A Multi-Dimensional Examination of Foraging Habitat Use by Gray Whales Using Long Time-Series and Acoustics Data
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Transect Surveys
2.2. Photo-Identification Mark-Recapture
2.3. Inter- and Intra-Annual Gray Whale Habitat Use
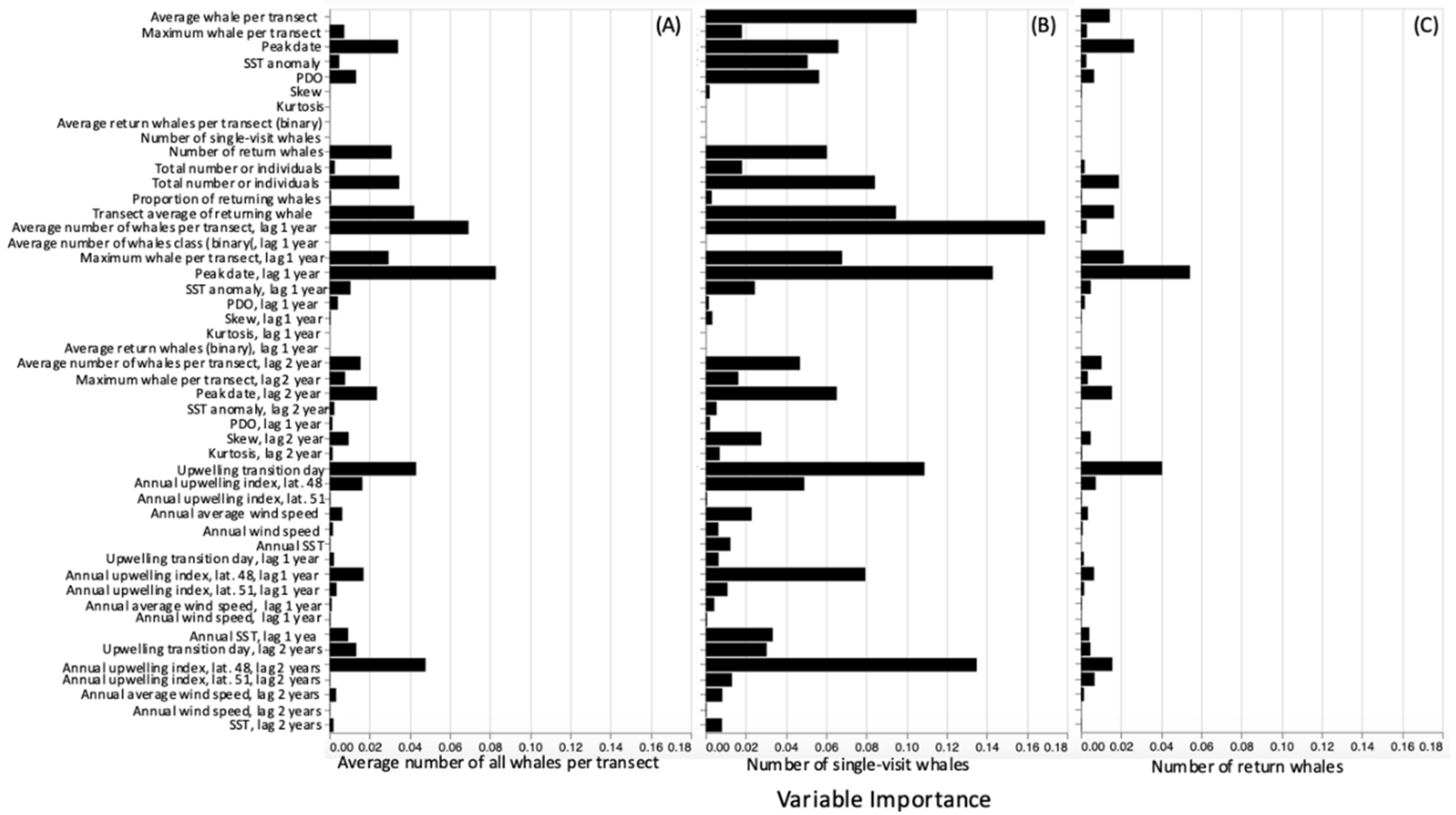
2.4. Statistical Analysis of Transect Data
2.5. Passive Acoustic Recording and Analysis
3. Results
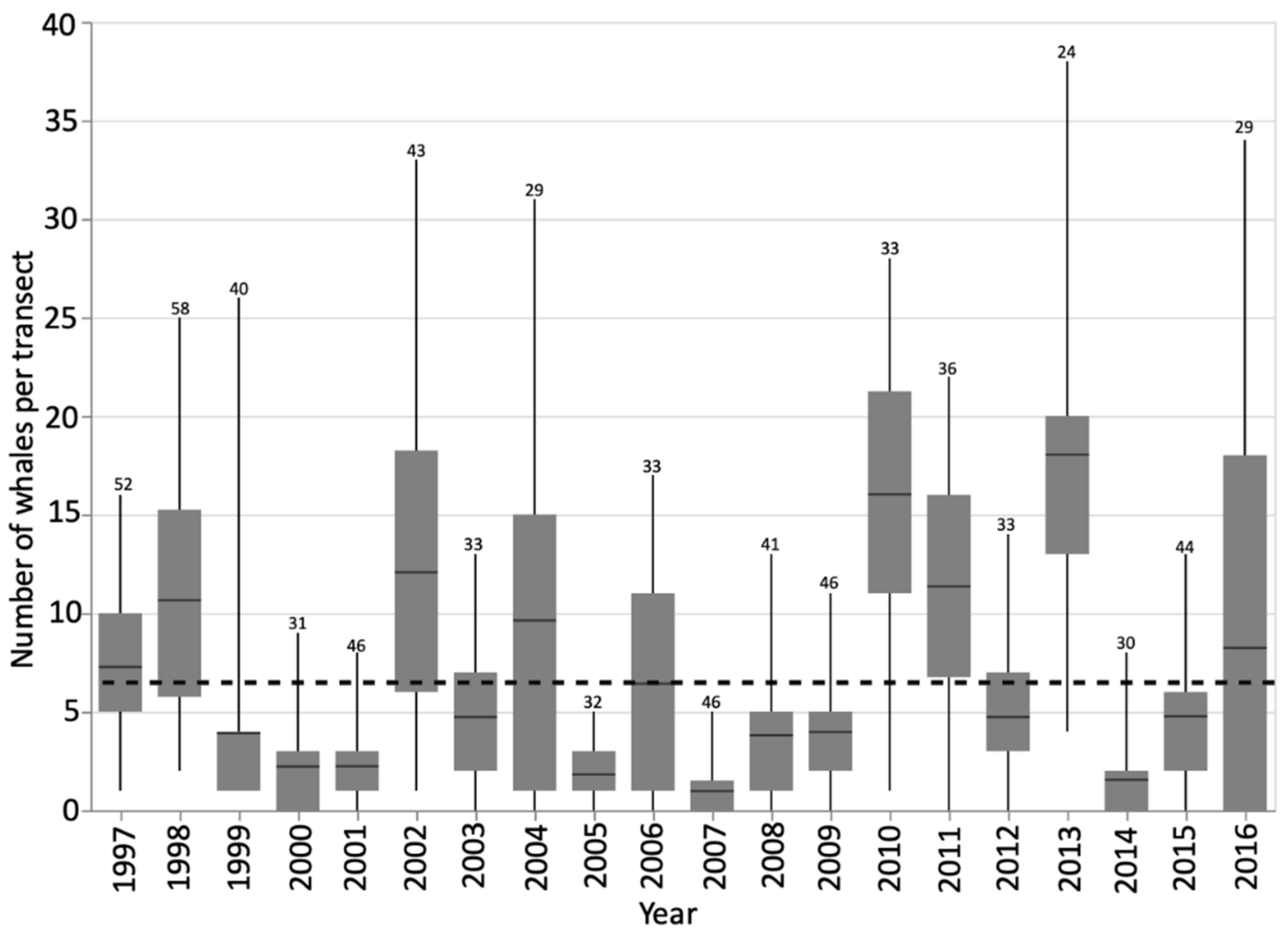
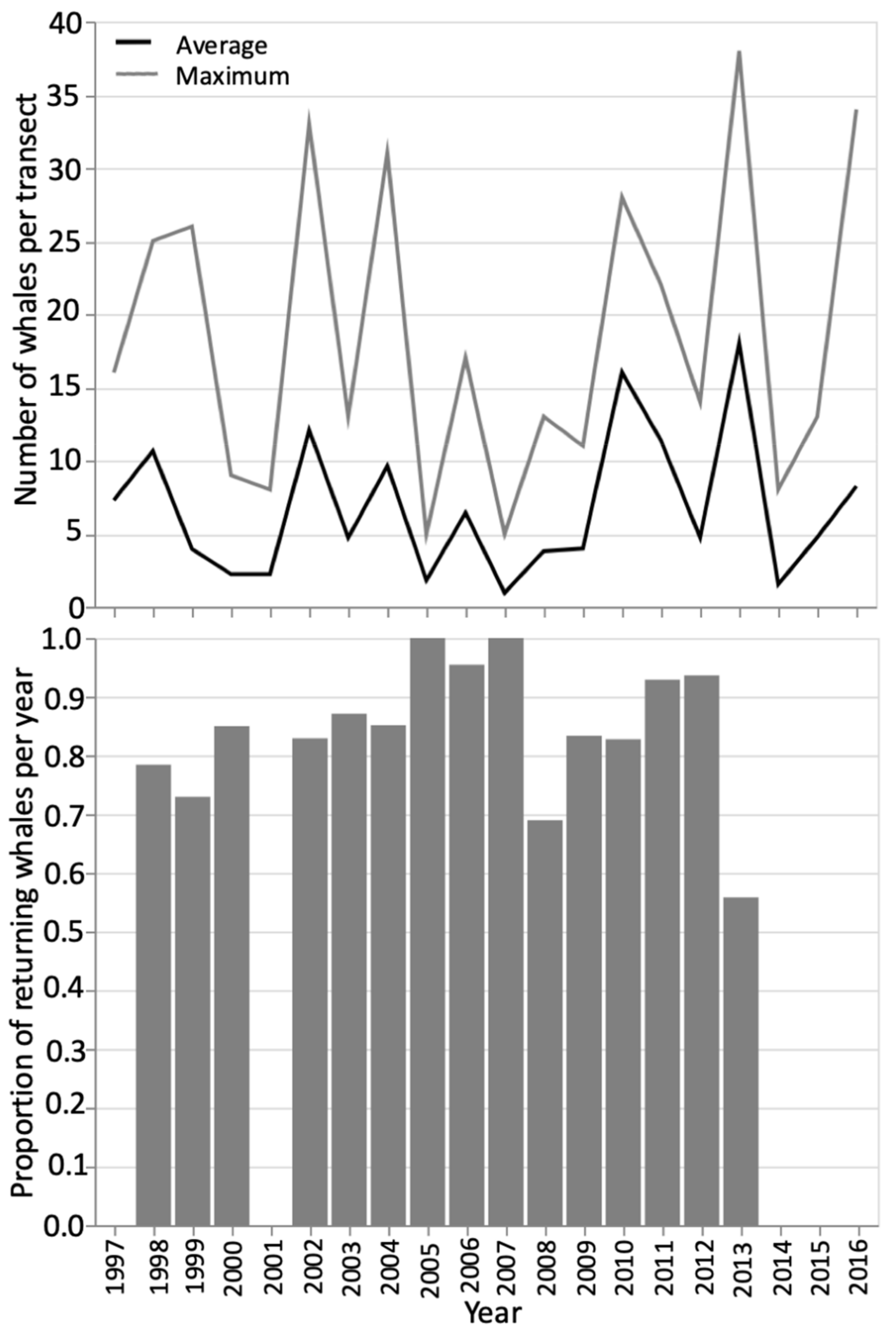
3.1. Foraging Whale Presence
3.2. Residency and Return Rates
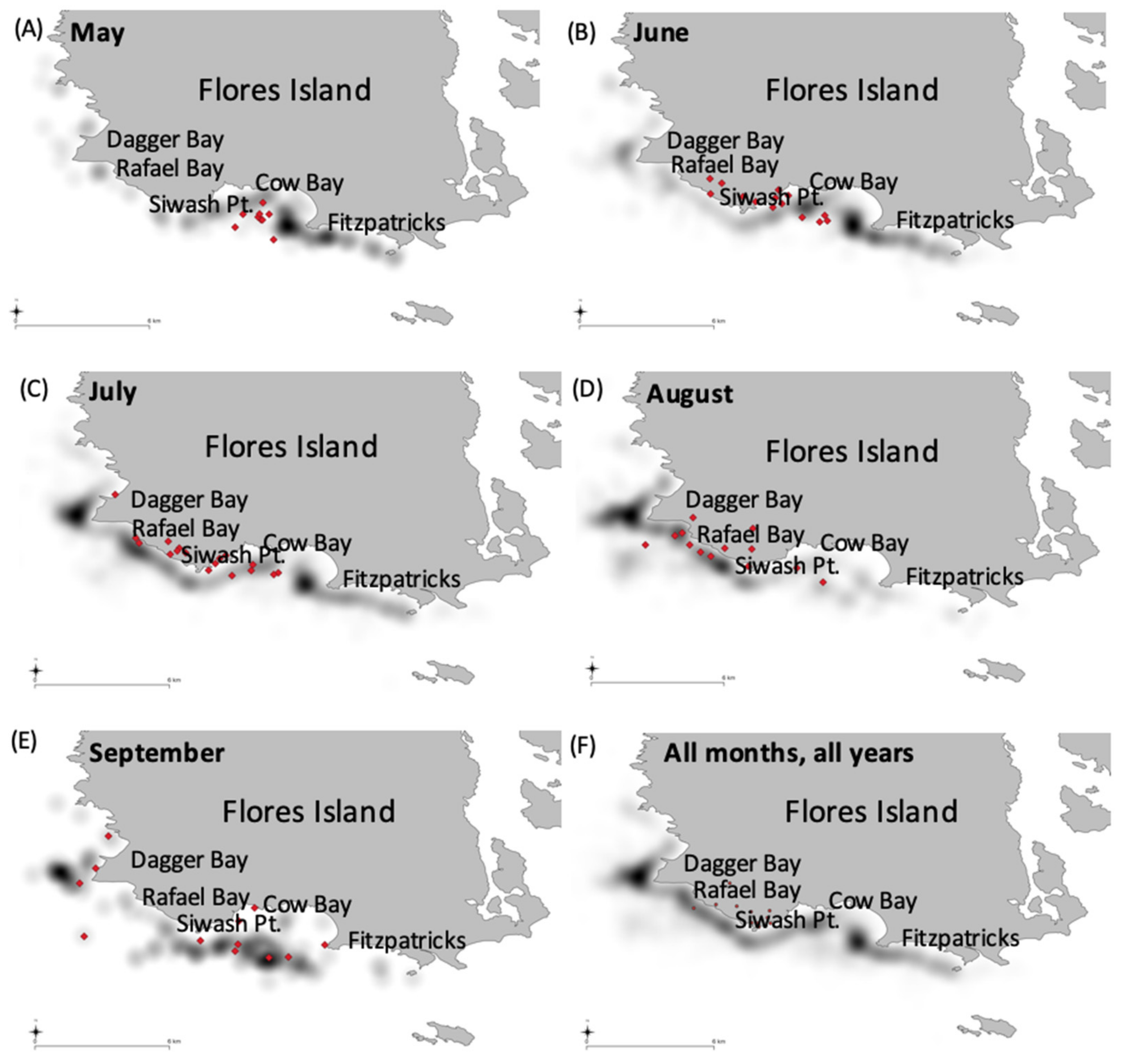
3.3. Spatial Use by Whales
3.4. Passive Acoustic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mueller, T.; Fagan, W.F. Search and navigation in dynamic environments—From individual behaviors to population distributions. Oikos 2008, 117, 654–664. [Google Scholar] [CrossRef]
- Augé, A.A.; Chilvers, B.L.; Moore, A.B.; Davis, L.S. Importance of studying foraging site fidelity for spatial conservation measures in a mobile predator. Anim. Conserv. 2014, 17, 61–71. [Google Scholar] [CrossRef]
- Keen, E.M.; Pilkington, J.; O’Mahony, É.; Thompson, K.L.; Hendricks, B.; Robinson, N.; Dundas, A.; Nichol, L.; Alidina, H.M.; Meuter, H.; et al. Fin whales of the Great Bear Rainforest: Balaenoptera physalus velifera in a Canadian Pacific fjord system. PLoS ONE 2021, 16, e0256815. [Google Scholar] [CrossRef]
- De Bruyn, M.; Hall, B.L.; Chauke, L.F.; Baroni, C.; Koch, P.L.; Hoelzel, A.R. Rapid Response of a Marine Mammal Species to Holocene Climate and Habitat Change. PLoS Genet. 2009, 5, e1000554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcangeli, A.; Orasi, A.; Carcassi, S.P.; Crosti, R. Exploring thermal and trophic preference of Balaenoptera physalus in the central Tyrrhenian Sea: A new summer feeding ground? Mar. Biol. 2014, 161, 427–436. [Google Scholar] [CrossRef]
- Arthur, B.; Hindell, M.; Bester, M.; Trathan, P.; Jonsen, I.; Staniland, I.; Ooasthuzien, W.C.; Wege, M.; Lea, M.-A. Return Customers: Foraging Site Fidelity and the Effect of Environmental Variability in Wide-Ranging Antarctic Fur Seals. PLoS ONE 2015, 10, e0120888. [Google Scholar] [CrossRef]
- Highsmith, R.C.; Coyle, K.O. Productivity of arctic amphipods relative to gray whale energy requirements. Mar. Ecol. Prog. Ser. 1992, 83, 141–150. [Google Scholar] [CrossRef]
- Highsmith, R.C.; Coyle, K.O.; Bluhm, B.A.; Konar, B. Gray whales in the Bering and Chukchi Seas. In Whales, Whaling and Ocean Ecosystems; Estes, J.A., Demaster, D.P., Doak, D.F., Williams, T.M., Brownell, R.L., Jr., Eds.; University of California Press: Berkley, CA, USA, 2006; pp. 303–313. [Google Scholar]
- Coyle, K.O.; Bluhm, B.A.; Konar, B.; Blanchard, A.L.; Highsmith, R.C. Amphipod prey of gray whales in the northern Bering Sea: Comparison of biomass and distribution between the 1980s and 2002–2003. Deep. Sea Res. II 2007, 54, 2906–2918. [Google Scholar] [CrossRef]
- Stafford, K.M.; Mellinger, D.K.; Moore, S.E.; Fox, C.G. Seasonal variability and detection range modeling of baleen whale calls in the Gulf of Alaska, 1999–2002. J. Acoust. Soc. Am. 2007, 122, 3378–3390. [Google Scholar] [CrossRef]
- Salvadeo, C.; Gómez-Gallardo, U.; Nájera-Caballero, M.; Urbán-Ramirez, J.; Lluch-Belda, D. The Effect of Climate Variability on Gray Whales (Eschrichtius robustus) within their wintering areas. PLoS ONE 2015, 10, e0134655. [Google Scholar] [CrossRef]
- Moore, S.E.; Urban, R.J.; Perryman, W.L.; Gulland, F.; Perez-Cortes, H.M.; Wade, P.; Rojas-Bracho, L.; Rowles, T. Are gray whales hitting K hard? Mar. Mamm. Sci. 2001, 17, 954–958. [Google Scholar] [CrossRef]
- Moore, S.E.; Grebmeier, J.M.; Davies, J.R. Gray whale distribution relative to forage habitat in the northern Bering Sea: Current conditions and retrospective summary. Can. J. of Zool. 2003, 81, 734–742. [Google Scholar] [CrossRef]
- Grebmeier, J.M.; Overland, J.E.; Moore, S.E.; Farley, E.V.; Carmack, E.C.; Cooper, L.W.; Frey, K.E.; Helle, J.H.; McLaughlin, F.A.; McNutt, S.L. A major ecosystem shift in the Northern Bering Sea. Science 2006, 311, 1461–1464. [Google Scholar] [CrossRef]
- LeBoeuf, B.J.; Perez-Cortes, M.H.; Urban, R.J.; Mate, B.R.; Ollervides, U.F. High gray whale mortality and low recruitment in 1999: Potential causes and implications. Int. J. Cet. Res. Manag. 2000, 2, 85–99. [Google Scholar]
- Swartz, S.L.; Taylor, B.L.; Rugh, D.J. Gray whale Eschrichtius robustus population and stock identity. Mamm. Rev. 2006, 36, 66–84. [Google Scholar] [CrossRef]
- National Ocean and Atmospheric Administration (NOAA). Gray whale Unusual Mortality Event (UME) of 2019–2020. 2020. Available online: https://www.fisheries.noaa.gov/national/marine-life-distress/2019-2022-gray-whale-unusual-mortality-event-along-west-coast-and (accessed on 1 September 2022).
- Hatler, D.F.; Darling, J.D. Recent observations of the gray whale in British Columbia. Can. Field Natural. 1974, 88, 449–459. [Google Scholar]
- Carruthers, E.H. Habitat, Population Structure and Energy Value of Benthic Amphipods, and Implications for Gray Whale Foraging in Clayoquot Sound, British Columbia. Master’s Thesis, Queens University, Kingston, ON, Canada, 2000. [Google Scholar]
- Duffus, D.A. The recreational use of grey whales in southern Clayoquot Sound, Canada. Appl. Geogr. 1996, 16, 179–190. [Google Scholar] [CrossRef]
- Dunham, J.S.; Duffus, D.A. Foraging patterns of gray whales in central Clayoquot Sound, British Columbia, Canada. Mar. Ecol. Prog. Ser. 2001, 223, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Dunham, J.S.; Duffus, D.A. Diet of gray whales (Eschrichtius robustus) in Clayoquot Sound, British Columbia, Canada. Mar. Mamm. Sci. 2002, 18, 419–437. [Google Scholar] [CrossRef]
- Feyrer, L.J.; Duffus, D.A. Threshold foraging by gray whales in response to fine scale variations in mysid density. Mar. Mamm. Sci. 2014, 31, 560–578. [Google Scholar] [CrossRef]
- Burnham, R.E.; Duffus, D.A. Patterns of Predator-Prey Dynamics Between Gray Whales (Eschrichtius robustus) and Mysid Species in Clayoquot Sound. Int. J. Cet. Res. Manag. 2018, 19, 95–103. [Google Scholar]
- Nelson, T.A.; Duffus, D.A.; Robertson, C.; Feyrer, L.J. Spatial-temporal patterns in intra-annual gray whale foraging: Characterizing interactions between predators and prey in Clayoquot Sound, B.C. Mar. Mamm. Sci. 2008, 24, 356–370. [Google Scholar] [CrossRef]
- Olsen, S.S. Gray Whales (Eschrichtius robustus) and Mysids (Family Mysidae): The Predator-Prey Relationship and a New Approach to Prey Quantification in Clayoquot Sound, British Columbia. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 2006. [Google Scholar]
- Burnham, R.E. Reproductive strategies conferring species dominance in marine mysid (Peracarida, Mysida) species in coastal waters off Vancouver Island, B.C. Crustaceana 2015, 88, 1421–1438. [Google Scholar] [CrossRef]
- Calambokidis, J.; Laake, J.L.; Klimek, A. Abundance and Population Structure of Seasonal Gray Whales in the Pacific Northwest, 1998–2008. Paper SC/62/BRG32 Submitted to The IWC Scientific Committee. 2010. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.435.5936&rep=rep1&type=pdf (accessed on 1 September 2020).
- International Whaling Commission, IWC. Annex G: Report of the Standing Working Group on the Aboriginal Whaling Management Plan (AWMP). In Annual Report of the International Whaling Commission 2010. 2010; pp. 80–87. Available online: https://iwc.int/annual-reports-iwc (accessed on 1 September 2020).
- Baumgartner, M.F.; Fratantoni, D.M. Diel periodicity in both sei whale vocalisation rates and the vertical migration of their copepod prey observed from ocean gliders. Lim. Ocean. 2008, 53, 2197–2209. [Google Scholar] [CrossRef] [Green Version]
- Vu, E.; Risch, D.; Clark, C.; Gaylord, S.; Hatch, L.; Thompson, M.; Van Parijs, S. Humpback whale song occurs extensively on feeding grounds in the western North Atlantic Ocean. Aquat. Bio. 2012, 14, 175–183. [Google Scholar] [CrossRef] [Green Version]
- D’Vincent, C.G.; Nilson, R.N.; Hanna, R.E. Vocalisation and coordinated feeding behavior of the humpback whale in Southeastern Alaska. Sci. Rep. Whales Res. Inst. 1985, 36, 41–47. [Google Scholar]
- Cerchio, S.; Dahlheim, M. Variation in feeding vocalisations of humpback whales Megaptera novaeangliae from southeast Alaska. Bioacoustics 2001, 11, 277–295. [Google Scholar] [CrossRef]
- Sharpe, F.A. Social Foraging of the Southeast Alaskan Humpback Whale, Megaptera Novaeangliae. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 2001. [Google Scholar]
- Oleson, E.M.; Calambokidis, J.; Burgess, W.C.; McDonald, M.A.; LeDuc, C.A.; Hildebrand, J.A. Behavioral context of call production by eastern North Pacific blue whales. Mar. Ecol. Prog. Ser. 2007, 330, 269–284. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.; Stafford, K.M.; Beedholm, K.; Lee, C.M.; Madsen, P.T. Singing behaviour of fin whales in the Davis Strait with implications for mating, migration and foraging. J. Acoust. Soc. Am. 2010, 128, 3200–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, S.E.; Miksis-Olds, J.L.; Denes, S.L. Assessing marine ecosystem acoustic diversity across ocean basins. Ecol. Inform. 2014, 21, 81–88. [Google Scholar] [CrossRef]
- Torres, L. A sense of scale: Foraging cetaceans’ use of scale-dependent multimodal sensory systems. Mar. Mamm. Sci. 2017, 33, 1170–1193. [Google Scholar] [CrossRef] [Green Version]
- Astthorsson, O.S.; Ralph, R. Growth and moulting of Neomysis integer (Crustacea: Mysidacea). Mar. Biol. 1984, 79, 55–61. [Google Scholar] [CrossRef]
- Mauchline, J. Growth in mysids and euphausiids. In Factors in Adult Growth; Schram, F.R., Wenner, A.M., Eds.; Routledge: Abingdon, UK, 1985; Volume 3, pp. i–xiii. [Google Scholar]
- Winkler, G.; Greve, W. Laboratory studies of the effect of temperature on growth, moulting and reproduction in the co-occurring mysids Neomysis integer and Praunus flexuosus. Mar. Ecol. Prog. Ser. 2002, 235, 177–188. [Google Scholar] [CrossRef]
- Fockedey, N.; Mees, J.; Vangheluwe, M.; Verslycke, T.; Janssen, C.R.; Vincx, M. Temperature and salinity effects on post-marsupial growth of Neomysis integer (Crustacea: Mysidacea). J. Exp. Mar. Bio. Ecol. 2005, 326, 27–47. [Google Scholar] [CrossRef]
- Clare, J.A. Characterizing Site Fidelity and Habitat Use of the Eastern North Pacific Gray Whale (Eschrichtius robustus) in Clayoquot Sound, British Columbia. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 2015. [Google Scholar]
- Calambokidis, J.; Darling, J.D.; Deeke, V.; Gearin, P.; Gosho, M.; Megill, W.; Tombach, C.M.; Goley, D.; Toropova, C.; Gisborne, B. Abundance, range and movements of a feeding aggregation of gray whales (Eschrichtius robustus) from California to southeastern Alaska in 1998. J. Cet. Res. Manag. 2002, 4, 267–276. [Google Scholar]
- International Union for Conservation of Nature (IUCN). Report of the Fourth Rangewise Workshop on the Status of North Pacific Gray Whales. J. Cet. Res. Manag. 2018, 19, 521–536. [Google Scholar]
- Feyrer, L.J. The foraging ecology of gray whales in Clayoquot Sound: Interactions between predator and prey across a continuum of scales. Master’s Thesis, University of Victoria, Victoria, Canada, 2010. [Google Scholar]
- Dahlheim, M.E. Bio-Acoustics of the Gray Whale. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1987. [Google Scholar]
- Wisdom, S.; Bowles, A.E.; Anderson, K.E. Development of behavior and sound repertoire of a rehabilitating gray whale calf. Aquat. Mamm. 2001, 27, 239–255. [Google Scholar]
- Youngson, B.T.; Darling, J.D. The occurrence of pulse, “knock” sounds amidst social/sexual behaviour of gray whales (Eschrichtius robustus) off Vancouver Island. Mar. Mamm. Sci. 2016, 32, 1482–1490. [Google Scholar] [CrossRef]
- Crane, N.L.; Lashkari, K. Sound production of gray whales, Eschrichtius robustus, along their migration route: A new approach to signal analysis. J. Acoust. Soc. Am. 1996, 100, 1878–1886. [Google Scholar] [CrossRef]
- Guazzo, R.A.; Helbe, T.A.; D’Spain, G.L.; Weller, D.W.; Wiggins, S.M.; Hildebrand, J.A. Migratory behaviour of eastern North Pacific gray whales tracked using a hydrophone array. PLoS ONE 2017, 12, e0185585. [Google Scholar] [CrossRef] [Green Version]
- Burnham, R.E.; Duffus, D.A.; Mouy, X. Gray whale (Eschrichtius robustus) call types recorded during migration off the west coast of Vancouver Island. Front. Mar. Sci. 2018, 5, 329. [Google Scholar] [CrossRef] [Green Version]
- Burnham, R.E.; Duffus, D.A. Maternal behaviors of gray whales (Eschrichtius robustus) on a summer foraging site. Mar. Mamm. Sci. 2020, 1, 9. [Google Scholar] [CrossRef]
- Ollervides, F. Effects of Boat Traffic on the Behavior of Gray Whales, Eschrichtius robustus, in Bahia Magdalena, Baja California Sur, Mexico: A Bioacoustic Assessment. Master‘s Thesis, Texas A and M University, Galveston, TX, USA, 1997. [Google Scholar]
- Ollervides, F.J. Gray Whales and Boat Traffic: Movement, Vocal, and Behavioral Responses in Bahia Magdalena, Mexico. Ph.D. Thesis, Texas A and M University, Galveston, TX, USA, 2001. [Google Scholar]
- Charles, S.M. Social Context of Gray Whale Eschrichtius robustus Sound Activity. Master‘s Thesis, Texas A and M University, Galveston, TX, USA, 2011. [Google Scholar]
- Burnham, R.E. The Importance of the Mid-Trophic Layers in Ecosystem Structure, Process and Function: The Relationship between the Eastern Pacific Gray Whale (Eschrichtius robustus) and Mysids (order Mysidacea) in Clayoquot Sound. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 2012. [Google Scholar]
- Hildebrand, L.; Bernard, K.S.; Torres, L.G. Do Gray Whales Count Calories? Comparing Energetic Values of Gray Whale Prey Across Two Different Feeding Grounds in the Eastern North Pacific. Front. Mar. Sci. 2021, 8, 683634. [Google Scholar] [CrossRef]
- Clark, C.W.; Dukas, R. Balancing foraging and antipredator demands: An advantage of sociality. Am. Natural. 1994, 144, 542–548. [Google Scholar] [CrossRef]
- Stimpert, A.; Wiley, D.; Au, W.W.L.; Johnson, M.P.; Arsenault, R. ‘Megapclicks’: Acoustic click trains and buzzes produced during night-time foraging of humpback whales (Megaptera novaeangliae). Biol. Lett. 2007, 3, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, W.A. Activities and underwater sounds of fin whales. Sci. Rep. Whales Res. Inst. 1981, 33, 83–117. [Google Scholar]
- Wiley, D.; Ware, C.; Bocconcelli, A.; Cholewiak, D.; Friedlaender, A.; Thompson, M.; Weinrich, M. Underwater components of humpback whale bubble-net feeding behaviour. Behaviour 2011, 148, 575–602. [Google Scholar]
- Mastick, N.C.; Wiley, D.; Cade, D.E.; Ware, C.; Parks, S.E.; Friedlaender, A.S. The effect of group size on individual behavior of bubble-net feeding humpback whales in the southern Gulf of Maine. Mar. Mamm. Sci. 2022, 38, 959–974. [Google Scholar] [CrossRef]
- Rannankari, L.; Burnham, R.E.; Duffus, D.A. Diurnal and seasonal acoustic trends in northward migrating eastern Pacific gray whales (Eschrichtius robustus). Aquat. Mamm. 2018, 44, 1–6. [Google Scholar] [CrossRef]
- Ponce, D.; Thode, A.M.; Guerra, M.; Urban, J.R.; Swartz, S. Relationship between visual counts and call detection rates of gray whales (Eschrichtius robustus) in Laguna San Ignacio, Mexico. J. Acoust. Soc. Am. 2012, 131, 2700–2713. [Google Scholar] [CrossRef]
- Hunt, G.; McKinnell, S. The interplay between top-down, bottom-up and wasp-waist control in marine ecosystems. Prog. Ocean. 2006, 68, 115–124. [Google Scholar] [CrossRef]
- Suryan, R.M.; Irons, D.B.; Brown, E.D.; Jodice, P.G.R.; Roby, D.D. Site-specific effects on productivity of an upper trophic-level marine predator: Bottom-up, top-down and mismatch effects on reproduction in a colonial seabird. Prog. Ocean. 2006, 68, 3030–3328. [Google Scholar] [CrossRef]
- Baum, J.; Worm, B. Cascading top-down effects of changing oceanic predator abundances. J. An. Ecol. 2009, 78, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Menge, B.A. Top-down and bottom-up community regulation in marine rocky intertidal habitats. J. Exp. Mar. Bio. Ecol. 2000, 250, 257–289. [Google Scholar] [CrossRef]
- Villegas-Amtmann, S.; Schwarz, L.K.; Sumich, J.L.; Costa, D.P. A bioenergetics model to evaluate demographic consequences of disturbance in marine mammals applied to gray whales. Ecosphere. 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Villegas-Amtmann, S.; Schwarz, L.K.; Gailey, G.; Sychenko, O.; Costa, D.P. East or west: The energetic coast of being a gray whale and the consequence of losing energy to disturbance. Endang. Species Res. 2017, 34, 167–183. [Google Scholar] [CrossRef] [Green Version]
- Soledade Lemos, L.; Burnett, J.D.; Chandler, T.E.; Sumich, J.L.; Torres, L.G. Intra- and inter annual variation in gray whale body condition on a foraging ground. Ecosphere 2020, 11, e03094. [Google Scholar] [CrossRef]
- Stewart, J.D.; Weller, D.J. Abundance of eastern North Pacific gray whales 2019/2020. U.S. Department of Commerce, NOAA Technical Memorandum NMFS-SWFSC-639. 2021. Available online: https://www.sanignaciograywhales.org/wp-content/uploads/2021/02/2021Stewart_GW-Abundance.pdf (accessed on 1 September 2022).




| Variable | Source |
|---|---|
| Skew | Calculated from transect survey data |
| Kurtosis | Calculated from transect survey data |
| Average return proportion | Transect survey and photo-identification data |
| PDO anomaly | Available at: psl.noaa.gov/pdo/ (accessed on 12 September 2022). |
| SST anomaly | Measures taken from Amphitrite Light station, available at: pac.dfo-mpo.gc.ca/science/oceans/data-donnees/lighthouses-phares/index-eng.html (accessed on 12 September 2022). |
| Sea surface temperature | La Perouse wave buoy (46,206): weather.gc.ca (accessed on 12 September 2022) |
| Wind speed | La Perouse wave buoy (46,206): weather.gc.ca (accessed on 12 September 2022) |
| Upwelling | 48° and 51° latitude: coastwatch.pfeg.noaa.gov (accessed on 12 September 2022) |
| Spring Transition Index (STI) | 48° and 51° latitude: coastwatch.pfeg.noaa.gov (accessed on 12 September 2022) |
| Average foraging whale number | Transect survey data |
| Peak foraging whale number | Transect survey data |
| Peak foraging whale date | Number of days elapsed from 24 May, transect data |
| Year | Count | Mean | Standard Dev. | Minimum Value | Maximum Value |
|---|---|---|---|---|---|
| All | 248 | −0.0161 | 4.155 | −17.000 | 15.000 |
| Annual | 7 | 0.609 | 10.652 | −16.508 | 13.314 |
| 1997 | 19 | 0.211 | 4.826 | −12.000 | 9.000 |
| 1998 | 21 | −0.571 | 8.500 | −17.000 | 11.000 |
| 1999 | 14 | 0.5000 | 11.992 | −25.000 | 24.000 |
| 2000 | 11 | 0.000 | 1.483 | −4.000 | 2.000 |
| 2001 | 19 | −0.105 | 2.923 | −5.000 | 7.000 |
| 2002 | 14 | −0.786 | 3.068 | −5.000 | 5.000 |
| 2003 | 12 | −0.250 | 3.494 | −9.000 | 4.000 |
| 2004 | 11 | 0.364 | 2.014 | −3.000 | 5.000 |
| 2005 | 11 | 0.091 | 1.221 | −2.000 | 2.000 |
| 2006 | 12 | −0.083 | 2.021 | −3.000 | 4.000 |
| 2007 | 16 | 0.063 | 1.389 | −3.000 | 3.000 |
| 2008 | 14 | −0.500 | 2.79 | −9.000 | 2.000 |
| 2009 | 16 | −0.563 | 3.596 | −9.000 | 3.000 |
| 2010 | 11 | −0.455 | 8.054 | −17.000 | 13.000 |
| 2011 | 13 | −0.923 | 4.092 | −7.000 | 7.000 |
| 2012 | 11 | 0.000 | 3.464 | −6.000 | 4.000 |
| 2013 | 9 | −0.889 | 8.594 | −12.000 | 15.000 |
| 2014 | 11 | −0.091 | 1.514 | −3.000 | 3.000 |
| 2015 | 12 | −0.250 | 1.288 | −2.000 | 2.000 |
| 2016 | 10 | 0.000 | 0.943 | −2.000 | 2.000 |
| Year | Total Individuals | Return Whales Proportion (%) | Classification |
|---|---|---|---|
| 1998 | 51 | 78.4 | high |
| 1999 | 37 | 73.0 | low |
| 2000 | 40 | 85.0 | low |
| 2002 | 41 | 82.9 | high |
| 2003 | 62 | 87.1 | low |
| 2004 | 27 | 85.2 | high |
| 2005 | 6 | 100.0 | low |
| 2006 | 22 | 95.4 | low |
| 2007 | 7 | 100.0 | low |
| 2008 | 29 | 69.0 | low |
| 2009 | 48 | 83.3 | low |
| 2010 | 58 | 83.8 | high |
| 2011 | 56 | 92.9 | high |
| 2012 | 47 | 93.6 | low |
| 2013 | 95 | 55.8 | high |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burnham, R.E.; Duffus, D.A. A Multi-Dimensional Examination of Foraging Habitat Use by Gray Whales Using Long Time-Series and Acoustics Data. Animals 2022, 12, 2735. https://doi.org/10.3390/ani12202735
Burnham RE, Duffus DA. A Multi-Dimensional Examination of Foraging Habitat Use by Gray Whales Using Long Time-Series and Acoustics Data. Animals. 2022; 12(20):2735. https://doi.org/10.3390/ani12202735
Chicago/Turabian StyleBurnham, Rianna E., and David A. Duffus. 2022. "A Multi-Dimensional Examination of Foraging Habitat Use by Gray Whales Using Long Time-Series and Acoustics Data" Animals 12, no. 20: 2735. https://doi.org/10.3390/ani12202735





