subsequent protein realimentation. Domest. Anim. Endocrinol. 2021, 74, 106512. [Google Scholar] [CrossRef]
- Doster, A.R. Porcine gastric ulcer. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 163–174. [Google Scholar] [CrossRef]
- Peralvo-Vidal, J.M.; Weber, N.R.; Nielsen, J.P.; Bache, J.K.; Haugegaard, S.; Pedersen, A. Øyan Risk factors for gastric ulceration in nursery pigs. Prev. Veter. Med. 2021, 189, 105298. [Google Scholar]
- De Witte, C.; Devriendt, B.; Flahou, B.; Bosschem, I.; Ducatelle, R.; Smet, A.; Haesebrouck, F. Helicobacter suis induces changes in gastric inflammation and acid secretion markers in pigs of different ages. Vet. Res. 2017, 48, 34. [Google Scholar] [CrossRef]
- Cybulski, P.; Woźniak, A.; Urban, J.; Stadejek, T. Gastric Lesions in Culled Sows: An Underestimated Welfare Issue in Modern Swine Production. Agriculture 2021, 11, 927. [Google Scholar] [CrossRef]
- Couret, D.; Jamin, A.; Simon, G.; Prunier, A.; Merlot, E. Maternal stress during late gestation has moderate but long-lasting effects on the immune system of the piglets. Veter. Immunol. Immunopathol. 2009, 131, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, K.M.; Piastowska-Ciesielska, A.; Donald, R.D.; Robson, S.K.; Ison, S.H.; Jarvis, S.; Brunton, P.J.; Russell, J.A.; Lawrence, A.B. Prenatal stress produces anxiety prone female offspring and impaired maternal behaviour in the domestic pig. Physiol. Behav. 2014, 129, 255–264. [Google Scholar] [CrossRef]
- Jarvis, S.; D’Eath, R.; Robson, S.K.; Lawrence, A. The effect of confinement during lactation on the hypothalamic–pituitary–adrenal axis and behaviour of primiparous sows. Physiol. Behav. 2006, 87, 345–352. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Baxter, E.; Rutherford, K.; D’Eath, R.; Arnott, G.; Turner, S.; Sandøe, P.; Moustsen, V.A.; Thorup, F.; Edwards, S.; Lawrence, A. The welfare implications of large litter size in the domestic pig II: Management factors. Anim. Welf. 2013, 22, 219–238. [Google Scholar] [CrossRef]
- Lynegaard, J.C.; Larsen, I.; Hansen, C.F.; Nielsen, J.P.; Amdi, C. Performance and risk factors associated with first antibiotic treatment in two herds, raising pigs without antibiotics. Porc. Health Manag. 2021, 7, 18. [Google Scholar] [CrossRef]
- Pluske, J.R.; Turpin, D.L.; Kim, J.C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Fouhse, J.M.; Yang, K.; More-Bayona, J.; Gao, Y.; Goruk, S.; Plastow, G.; Field, C.; Barreda, D.R.; Willing, B.P. Neonatal Exposure to Amoxicillin Alters Long-Term Immune Response Despite Transient Effects on Gut-Microbiota in Piglets. Front. Immunol. 2019, 10, 2059. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.; Bobe, A.M.; Miyoshi, S.; Huang, Y.; Hubert, N.; Delmont, T.; Eren, A.M.; Leone, V.; Chang, E.B. Peripartum Antibiotics Promote Gut Dysbiosis, Loss of Immune Tolerance, and Inflammatory Bowel Disease in Genetically Prone Offspring. Cell Rep. 2017, 20, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.A.C.; Manzanilla, E.G.; Diana, A.; Boyle, L.A. Cross-Fostering Implications for Pig Mortality, Welfare and Performance. Front. Veter. Sci. 2018, 5, 123. [Google Scholar]
- Pajžlar, L.; Skok, J. Cross-fostering into smaller or older litter makes piglets integration difficult: Suckling stability-based rationale. Appl. Anim. Behav. Sci. 2019, 220, 104856. [Google Scholar] [CrossRef]
- Schmitt, O.; O’Driscoll, K.; Boyle, L.A.; Baxter, E. Artificial rearing affects piglets pre-weaning behaviour, welfare and growth performance. Appl. Anim. Behav. Sci. 2019, 210, 16–25. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; He, T.; Long, S.; Guo, Y.; Chen, Z. Effect of Different Cross-Fostering Strategies on Growth Performance, Stress Status and Immunoglobulin of Piglets. Animals 2021, 11, 499. [Google Scholar] [CrossRef]
- Foxcroft, G.R.; Dixon, W.T.; Novak, S.; Putman, C.T.; Town, S.C.; Vinsky, M.D.A. The biological basis for prenatal programming of postnatal performance in pigs1,2. J. Anim. Sci. 2006, 84, E105–E112. [Google Scholar] [CrossRef]
- Baxter, E.M.; Edwards, S.A. Chapter 3—Piglet mortality and morbidity: Inevitable or unacceptable? In Advances in Pig Welfare; Woodhead Publishing: Sawston, UK, 2018; pp. 73–100. [Google Scholar]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.-J.; Kim, D.W.; Na Kang, B.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Obregon-Gutierrez, P.; Aragon, V.; Correa-Fiz, F. Sow contact is a major driver in the development of the nasal microbiota of piglets. Pathogens 2021, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Schlosser-Brandenburg, J.; Ebner, F.; Klopfleisch, R.; Kühl, A.A.; Zentek, J.; Pieper, R.; Hartmann, S. Influence of Nutrition and Maternal Bonding on Postnatal Lung Development in the Newborn Pig. Front. Immunol. 2021, 12, 3144. [Google Scholar] [CrossRef]
- Heim, G.; Mellagi, A.P.; Bierhals, T.; de Souza, L.; de Fries, H.; Piuco, P.; Seidel, E.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F. Effects of cross-fostering within 24h after birth on pre-weaning behaviour, growth performance and survival rate of biological and adopted piglets. Livest. Sci. 2012, 150, 121–127. [Google Scholar] [CrossRef]
- Díaz, J.A.C.; Diana, A.; Boyle, L.A.; Leonard, F.C.; McElroy, M.; McGettrick, S.; Moriarty, J.; Manzanilla, E.G. Delaying pigs from the normal production flow is associated with health problems and poorer performance. Porc. Health Manag. 2017, 3, 13. [Google Scholar]
- Albernaz-Gonçalves, R.; Olmos, G.; Hötzel, M.J. My pigs are ok, why change? Animal welfare accounts of pig farmers. Animal 2021, 15, 100154. [Google Scholar] [CrossRef]
- Kobek-Kjeldager, C.; Moustsen, V.A.; Theil, P.K.; Pedersen, L.J. Managing large litters: Selected measures of performance in 10 intermediate nurse sows and welfare of foster piglets. Appl. Anim. Behav. Sci. 2020, 233, 105149. [Google Scholar] [CrossRef]
- Weary, D.M.; Jasper, J.; Hötzel, M.J. Understanding weaning distress. Appl. Anim. Behav. Sci. 2008, 110, 24–41. [Google Scholar] [CrossRef]
- Sjölund, M.; Postma, M.; Collineau, L.; Lösken, S.; Backhans, A.; Belloc, C.; Emanuelson, U.; Beilage, E.; Stärk, K.; Dewulf, J. Quantitative and qualitative antimicrobial usage patterns in farrow-to-finish pig herds in Belgium, France, Germany and Sweden. Prev. Veter. Med. 2016, 130, 41–50. [Google Scholar] [CrossRef]
- Sarrazin, S.; Joosten, P.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; AWagenaar, J.; Heederik, D.J.J.; Dewulf, J.; Wagenaar, J.; Graveland, H.; et al. Quantitative and qualitative analysis of antimicrobial usage patterns in 180 selected farrow-to-finish pig farms from nine European countries based on single batch and purchase data. J. Antimicrob. Chemother. 2019, 74, 807–816. [Google Scholar] [CrossRef]
- Postma, M.; Backhans, A.; Collineau, L.; Loesken, S.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Nielsen, E.O. Evaluation of the relationship between the biosecurity status, production parameters, herd characteristics and antimicrobial usage in farrow-to-finish pig production in four EU countries. Porc. Health Manag. 2016, 2, 9. [Google Scholar] [CrossRef]
- Van Rennings, L.; Von Münchhausen, C.; Ottilie, H.; Hartmann, M.; Merle, R.; Honscha, W.; Käsbohrer, A.; Kreienbrock, L. Cross-Sectional Study on Antibiotic Usage in Pigs in Germany. PLoS ONE 2015, 10, e0119114. [Google Scholar]
- Jensen, P. Observations on the maternal behaviour of free-ranging domestic pigs. Appl. Anim. Behav. Sci. 1986, 16, 131–142. [Google Scholar] [CrossRef]
- Widowski, T.M.; Torrey, S.; Bench, C.J.; Gonyou, H.W. Development of ingestive behaviour and the relationship to belly nosing in early-weaned piglets. Appl. Anim. Behav. Sci. 2008, 110, 109–127. [Google Scholar] [CrossRef]
- Poletto, R.; Steibel, J.; Siegford, J.; Zanella, A. Effects of early weaning and social isolation on the expression of glucocorticoid and mineralocorticoid receptor and 11β-hydroxysteroid dehydrogenase 1 and 2 mRNAs in the frontal cortex and hippocampus of piglets. Brain Res. 2006, 1067, 36–42. [Google Scholar] [CrossRef]
- Hotzel, M.J.; De Souza, G.P.; Costa, O.A.D.; Filho, L.C.P.M. Disentangling the effects of weaning stressors on piglets’ behaviour and feed intake: Changing the housing and social environment. Appl. Anim. Behav. Sci. 2011, 135, 44–50. [Google Scholar] [CrossRef]
- Lewis, N.J. Transport of early weaned piglets. Appl. Anim. Behav. Sci. 2008, 110, 128–135. [Google Scholar] [CrossRef]
- Rioja-Lang, F.C.; Brown, J.A.; Brockhoff, E.J.; Faucitano, L. A Review of Swine Transportation Research on Priority Welfare Issues: A Canadian Perspective. Front. Veter. Sci. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Arndt, H.; Volkmann, N.; Spindler, B.; Hartung, J.; Kemper, N. Do Pigs Have Adequate Space in Animal Transportation Vehicles? Planimetric Measurement of the Floor Area Covered by Finishing Pigs in Various Body Positions. Front. Veter. Sci. 2019, 5, 330. [Google Scholar] [CrossRef]
- Garcia, A.; Pirner, G.; Picinin, G.; May, M.; Guay, K.; Backus, B.; Sutherland, M.; McGlone, J. Effect of Provision of Feed and Water during Transport on the Welfare of Weaned Pigs. Animals 2015, 5, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Marchant-Forde, J.N.; Lay, D.C., Jr.; McMunn, K.A.; Cheng, H.W.; Pajor, E.A.; Marchant-Forde, R.M. Postnatal piglet husbandry practices and well-being: The effects of alternative techniques delivered in combination. J. Anim. Sci. 2014, 92, 1150–1160. [Google Scholar] [CrossRef]
- Weary, D.M.; Fraser, D. Partial tooth-clipping of suckling pigs: Effects on neonatal competition and facial injuries. Appl. Anim. Behav. Sci. 1999, 65, 21–27. [Google Scholar] [CrossRef]
- Tallet, C.; Rakotomahandry, M.; Herlemont, S.; Prunier, A. Evidence of Pain, Stress, and Fear of Humans During Tail Docking and the Next Four Weeks in Piglets (Sus scrofa domesticus). Front. Veter. Sci. 2019, 6, 462. [Google Scholar] [CrossRef]
- Di Giminiani, P.; Nasirahmadi, A.; Malcolm, E.M.; Leach, M.C.; Edwards, S.A. Docking piglet tails: How much does it hurt and for how long? Physiol. Behav. 2017, 182, 69–76. [Google Scholar] [CrossRef][Green Version]
- Patterson, R.L.S. 5α-androst-16-ene-3-one: Compound responsible for taint in boar fat. J. Sci. Food. Agric. 1968, 19, 31–38. [Google Scholar] [CrossRef]
- Ison, S.H.; Clutton, R.E.; Di Giminiani, P.; Rutherford, K.M.D. A Review of Pain Assessment in Pigs. Front. Veter. Sci. 2016, 3, 108. [Google Scholar] [CrossRef] [PubMed]
- Mainau, E.; Temple, D.; Manteca, X. Experimental study on the effect of oral meloxicam administration in sows on pre-weaning mortality and growth and immunoglobulin G transfer to piglets. Prev. Veter. Med. 2016, 126, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Mainau, E.; de Miguel, R.; Temple, D.; Salas, M.; Manteca, X. Oral Meloxicam Administration in Sows at Farrowing and Its Effects on Piglet Immunity Transfer and Growth. Front. Veter. Sci. 2021, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Yngvesson, J. Aggression between unacquainted pigs—Sequential assessment and effects of familiarity and weight. Appl. Anim. Behav. Sci. 1998, 58, 49–61. [Google Scholar] [CrossRef]
- Rydhmer, L. Advances in understanding the genetics of pig behaviour. In Understanding the Behaviour and Improving the Welfare of Pigs; Edwards, S., Ed.; Burleigh Dodds Science Publishing: London, UK, 2020; pp. 1–23. [Google Scholar]
- Verdon, M.; Rault, J. Aggression in group housed sows and fattening pigs. In Advances in Pig Welfare; Elsevier Ltd.: Cambridge, UK, 2018; pp. 237–262. [Google Scholar] [CrossRef]
- Tallet, C.; Brajon, S.; Devillers, N.; Lensink, J. Pig-human interactions: Creating a positive perception of humans to ensure pig welfare. In Advances in Pig Welfare; Špinka, M., Camerlink, I., Eds.; Elsevier Ltd.: Cambridge, UK, 2018; pp. 381–398. [Google Scholar]
- Collineau, L.; Rojo-Gimeno, C.; Léger, A.; Backhans, A.; Loesken, S.; Okholm Nielsen, E.; Postma, M.; Emanuelson, U.; Beilage, E.; Sjölund, M.; et al. Herd-specific interventions to reduce antimicrobial usage in pig production without jeopardising technical and economic performance. Prev. Veter. Med. 2017, 144, 167–178. [Google Scholar] [CrossRef]
- Diana, A.; Boyle, L.A.; Leonard, F.C.; Carroll, C.; Sheehan, E.; Murphy, D.; Manzanilla, E.G. Removing prophylactic antibiotics from pig feed: How does it affect their performance and health? BMC Veter. Res. 2019, 15, 67. [Google Scholar] [CrossRef]
- Raasch, S.; Collineau, L.; Postma, M.; Backhans, A.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Stärk, K. Effectiveness of alternative measures to reduce antimicrobial usage in pig production in four European countries. Porc. Health Manag. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Gimeno, C.; Postma, M.; Dewulf, J.; Hogeveen, H.; Lauwers, L.; Wauters, E. Farm-economic analysis of reducing antimicrobial use whilst adopting improved management strategies on farrow-to-finish pig farms. Prev. Veter. Med. 2016, 129, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of antibiotic use in global pig production: A systematic review. Veter. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef]
- Nielsen, C.L.; Kongsted, H.; Sørensen, J.T.; Krogh, M.A. Antibiotic and medical zinc oxide usage in Danish conventional and welfare-label pig herds in 2016–2018. Prev. Veter. Med. 2021, 189, 105283. [Google Scholar] [CrossRef]
- Scali, F.; Santucci, G.; Maisano, A.M.; Giudici, F.; Guadagno, F.; Tonni, M.; Amicabile, A.; Formenti, N.; Giacomini, E.; Lazzaro, M.; et al. The Use of Antimicrobials in Italian Heavy Pig Fattening Farms. Antibiotics 2020, 9, 892. [Google Scholar] [CrossRef]
- Mencía-Ares, O.; Argüello, H.; Puente, H.; Gómez-García, M.; Manzanilla, E.G.; Álvarez-Ordóñez, A.; Carvajal, A.; Rubio, P. Antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. is influenced by production system, antimicrobial use, and biosecurity measures on Spanish pig farms. Porc. Health Manag. 2021, 7, 27. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Barnett, J.L.; Hemsworth, P.H.; Cronin, G.M.; Jongman, E.C.; Hutson, G.D. A review of the welfare issues for sows and piglets in relation to housing. Aust. J. Agric. Res. 2001, 52, 1–28. [Google Scholar] [CrossRef]
- Bench, C.; Rioja-Lang, F.; Hayne, S.; Gonyou, H. Group gestation sow housing with individual feeding—II: How space allowance, group size and composition, and flooring affect sow welfare. Livest. Sci. 2013, 152, 218–227. [Google Scholar] [CrossRef]
- Bulens, A.; Van Beirendonck, S.; Van Thielen, J.; Buys, N.; Driessen, B. Hiding walls for fattening pigs: Do they affect behavior and performance? Appl. Anim. Behav. Sci. 2017, 195, 32–37. [Google Scholar] [CrossRef]
- Pluym, L.M.; Maes, D.; Van Weyenberg, S.; Van Nuffel, A. Risk factors for development of lameness in gestating sows within the first days after moving to group housing. Veter. J. 2017, 220, 28–33. [Google Scholar] [CrossRef]
- Singh, C.; Verdon, M.; Cronin, G.M.; Hemsworth, P. The behaviour and welfare of sows and piglets in farrowing crates or lactation pens. Animal 2017, 11, 1210–1221. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Ko, H.-L.; Chong, Q.; Escribano, D.; Camerlink, I.; Manteca, X.; Llonch, P. Pre-weaning socialization and environmental enrichment affect life-long response to regrouping in commercially-reared pigs. Appl. Anim. Behav. Sci. 2020, 229, 105044. [Google Scholar] [CrossRef]
- Lidfors, L.; Hultman, P.; Zupan, M. Providing additional objects to straw reduces piglets’ redirected behaviour post-weaning but influences weight gain pre-weaning negatively. Acta Agric. Scand. Sect. A Anim. Sci. 2020, 69, 239–248. [Google Scholar] [CrossRef]
- Chou, J.-Y.; D’Eath, R.; Sandercock, D.A.; O’Driscoll, K. Enrichment use in finishing pigs and its relationship with damaging behaviours: Comparing three wood species and a rubber floor toy. Appl. Anim. Behav. Sci. 2020, 224, 104944. [Google Scholar] [CrossRef]
- Lahrmann, H.P.; Hansen, C.F.; D’Eath, R.B.; Busch, M.E.; Nielsen, J.P.; Forkman, B. Early intervention with enrichment can prevent tail biting outbreaks in weaner pigs. Livest. Sci. 2018, 214, 272–277. [Google Scholar] [CrossRef]
- Wen, C.; van Dixhoorn, I.; Schokker, D.; Woelders, H.; Stockhofe-Zurwieden, N.; Rebel, J.M.J.; Smidt, H. Environmentally enriched housing conditions affect pig welfare, immune system and gut microbiota in early life. Anim. Microbiome 2021, 3, 52. [Google Scholar] [CrossRef]
- Westin, R.; Holmgren, N.; Hultgren, J.; Algers, B. Large quantities of straw at farrowing prevents bruising and increases weight gain in piglets. Prev. Veter. Med. 2014, 115, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, C.; Hao, Y.; Gu, X. Effects of Different Farrowing Environments on the Behavior of Sows and Piglets. Animals 2020, 10, 320. [Google Scholar] [CrossRef]
- Tatemoto, P.; Bernardino, T.; Alves, L.; Souza, A.C.D.O.; Palme, R.; Zanella, A.J. Environmental enrichment for pregnant sows modulates HPA-axis and behavior in the offspring. Appl. Anim. Behav. Sci. 2019, 220, 104854. [Google Scholar] [CrossRef]
- Herskin, M.S.; Jensen, H.E.; Jespersen, A.; Forkman, B.; Jensen, M.B.; Canibe, N.; Pedersen, L.J. Impact of the amount of straw provided to pigs kept in intensive production conditions on the occurrence and severity of gastric ulceration at slaughter. Res. Veter. Sci. 2016, 104, 200–206. [Google Scholar] [CrossRef]
- Swan, K.-M.; Telkänranta, H.; Munsterhjelm, C.; Peltoniemi, O.; Valros, A. Access to chewable materials during lactation affects sow behaviour and interaction with piglets. Appl. Anim. Behav. Sci. 2021, 234, 105174. [Google Scholar] [CrossRef]
- Tatemoto, P.; Bernardino, T.; Alves, L.; Zanella, A.J. Sham-Chewing in Sows Is Associated with Decreased Fear Responses in Their Offspring. Front. Veter. Sci. 2019, 6, 390. [Google Scholar] [CrossRef]
- Liu, X.; Song, P.; Yan, H.; Zhang, L.; Wang, L.; Zhao, F.; Gao, H.; Hou, X.; Shi, L.; Li, B.; et al. A Comparison of the Behavior, Physiology, and Offspring Resilience of Gestating Sows When Raised in a Group Housing System and Individual Stalls. Animals 2021, 11, 2076. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; Gentz, M.; Hahne, M.; Lambertz, C.; Gauly, M.; Burfeind, O.; Traulsen, I. Effects of Different Farrowing and Rearing Systems on Post-Weaning Stress in Piglets. Agriculture 2020, 10, 230. [Google Scholar] [CrossRef]
- Tarazona, A.M.; Ceballos, M.C.; Broom, D.M. Human Relationships with Domestic and Other Animals: One Health, One Welfare, One Biology. Animals 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.-L.; Waiblinger, S.; Boivin, X.; Hemsworth, P. The Power of a Positive Human–Animal Relationship for Animal Welfare. Front. Veter. Sci. 2020, 7, 857. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Bi, Y.; Zhao, P.; Sun, H.; Li, J.; Liu, H.; Zhang, R.; Li, X.; Bao, J. Effects of Long-Term Gentle Handling on Behavioral Responses, Production Performance, and Meat Quality of Pigs. Animals 2020, 10, 330. [Google Scholar] [CrossRef]
- De Meyer, D.; Amalraj, A.; Van Limbergen, T.; Fockedey, M.; Edwards, S.; Moustsen, V.A.; Chantziaras, I.; Maes, D. Short Communication: Effect of positive handling of sows on litter performance and pre-weaning piglet mortality. Animal 2020, 14, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.; Keeling, L.J.; da Costa, M.J.R.P. Individual variation over time in piglet’s reactions to early handling and its association to weight gain. Appl. Anim. Behav. Sci. 2019, 215, 7–12. [Google Scholar] [CrossRef]
- Suzuki, K.; Shinkai, H.; Yoshioka, G.; Matsumoto, T.; Tanaka, J.; Hayashi, N.; Kitazawa, H.; Uenishi, H. NOD2 Genotypes Affect the Symptoms and Mortality in the Porcine Circovirus 2-Spreading Pig Population. Genes 2021, 12, 1424. [Google Scholar] [CrossRef]
- Borjigin, L.; Shimazu, T.; Katayama, Y.; Li, M.; Satoh, T.; Watanabe, K.; Kitazawa, H.; Roh, S.-G.; Aso, H.; Katoh, K.; et al. Immunogenic properties of Landrace pigs selected for resistance to mycoplasma pneumonia of swine. Anim. Sci. J. 2015, 87, 321–329. [Google Scholar] [CrossRef]
- Uemoto, Y.; Ichinoseki, K.; Matsumoto, T.; Oka, N.; Takamori, H.; Kadowaki, H.; Kojima-Shibata, C.; Suzuki, E.; Okamura, T.; Aso, H.; et al. Genome-wide association studies for production, respiratory disease, and immune-related traits in Landrace pigs. Sci. Rep. 2021, 11, 15823. [Google Scholar]
- Andersson, E.; Frössling, J.; Westin, R.; Algers, B.; Gunnarsson, S. Associations between litter size and medical treatment of sows during farrowing and lactation. Acta Agric. Scand. Sect. A Anim. Sci. 2020, 69, 176–182. [Google Scholar] [CrossRef]
- Velarde, A.; Fàbrega, E.; Blanco-Penedo, I.; Dalmau, A. Animal welfare towards sustainability in pork meat production. Meat Sci. 2015, 109, 13–17. [Google Scholar] [CrossRef]
- Lovendahl, P.; Damgaard, L.H.; Nielsen, B.L.; Thodberg, K.; Su, G.; Rydhmer, L. Aggressive behaviour of sows at mixing and maternal behaviour are heritable and genetically correlated traits. Livest. Prod. Sci. 2005, 93, 73–85. [Google Scholar] [CrossRef]
- Larzul, C. How to Improve Meat Quality and Welfare in Entire Male Pigs by Genetics. Animals 2021, 11, 699. [Google Scholar] [CrossRef]
- Menchaca, A. Sustainable Food Production: The Contribution of Genome Editing in Livestock. Sustainability 2021, 13, 6788. [Google Scholar] [CrossRef]
- Woods, A. Decentring antibiotics: UK responses to the diseases of intensive pig production (ca. 1925–1965). Palgrave Commun. 2019, 5, 41. [Google Scholar] [CrossRef]
- Kirchhelle, C. Swann Song: Antibiotic Regulation in British Livestock Production (1953–2006). Bull. Hist. Med. 2018, 92, 317–350. [Google Scholar] [CrossRef]
- Tompson, A.C.; Manderson, L.; Chandler, C.I.R. Understanding antibiotic use: Pratices, structures and networks. JAC-Antimicrob. Resist. 2021, 3, dlab150. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.E.; González-Montaña, J.R.; Lomillos, J.M. Consumers’ concerns and perceptions of farm animal welfare. Animals 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.; Stewart, G.B.; Panzone, L.A.; Kyriazakis, I.; Frewer, L.J. Citizens, consumers and farm animal welfare: A meta-analysis of willingness-to-pay studies. Food Policy 2017, 68, 112–127. [Google Scholar] [CrossRef]
- Hötzel, M.J.; Yunes, M.C.; Vandresen, B.; Albernaz-Gonçalves, R.; Woodroffe, R.E. On the Road to End Pig Pain: Knowledge and Attitudes of Brazilian Citizens Regarding Castration. Animals 2020, 10, 1826. [Google Scholar] [CrossRef]
- Molnár, M.; Fraser, D. Protecting farm animal welfare during intensification: Farmer perceptions of economic and regulatory pressures. Anim. Welf. 2020, 29, 133–141. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albernaz-Gonçalves, R.; Olmos Antillón, G.; Hötzel, M.J. Linking Animal Welfare and Antibiotic Use in Pig Farming—A Review. Animals 2022, 12, 216. https://doi.org/10.3390/ani12020216
Albernaz-Gonçalves R, Olmos Antillón G, Hötzel MJ. Linking Animal Welfare and Antibiotic Use in Pig Farming—A Review. Animals. 2022; 12(2):216. https://doi.org/10.3390/ani12020216
Chicago/Turabian StyleAlbernaz-Gonçalves, Rita, Gabriela Olmos Antillón, and Maria José Hötzel. 2022. "Linking Animal Welfare and Antibiotic Use in Pig Farming—A Review" Animals 12, no. 2: 216. https://doi.org/10.3390/ani12020216
APA StyleAlbernaz-Gonçalves, R., Olmos Antillón, G., & Hötzel, M. J. (2022). Linking Animal Welfare and Antibiotic Use in Pig Farming—A Review. Animals, 12(2), 216. https://doi.org/10.3390/ani12020216







