Feline Soft Tissue Sarcomas: A Review of the Classification and Histological Grading, with Comparison to Human and Canine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Terminology
3. Prevalence of Soft Tissue Sarcomas in Cats
4. Clinical Behaviour of Soft Tissue Sarcomas in Cats and Dogs
5. Which Histological Subtypes Should Be Included in the STS Group?
- (a)
- human:
- (b)
- canine:
- (c)
- feline:
6. Histological Grading of STS
- (a)
- Human:
- (b)
- canine:
- (c)
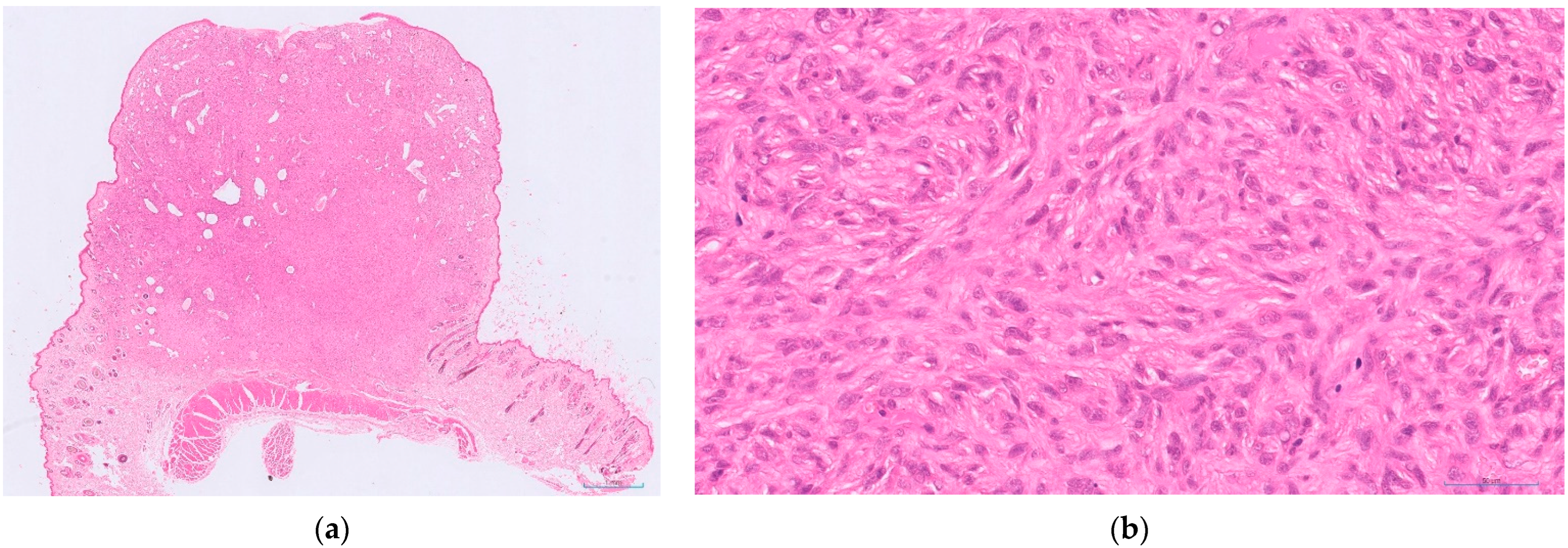
- feline injection site sarcoma:
7. Other Feline Soft Tissue Sarcomas
8. Impact of Histological Grading on Prognostication of Feline STS
9. Conclusions—Where Next for Feline Soft Tissue Sarcomas?
Funding
Acknowledgments
Conflicts of Interest
References
- Bostock, D.E.; Dye, M.T. Prognosis after surgical excision of canine fibrous connective tissue sarcomas. Vet. Pathol. 1980, 17, 581–588. [Google Scholar] [CrossRef]
- Chase, D.; Bray, J.; Ide, A.; Polton, G. Outcome following removal of canine spindle cell tumours in first opinion practice: 104 cases. J. Small Anim. Pract. 2009, 50, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.M.; McSporran, K.D.; Bacon, N.J.; Schulman, F.Y.; Foster, R.A.; Powers, B.E. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet. Pathol. 2011, 48, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Patnaik, A.K. Canine extraskeletal osteosarcoma and chondrosarcoma: A clinicopathologic study of 14 cases. Vet. Pathol. 1990, 27, 46–55. [Google Scholar] [CrossRef]
- Kuntz, C.A.; Dernell, W.S.; Powers, B.E.; Devitt, C.; Straw, R.C.; Withrow, S.J. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986–1996). J. Am. Vet. Med. Assoc. 1997, 211, 1147–1151. [Google Scholar]
- Roccabianca, P.; Schulman, F.Y.; Avallone, G.; Foster, R.A.; Scruggs, J.L.; Dittmer, K.; Kiupel, M. Surgical Pathology of Tumors of Domestic Animals: Volume 3: Tumors of Soft Tissue, 3rd ed.; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2021. [Google Scholar]
- McSporran, K.D. Histologic grade predicts recurrence for marginally excised canine subcutaneous soft tissue sarcomas. Vet. Pathol. 2009, 46, 928–933. [Google Scholar] [CrossRef]
- The Davis-Thompson Foundation. Available online: https://davisthompsonfoundation.org (accessed on 7 October 2022).
- Williamson, M.M.; Middleton, D.J. Cutaneous soft tissue tumours in dogs: Classification, differentiation, and histogenesis. Vet. Dermatol. 1998, 9, 43–48. [Google Scholar] [CrossRef]
- Gaitero, L.; Anor, S.; Fondevila, D.; Pumarola, M. Canine cutaneous spindle cell tumours with features of peripheral nerve sheath tumours: A histopathological and immunohistochemical study. J. Comp. Pathol. 2008, 139, 16–23. [Google Scholar] [CrossRef]
- Perez, J.; Bautista, M.J.; Rollon, E.; de Lara, F.C.; Carrasco, L.; Martin de las Mulas, J. Immunohistochemical characterization of hemangiopericytomas and other spindle cell tumors in the dog. Vet. Pathol. 1996, 33, 391–397. [Google Scholar] [CrossRef]
- Chijiwa, K.; Uchida, K.; Tateyama, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet. Pathol. 2004, 41, 307–318. [Google Scholar] [CrossRef]
- Mazzei, M.; Millanta, F.; Citi, S.; Lorenzi, D.; Poli, A. Haemangiopericytoma: Histological spectrum, immunohistochemical characterization and prognosis. Vet. Dermatol. 2002, 13, 15–21. [Google Scholar] [CrossRef]
- O’ Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Longevity and mortality of cats attending primary care veterinary practices in England. J. Feline Med. Surg. 2015, 17, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Nelson, S.L.; Turk, J.R.; Pace, L.W.; Brown, T.P.; Shaw, D.P.; Fischer, J.R.; Gosser, H.S. Cutaneous neoplasia in 340 cats. Vet. Pathol. 1991, 28, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Graf, R.; Grüntzig, K.; Hässig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Guscetti, F.; Folkers, G.; Otto, V.; et al. Swiss Feline Cancer Registry: A retrospective study of the occurrence of tumours in cats in Switzerland from 1965 to 2008. J. Comp. Pathol. 2015, 153, 266–277. [Google Scholar] [CrossRef]
- Ho, N.T.; Smith, K.C.; Dobromylskyj, M.J. Retrospective study of more than 9000 feline cutaneous tumours in the UK: 2006–2013. J. Feline Med. Surg. 2018, 20, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Grüntzig, K.; Boo, G.; Hässig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Guscetti, F.; Folkers, G.; et al. Swiss Feline Cancer Registry 1965–2008, the influence of sex, breed and age on tumour types and tumour locations. J. Comp. Pathol. 2016, 154, 195–210. [Google Scholar] [CrossRef] [Green Version]
- Manuali, E.; Forte, C.; Vichi, G.; Genovese, D.A.; Mancini, D.; De Leo, A.A.P.; Cavicchioli, L.; Pierucci, P.; Zappulli, V. Tumours in European Shorthair cats: A retrospective study of 680 cases. J. Feline Med. Surg. 2020, 22, 1095–1102. [Google Scholar] [CrossRef]
- Schmidt, J.M.; North, S.M.; Freeman, K.P.; Ramiro-Ibañez, F. Feline paediatric oncology: Retrospective assessment of 233 tumours from cats up to one year (1993 to 2008). J. Small Anim. Pract. 2010, 51, 306–311. [Google Scholar] [CrossRef]
- Hardy, W.D.; Zuckerman, E.E.; MacEwen, E.G.; Hayes, A.A.; Essex, M. A feline leukaemia virus- and sarcoma virus-induced tumour-specific antigen. Nature 1977, 270, 249–251. [Google Scholar] [CrossRef]
- Macy, D.W.; Reeds, K.B. Chapter Three—Veterinary Cancer Etiology, Section B: Viral Carcinogenesis. In Cancer Management in Small Animal Practice; Henry, C.J., Higginbotham, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 16–27. [Google Scholar]
- Davidson, E.B.; Gregory, C.R.; Kass, P.H. Surgical excision of soft tissue fibrosarcomas in cats. Vet. Surg. 1997, 26, 265–269. [Google Scholar] [CrossRef]
- Mauldin, G.N. Soft tissue sarcomas. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 139–148. [Google Scholar] [CrossRef]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef]
- Liptak, J.M.; Forrest, L.J. Soft Tissue Sarcomas. In Small Animal Clinical Oncology, 4th ed.; Withrow, S.J., Vail, D.M., Eds.; Sanders Elsevier: St Louis, MO, USA, 2007; pp. 425–454. [Google Scholar]
- Dean, R.S.; Pfeiffer, D.U.; Adams, V.J. The incidence of feline injection site sarcomas in the United Kingdom. BMC Vet. Res. 2013, 9, 17. [Google Scholar] [CrossRef]
- Ladlow, J. Injection site-associated sarcoma in the cat: Treatment recommendations and results to date. J. Feline Med. Surg. 2013, 15, 409–418. [Google Scholar] [CrossRef]
- Martano, M.; Morello, E.; Buracco, P. Feline injection-site sarcoma: Past, present and future perspectives. Vet. J. 2011, 188, 136–141. [Google Scholar] [CrossRef]
- Feline Injection Site Sarcoma. Available online: www.abcdcastvets.org/feline-injection-site-sarcoma-2/ (accessed on 4 September 2022).
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalised medicine. CA Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef] [Green Version]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; de Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer. 1984, 33, 37–42. [Google Scholar] [CrossRef]
- Avallone, G.; Helmbold, P.; Caniatti, M.; Stefanello, D.; Nayak, R.C.; Roccabianca, P. The spectrum of canine cutaneous perivascular wall tumors: Morphologic, phenotypic, and clinical characterization. Vet. Pathol. 2007, 44, 607–620. [Google Scholar] [CrossRef] [Green Version]
- Chiti, L.E.; Ferrari, R.; Roccabianca, P.; Boracchi, P.; Godizzi, F.; Busca, G.A.; Stefanello, D. Surgical margins in canine cutaneous soft tissue sarcomas: A dichotomous classification system does not accurately predict the risk of local recurrence. Animals 2021, 11, 2367. [Google Scholar] [CrossRef]
- Stefanello, D.; Morello, W.; Roccabianca, P.; Iussich, S.; Nassuato, C.; Martano, M.; Squassino, C.; Avallone, G.; Romussi, S.; Buracco, P. Marginal excision of low-grade spindle cell sarcoma of canine extremities: 35 dogs (1996–2006). Vet. Surg. 2008, 37, 461–465. [Google Scholar] [CrossRef]
- Bacon, N.J.; Dernell, W.S.; Ehrhart, N.; Powers, B.E.; Withrow, S.J. Evaluation of primary re-excision after recent inadequate resection of soft tissue sarcomas in dogs: 41 cases (1999–2004). J. Am. Vet. Med. Assoc. 2007, 230, 548–554. [Google Scholar] [CrossRef]
- Couto, S.S.; Griffey, S.M.; Duarte, P.C.; Madewell, B.R. Feline vaccine-associated fibrosarcoma: Morphological distinctions. Vet. Pathol. 2002, 39, 33–40. [Google Scholar] [CrossRef]
- Eckstein, C.; Guscetti, F.; Roos, M.; Martín de las Mulas, J.; Kaser-Hotz, B.; Rohrer Bley, C. A retrospective analysis of radiation therapy for the treatment of feline vaccine-associated sarcoma. Vet. Comp. Oncol. 2009, 7, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Porcellato, I.; Menchetti, L.; Brachelente, C.; Sforna, M.; Reginato, A.; Lepri, E.; Mechelli, L. Feline Injection-Site Sarcoma. Vet. Pathol. 2017, 54, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, C.S.; de Queiroz, G.F.; Pinto, A.C.; Dagli, M.L.; Matera, J.M. Feline injection site sarcoma: Immunohistochemical characteristics. J. Feline Med. Surg. 2019, 21, 314–321. [Google Scholar] [CrossRef]
- Dobromylskyj, M.J.; Richards, V.; Smith, K.C. Prognostic factors and proposed grading system for cutaneous and subcutaneous soft tissue sarcomas in cats, based on a retrospective study. J. Feline Med. Surg. 2021, 23, 168–174. [Google Scholar] [CrossRef]
- Schulman, F.Y.; Johnson, T.O.; Facemire, P.R.; Fanburg-Smith, J.C. Feline Peripheral Nerve Sheath Tumors: Histologic, Immunohistochemical, and Clinicopathologic Correlation (59 Tumors in 53 Cats). Vet. Pathol. 2009, 46, 1166–1180. [Google Scholar] [CrossRef] [Green Version]
- Mandara, M.T.; Fabriani, E.; Pavone, S.; Pumarola, M. Feline cutaneous nerve sheath tumours; histological features and immunohistochemical evaluations. Res. Vet. Sci. 2013, 95, 548–555. [Google Scholar] [CrossRef]
- Stoll, A.L.; Suárez-Bonnet, A.; Summers, B.A.; Priestnall, S.L. Malignant cutaneous peripheral nerve sheath tumour with rhabdomyosarcomatous differentiation (triton tumour) in a domestic cat. J. Comp. Path. 2018, 165, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.J.; Njaa, B.L.; Lamm, C.G. Immunohistochemical expression of c-KIT protein in feline soft tissue fibrosarcomas. Vet. Pathol. 2009, 46, 934–939. [Google Scholar] [CrossRef]
- de Cecco, B.S.; Argenta, F.F.; Bianchi, R.M.; De Lorenzo, C.; Wronski, J.G.; Bandinelli, M.B.; da Costa, F.V.; Driemeier, D.; Pavarini, S.P.; Sonne, L. Feline giant-cell pleomorphic sarcoma: Cytologic, histologic and immunohistochemical characterization. J. Feline Med. Surg. 2021, 23, 738–744. [Google Scholar] [CrossRef]
- Veterinary Cancer Guidelines and Protocols. Available online: https://vcgp.org (accessed on 4 September 2022).
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of histological grading systems in veterinary medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef]
- Meuten, D.J.; Moore, F.M.; Donovan, T.A.; Bertram, C.A.; Klopfleisch, R.; Foster, R.A.; Smedley, R.C.; Dark, M.J.; Milovancev, M.; Stromberg, P.; et al. International Guidelines for Veterinary Tumor Pathology: A Call to Action. Vet. Pathol. 2021, 58, 766–794. [Google Scholar] [CrossRef] [PubMed]
- La Perle, K.M.D. Machine Learning and Veterinary Pathology: Be Not Afraid! Vet. Pathol. 2019, 56, 506–507. [Google Scholar] [CrossRef]
- Rai, T.; Morisi, A.; Bacci, B.; Bacon, N.J.; Dark, M.J.; Aboellail, T.; Thomas, S.A.; Bober, M.; La Ragione, R.; Wells, K. Deep learning for necrosis detection using canine perivascular wall tumour whole slide images. Sci. Rep. 2022, 12, 10634. [Google Scholar] [CrossRef]
- Bertram, C.A.; Aubreville, M.; Donovan, T.A.; Bartel, A.; Wilm, F.; Marzahl, C.; Assenmacher, C.A.; Becker, K.; Bennett, M.; Corner, S.; et al. Computer-assisted mitotic count using a deep learning-based algorithm improves interobserver reproducibility and accuracy. Vet. Pathol. 2022, 59, 211–226. [Google Scholar] [CrossRef] [PubMed]



| Differentiation Score | |
| 1 | Sarcomas mostly resembling normal adult mesenchymal tissue |
| 2 | Sarcomas with known histological type but poor differentiation |
| 3 | Undifferentiated sarcomas, sarcomas of unknown type |
| Mitotic Score 1 | |
| 1 | 0–9 |
| 2 | 10–19 |
| 3 | More than 19 |
| Tumour Necrosis Score | |
| 0 | No necrosis |
| 1 | Equal to or less than 50% necrosis |
| 2 | More than 50% necrosis |
| Histological Grade 2 | |
| I | Equal to or less than 3 |
| II | 4–5 |
| III | Equal to or more than 6 |
| Inflammation Score | |
| 1 | None, minimal or very mild |
| 2 | Mild to moderate |
| 3 | Severe |
| Mitotic Score 1 | |
| 1 | 0–9 |
| 2 | 10–19 |
| 3 | More than 19 |
| Tumour Necrosis Score 2 | |
| 0 | No necrosis |
| 1 | Equal to or less than 50% necrosis |
| 2 | More than 50% necrosis |
| Histological Gradem 3 | |
| I | Equal to or less than 3 |
| II | 4–5 |
| III | Equal to or more than 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobromylskyj, M. Feline Soft Tissue Sarcomas: A Review of the Classification and Histological Grading, with Comparison to Human and Canine. Animals 2022, 12, 2736. https://doi.org/10.3390/ani12202736
Dobromylskyj M. Feline Soft Tissue Sarcomas: A Review of the Classification and Histological Grading, with Comparison to Human and Canine. Animals. 2022; 12(20):2736. https://doi.org/10.3390/ani12202736
Chicago/Turabian StyleDobromylskyj, Melanie. 2022. "Feline Soft Tissue Sarcomas: A Review of the Classification and Histological Grading, with Comparison to Human and Canine" Animals 12, no. 20: 2736. https://doi.org/10.3390/ani12202736
APA StyleDobromylskyj, M. (2022). Feline Soft Tissue Sarcomas: A Review of the Classification and Histological Grading, with Comparison to Human and Canine. Animals, 12(20), 2736. https://doi.org/10.3390/ani12202736








