Effect of Lipids in Yak Muscle under Different Feeding Systems on Meat Quality Based on Untargeted Lipidomics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Determination of Meat Quality and Total Lipid Content
2.3. Determination of Volatile Flavor Compounds
2.4. Determination of Lipidomics
2.5. Analysis of the Correlation of Lipids with Meat Quality of Yak Longissimus Dorsi (LD)
2.6. Statistical Analysis
3. Results
3.1. Meat Quality and Total Lipid Content of Yak LD
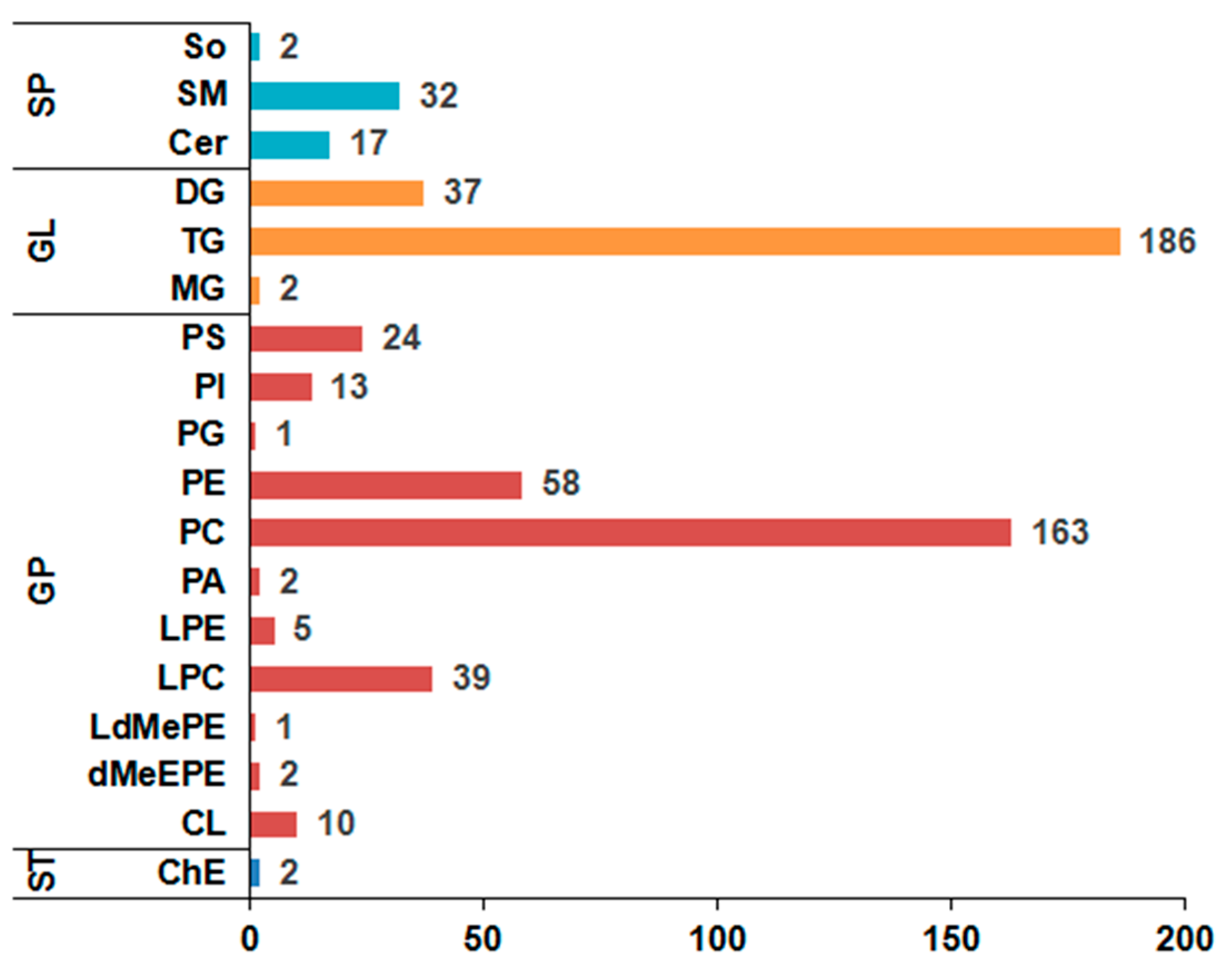
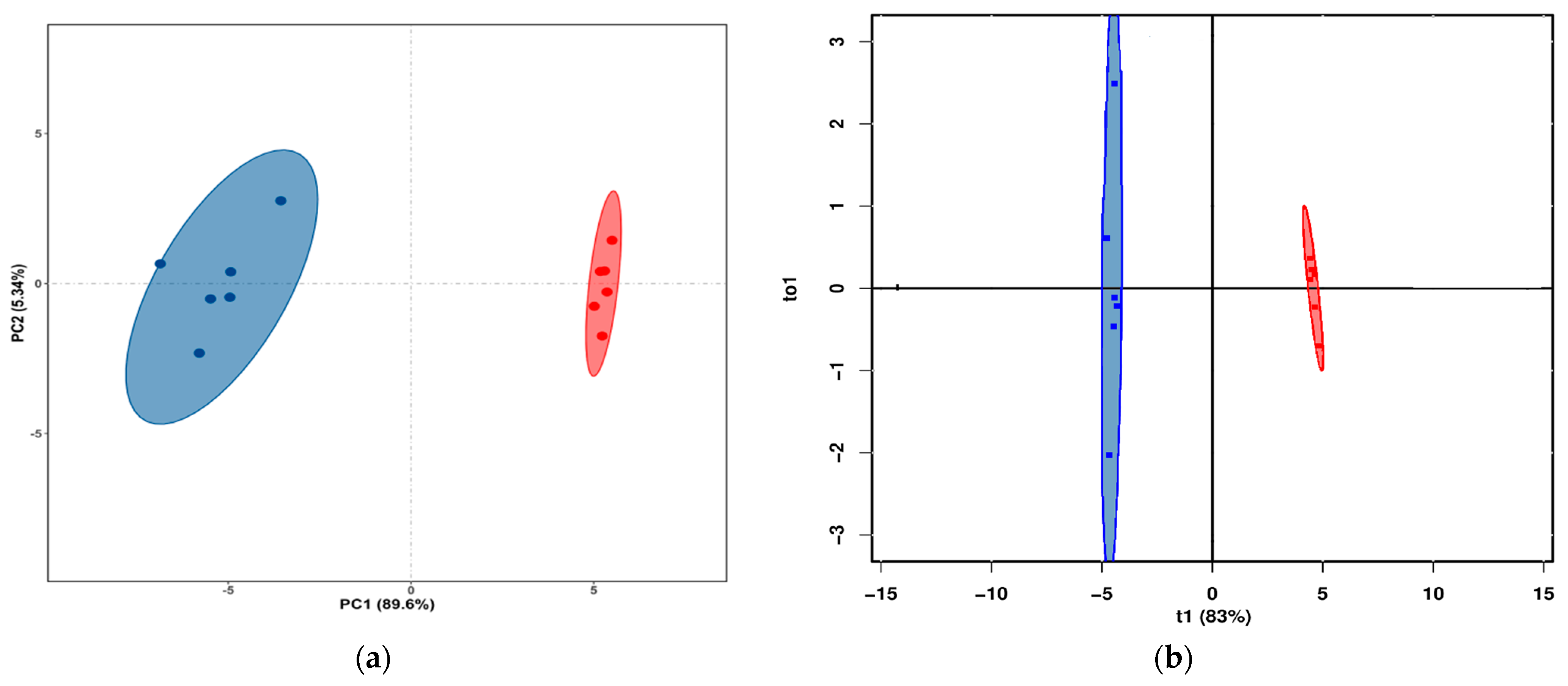
3.2. Lipids in Yak LD
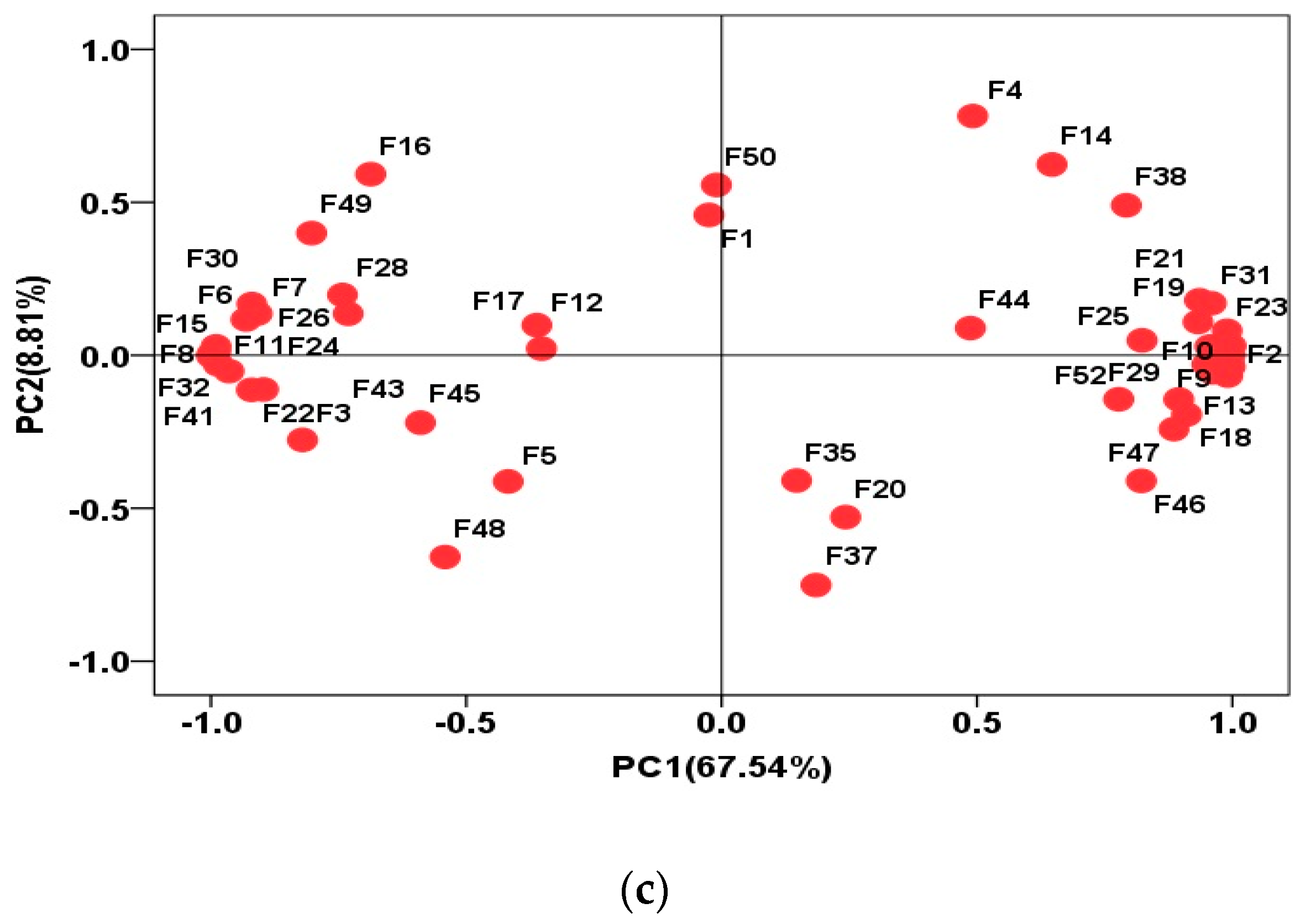
3.3. Volatile Flavor Compounds in Yak LD
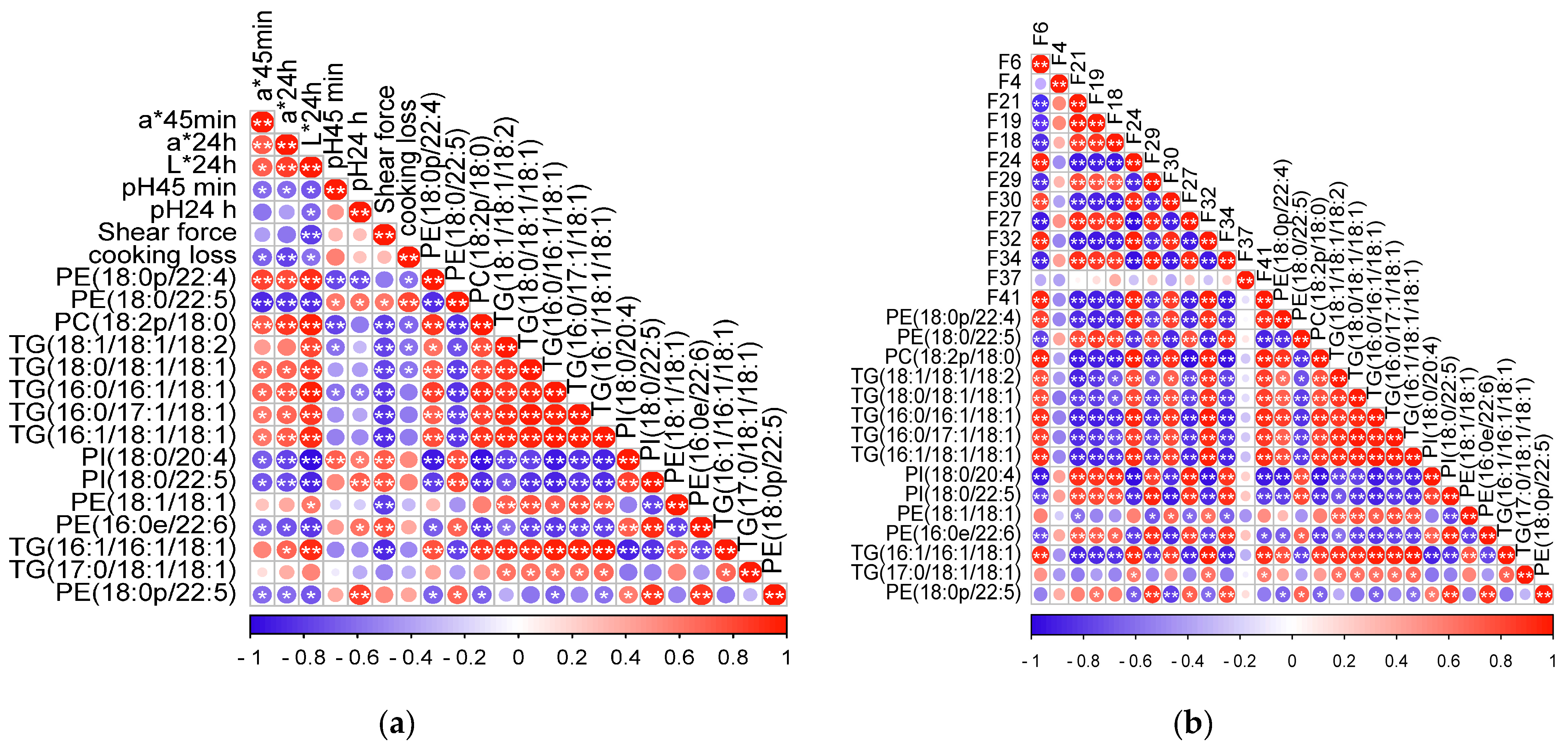
3.4. Results of Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masoodi, M.; Kuda, O.; Rossmeisl, M.; Flachs, P.; Kopecky, J. Lipid signaling in adipose tissue: Connecting inflammation & metabolism. Biochim. Biophys. Acta 2015, 1851, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Realini, C.E.; Pavan, E.; Johnson, P.L.; Font-I-Furnols, M.; Jacob, N.; Agnew, M.; Craigie, C.R.; Moon, C.D. Consumer liking of M. longissimus lumborum from New Zealand pasture-finished lamb is influenced by intramuscular fat. Meat Sci. 2021, 173, 108380. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J.; Lebret, B. Influence of intramuscular fat content on the quality of pig meat-1. Composition of the lipid fraction and sensory characteristics of M. longissimus lumborum. Meat Sci. 1999, 53, 59–65. [Google Scholar] [CrossRef]
- Listrat, A.; Gagaoua, M.; Normand, J.; Gruffat, D.; Andueza, D.; Mairesse, G.; Mourot, B.P.; Chesneau, G.; Gobert, C.; Picard, B. Contribution of connective tissue components, muscle fibres and marbling to beef tenderness variability in longissimus thoracis, rectus abdominis, semimembranosus and semitendinosus muscles. J. Sci. Food Agric. 2020, 100, 2502–2511. [Google Scholar] [CrossRef]
- Hoa, V.B.; Seol, K.H.; Seo, H.W.; Seong, P.N.; Kang, S.M.; Kim, Y.S.; Moon, S.S.; Kim, J.H.; Cho, S.H. Meat quality characteristics of pork bellies in relation to fat level. Anim. Biosci. 2021, 34, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Whitfield, F.B. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.H.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef] [Green Version]
- Madruga, M.; Dantas, I.; Queiroz, A.; Brasil, L.; Ishihara, Y. Volatiles and water- and fat-soluble precursors of Saanen goat and cross Suffolk lamb flavour. Molecules 2013, 18, 2150–2165. [Google Scholar] [CrossRef]
- Madruga, M.S.; Elmore, J.S.; Oruna-Concha, M.J. Determination of some water-soluble aroma precursors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 2010, 123, 513–520. [Google Scholar] [CrossRef]
- Cameron, N.D.; Enser, M.; Nute, G.R. Genotype with nutrition interaction on fatty acid composition of intramuscular fat and the relationship with flavour of pig meat. Meat Sci. 2000, 55, 187–195. [Google Scholar] [CrossRef]
- Piao, M.Y.; Lee, H.J.; Yong, H.I.; Beak, S.H.; Kim, H.J.; Jo, C.; Wiryawan, K.G.; Baik, M. Comparison of reducing sugar content, sensory traits, and fatty acids and volatile compound profiles of the longissimus thoracis among Korean cattle, Holsteins, and Angus steers. Asian-Australas. J. Anim. Sci. 2019, 32, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; He, Y.; Li, H.; Wu, F.; Qiu, Q.H.; Niu, W.J.; Gao, Z.B.; Su, H.W.; Cao, B.H. Rumen fermentation, intramuscular fat fatty acid profiles and related rumen bacterial populations of Holstein bulls fed diets with different energy levels. Appl. Microbiol. Biotechnol. 2019, 103, 4931–4942. [Google Scholar] [CrossRef]
- Sobczuk-Szul, M.; Mochol, M.; Nogalski, Z.; Pogorzelska-Przybyłek, P. Fatty acid profile as affected by fat depot and the sex category of Polish Holstein-Friesian × Limousin fattening cattle fed silage ad libitum. Anim. Sci. J. 2021, 92, e13516. [Google Scholar] [CrossRef]
- Momot, M.; Nogalski, Z.; Pogorzelska-Przybyłek, P.; Sobczuk-Szul, M. Influence of Genotype and Slaughter Age on the Content of Selected Minerals and Fatty Acids in the Longissimus Thoracis Muscle of Crossbred Bulls. Animals 2020, 10, 2004. [Google Scholar] [CrossRef]
- Sobczuk-Szul, M.; Mochol, M.; Nogalski, Z.; Pogorzelska-Przybyłek, P.; Momot, M. Fattening of Polish Holstein-Friesian × Limousin Bulls under Two Production Systems and Its Effect on the Fatty Acid Profiles of Different Fat Depots. Animals 2021, 11, 3078. [Google Scholar] [CrossRef]
- Scollan, N.D.; Choi, N.J.; Kurt, E.; Fisher, A.V.; Enser, M.; Wood, J.D. Manipulating of fatty acid composition of muscle and adipose tissue in beef cattle. Br. J. Nutr. 2001, 85, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.T.; Luo, X.L.; Xia, B.X.; Guan, J.Q.; Nie, Y.Y.; Li, L.; Duan, J.Y.; Suman, S.P.; Sun, Q. Post-mortem oxidative stability of three yak (Bos grunniens) muscles as influenced by animal age. Meat Sci. 2015, 105, 121–125. [Google Scholar] [CrossRef]
- Lang, Y.M.; Sha, K.; Zhang, R.; Xie, P.; Luo, X.; Sun, B.Z. Effect of electrical stimulation and hot boning on the eating quality of Gannan yak longissimus lumborum. Meat Sci. 2016, 112, 3–8. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhao, Y.; Zhu, D.; Pang, X.M.; Liu, Y.; Frew, R.; Chen, G. Lipidomics profiling of goat milk, soymilk and bovine milk by UPLC-Q-Exactive Orbitrap Mass Spectrometry. Food Chem. 2017, 224, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Blaženovi, I.; Shen, T.; Mehta, S.S.; Kind, T.; Ji, J.; Piparo, M.; Cacciola, F.; Mondello, L.; Fiehn, O. Increasing Compound Identification Rates in Untargeted Lipidomics Research with Liquid Chromatography Drift Time-Ion Mobility Mass Spectrometry. Anal. Chem. 2018, 90, 10758–10764. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Quiles-Zafra, R. Lipidomics: An omics discipline with a key role in nutrition. Talanta 2020, 219, 121197. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimentional Mass Spectrometry-Based Shotgun Lipidomics and Novel Strategies for Lipidomics Analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico, J.E.; Samii, S.S.; Zang, Y.; Deme, P.; Haughey, N.J.; Grilli, E.; McFadden, J.W. Characterization of the Plasma Lipidome in Dairy Cattle Transitioning from Gestation to Lactation: Identifying Novel Biomarkers of Metabolic Impairment. Metabolites 2021, 11, 290. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.D.; Hong, L.L.; Yu, Z.; Yu, Z.; Chang, H.; Zhu, Q.P. Comparative lipidomics analysis of human, bovine and caprine milk by UHPLC-Q-TOF-MS. Food Chem. 2020, 310, 125865. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.Q.; Yang, Y.Y.; Zhu, J.W.; He, W.Z.; Zhao, Q.Y.; Tang, C.H.; Qin, Y.C.; Zhang, J.M. Comparative characterization of lipids and volatile compounds of Beijing Heiliu and Laiwu Chinese black pork as markers. Food Res. Int. 2021, 146, 110433. [Google Scholar] [CrossRef]
- Artegoitia, V.M.; Foote, A.P.; Lewis, R.M.; Freetly, H.C. Metabolomics Profile and Targeted Lipidomics in Multiple Tissues Associated with Feed Efficiency in Beef Steers. ACS Omega 2019, 4, 3973–3982. [Google Scholar] [CrossRef] [Green Version]
- Ueda, S.; Sasaki, R.; Nakabayashi, R.; MYamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle. Metabolites 2021, 11, 203. [Google Scholar] [CrossRef]
- Wang, X.; Liang, C.; Li, A.; Cheng, G.; Long, F.; Khan, R.; Wang, J.; Zhang, Y.; Wu, S.; Wang, Y.; et al. RNA-Seq and lipidomics reveal different adipogenic processes between bovine perirenal and intramuscular adipocytes. Adipocyte 2022, 11, 448–462. [Google Scholar] [CrossRef]
- Jia, W.; Wu, X.X.; Zhang, R.; Shi, L. UHPLC-Q-Orbitrap-based lipidomics reveals molecular mechanism of lipid changes during preservatives treatment of Hengshan goat meat sausages. Food Chem. 2022, 369, 130948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Na, X.K.; Lai, B.; Song, Y.K.; Wang, H.T.; Tan, M.Q. Effects of fluorescent carbon dots from the baked lamb on energy and lipid metabolism. Food Chem. 2021, 338, 127832. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.G.; Zhao, J.H.; Luo, Z.; Lin, Q.L.; Luo, F.J.; Yang, T. Systematic evaluation of the physicochemical properties and the volatile flavors of yak meat during chilled and controlled freezing-point storage. J. Food Sci. Technol. 2020, 57, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Bonnet, M.; Picard, B. Protein array-based approach to evaluate biomarkers of beef tenderness and marbling in cows: Understanding of the underlying mechanisms and prediction. Foods 2020, 9, 1180. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Gondret, F.; Baeza, E. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.Y.; Zhang, Y.M.; Zhu, L.X.; Han, M.S.; Gao, S.J.; Luo, X. Effect of packaging atmospheres on storage quality characteristics of heavily marbled beef longissimus steaks. Meat Sci. 2016, 117, 50–56. [Google Scholar] [CrossRef]
- Malva, A.D.; Marino, R.; Santillo, A. Proteomic approach to investigate the impact of different dietary supplementation on lamb meat tenderness. Meat Sci. 2017, 131, 74–81. [Google Scholar] [CrossRef]
- Purchas, R.W. An assessment of the role of pH differences in determining the relative tenderness of meat from bulls and steers. Meat Sci. 1990, 27, 129–140. [Google Scholar] [CrossRef]
- Wheeler, T.L.; Cundiff, L.V.; Koch, R.M. Effect of marbling degree on beef palatability in Bos taurus and Bos indicus cattle. J. Anim. Sci. 1994, 72, 3145–3151. [Google Scholar] [CrossRef]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sañudo, C.; Escudero, A. Relationship between odour-active compounds and flavour perception in meat from lambs fed different diets. Meat Sci. 2010, 85, 700–706. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Insani, E.M.; Biolatto, A.; Sancho, A.M.; García, P.T.; Pensel, N.A.; Josifovich, J.A. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Sci. 2005, 70, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Van, B.A.H.; Ryuk, S.; Lan, N.T.K. Influence of particular breed on meat quality parameter, sensory characteristics, and volatile components. Food Sci. Biotechnol. 2013, 22, 651–658. [Google Scholar] [CrossRef]
- Sanudo, C.; Enser, M.E.; Campo, M.M. Fatty acid composition and sensory characteristics of lamb carcasses from Britain and Spain. Meat Sci. 2000, 54, 339–346. [Google Scholar] [CrossRef]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef]
- Mezgebo, G.B.; Monahan, F.J.; McGee, M.; O’Riordan, E.G.; Richardson, I.R.; Brunton, N.P.; Moloney, A.P. Fatty acid, volatile and sensory characteristics of beef as affected by grass silage or pasture in the bovine diet. Food Chem. 2017, 235, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Ba, H.V.; Park, K.; Dashmaa, D.; Hwang, I. Effect of muscle type and vacuum chiller ageing period on the chemical compositions, meat quality, sensory attributes and volatile compounds of Korean native cattle beef. Anim. Sci. J. 2014, 85, 164–173. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Dai, X.R.; Du, M.Y. Formation of volatile flavor components during sour meat processing. Food Sci. 2010, 31, 98–104. [Google Scholar]
- Sabio, E.; Vidal-Aargbn, M.C.; Bemalet, M.J. Volati1e compounds present in six types of dry-cured ham form south European countries. Food Chem. 1998, 61, 493–503. [Google Scholar] [CrossRef]
- Young, O.A.; Lane, G.A.; Priolo, A. Pastoral and species flavour in lamb raised on pasture, lucerne or maize. J. Sci. Food Agric. 2003, 83, 93–104. [Google Scholar] [CrossRef]
- Floers, M.; Grimm, C.C.; Toldra, F. Correlations of sensory and volatile compounds of Spanish “Serrano” dry-cured ham as a function of two Processing times. J. Agric. Food Chem. 1997, 45, 2178–2186. [Google Scholar] [CrossRef]
- Shahidi, F.; Rubin, L.J.; Souza, L.A. Meat flavor volatiles: A review of the composition, techniques of analysis, and sensory evaluation. Crit. Rev. Food Sci. 1986, 24, 141–243. [Google Scholar] [CrossRef] [PubMed]
- Morbn, L.; GiraIdez, F.J.; Panseri, S. Effect of dietary carnosic acid on the fatty acid profile and flavour stability of meat from fattening lambs. Food Chem. 2013, 138, 2407–2414. [Google Scholar] [CrossRef]
- Lin, J.J.; Zhao, W.H.; Liu, Q.Y.; He, D.H.; Lei, M.L.; Bai, W.D. Research Progress on the Effect of Lipid Hydrolysis and Oxidation on the Flavor of Dry-Cured Fish. Food Ind. 2021, 42, 206–209. [Google Scholar]
- Ma, Q.L.; Hamid, N.; Bekhit, A.E.D. Evaluation of pre-rigor injection of beef with proteases on cooked meat volatile profile after 1 day and 21 days post-mortem storage. Meat Sci. 2012, 92, 430–439. [Google Scholar] [CrossRef]


| Variable | GF Group (Mean ± SD, n = 6) | SF Group (Mean ± SD, n = 6) |
|---|---|---|
| a*45min | 29.10 ± 0.29 | 30.67 ± 0.99 ** |
| b*45min | 6.69 ± 0.07 | 6.45 ± 0.23 |
| L*45min | 6.93 ± 0.39 | 7.27 ± 0.24 |
| a*24h | 31.79 ± 0.46 | 33.49 ± 0.64 ** |
| b*24h | 9.07 ± 0.36 | 8.74 ± 0.19 |
| L*24h | 7.31 ± 0.04 | 8.55 ± 0.07 ** |
| pH45min | 6.45 ± 0.11 | 6.28 ± 0.07 * |
| pH24h | 5.76 ± 0.05 | 5.65 ± 0.09 * |
| Shear force (kg) | 17.13 ± 0.75 | 14.77 ± 0.01 ** |
| Cooking loss (%) | 30.51 ± 0.91 | 28.24 ± 1.88 * |
| Water loss rate (%) | 20.53 ± 1.03 | 19.50 ± 1.01 |
| Total lipids content (%) | 1.73 ± 0.10 | 2.71 ± 0.11 ** |
| Lipid | ID | Class | Formula | Fatty Acid | FC |
|---|---|---|---|---|---|
| PE(18:0/22:4) | 86 | PE | C45 H80 O7 N1 P1 | C18:0, C22:4 | 3.09 |
| PE(18:0/22:5) | 147 | PE | C45 H78 O8 N1 P1 | C18:0, C22:5 | 0.56 |
| PC(18:2/18:0) | 337 | PC | C44 H84 O7 N1 P1 | C18:2, C18:0 | 2.33 |
| TG(18:1/18:1/18:2) | 84 | TG | C57 H102 O6 | C18:1, C18:1, C18:2 | 2.65 |
| TG(18:0/18:1/18:1) | 51 | TG | C57 H106 O6 | C18:0, C18:1, C18:1 | 1.95 |
| TG(16:0/16:1/18:1) | 37 | TG | C53 H98 O6 | C16:0, C16:1, C18:1 | 3.70 |
| TG(16:0/17:1/18:1) | 41 | TG | C54 H100 O6 | C16:0, C17:1, C18:1 | 2.43 |
| TG(16:1/18:1/18:1) | 94 | TG | C55 H100 O6 | C16:1, C18:1, C18:1 | 3.54 |
| PI(18:0/20:4) | 78 | PI | C47 H81 O13 P1 | C18:0, C20:4 | 0.43 |
| PI(18:0/22:5) | 74 | PI | C49 H83 O13 P1 | C18:0, C22:5 | 0.13 |
| PE(18:1/18:1) | 67 | PE | C41 H76 O8 N1 P1 | C18:1, C18:1 | 1.84 |
| PE(16:0/22:6) | 387 | PE | C43 H74 O7 N1 P1 | C16:0, C22:6 | 0.61 |
| TG(16:1/16:1/18:1) | 38 | TG | C53 H96 O6 | C16:1, C16:1, C18:1 | 3.25 |
| TG(17:0/18:1/18:1) | 49 | TG | C56 H104 O6 | C17:0, C18:1, C18:1 | 1.96 |
| PE(18:0/22:5) | 81 | PE | C45 H78 O7 N1 P1 | C18:0, C22:5 | 0.66 |
| Volatile Flavor Compound | GF Group (Mean ± SD, n = 6) | SF Group (Mean ± SD, n = 6) |
|---|---|---|
| Acetaldehyde (F1) | 2.91 ± 0.29 | 2.92 ± 0.11 |
| 3-methyl butyraldehyde (F2) | 1.68 ± 0.12 | ND |
| Caproaldehyde (F3) | 1.91 ± 0.15 | 2.26 ± 0.14 ** |
| Heptaldehyde (F4) | 2.31 ± 0.30 | 2.06 ± 0.08 |
| Caprylaldehyde (F5) | 1.83 ± 0.33 | 2.10 ± 0.09 |
| Pelargonic aldehyde (F6) | 12.59 ± 0.89 | 17.39 ± 0.84 ** |
| Capric aldehyde (F7) | 0.18 ± 0.03 | 0.29 ± 0.02 ** |
| 2-nonene aldehyde (F8) | ND | 1.27 ± 0.06 |
| 2-decyl olefine aldehyde (F9) | 2.16 ± 0.16 | ND |
| 2-undecenal (F10) | 1.61 ± 0.18 | ND |
| Myristic aldehyde (F11) | ND | 1.52 ± 0.06 |
| Hexadecanal (F12) | 3.10 ± 0.48 | 3.34 ± 0.10 |
| Stearic aldehyde (F13) | 1.63 ± 0.13 | ND |
| Benzaldehyde (F14) | 0.31 ± 0.07 | 0.22 ± 0.02 * |
| 3-ethyl benzaldehyde (F15) | ND | 0.51 ± 0.04 |
| Acetone (F16) | 1.40 ± 0.10 | 1.56 ± 0.08 * |
| 2-butanone (F17) | 0.57 ± 0.06 | 0.61 ± 0.05 |
| 3-hydroxy-2-butanone (F18) | 10.80 ± 0.92 | 7.31 ± 0.61 ** |
| 2-heptanone (F19) | 1.53 ± 0.08 | 1.03 ± 0.11 ** |
| 6-methyl-5-hepten-2-one (F20) | 0.73 ± 0.16 | 0.67 ± 0.05 |
| Acetophenone (F21) | 3.95 ± 0.42 | 2.27 ± 0.15 ** |
| Ethyl alcohol (F22) | 0.83 ± 0.10 | 1.44 ± 0.14 ** |
| 3-methyl-1-butanol (F23) | 1.78 ± 0.06 | ND |
| 1-pentanol (F24) | 2.66 ± 0.13 | 4.57 ±0.14 ** |
| 3-methyl-2-buten-1-ol (F25) | 0.76 ± 0.06 | 0.58 ± 0.08 ** |
| 1-hexanol (F26) | 2.09 ± 0.15 | 2.43 ± 0.15 ** |
| 1-heptanol (F27) | 2.78 ± 0.11 | 1.82 ± 0.16 ** |
| 2-ethylhexanol (F28) | 2.10 ± 0.17 | 2.49 ± 0.17 ** |
| 1-octanol (F29) | 2.19 ± 0.22 | 1.43 ± 0.14 ** |
| 1-octen-3-ol (F30) | 7.83 ± 1.51 | 13.40 ± 0.57 ** |
| 2-hexadecanol (F31) | 0.14 ± 0.03 | ND |
| Ethyl caprate (F32) | 2.33 ± 0.15 | 5.20 ± 0.28 ** |
| N-decyl Acetate (F33) | 0.10 ± 0.02 | ND |
| Vinyl acetate (F34) | 3.04 ± 0.29 | 1.46 ± 0.14 ** |
| Vinyl Hexanoate (F35) | 0.75 ± 0.09 | 0.73 ± 0.06 |
| Octadecane (F36) | 0.51 ± 0.04 | ND |
| 2,2,4,4,6,8,8-heptamethylnonane (F37) | 2.60 ± 0.40 | 2.44 ± 0.25 |
| n-tridecane (F38) | 0.10 ± 0.03 | 0.05 ± 0.01 ** |
| DL-limonene (F39) | 0.81 ± 0.08 | ND |
| Decane (F40) | 0.27 ± 0.05 | ND |
| Pentylcyclopropane (F41) | 1.53 ± 0.18 | 2.78 ± 0.14 ** |
| Styrene (F42) | 0.60 ± 0.09 | ND |
| 2-ethylfuran(F43) | 0.56 ± 0.08 | 0.82 ± 0.07 ** |
| 2-pentylfuran (F43) | 1.69 ± 0.10 | 1.56 ± 0.13 |
| Toluene (F45) | 0.79 ± 0.08 | 0.90 ± 0.08 |
| Phenol (F46) | 0.30 ± 0.14 | ND |
| Naphthalene (F47) | 0.81 ± 0.29 | 1.16 ± 0.20 |
| m-xylene (F48) | 1.16 ± 0.21 | 0.55 ± 0.09 ** |
| Acetic acid (F49) | 2.21 ± 0.21 | 2.75 ± 0.16 |
| Butyric acid (F50) | 1.41 ± 0.19 | 1.40 ± 0.15 |
| 4-hydroxybutyric acid (F51) | 0.49 ± 0.10 | ND |
| Hexanoic acid (F52) | 2.40 ± 0.29 | 1.78 ± 0.16 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, L.; Pei, J.; Wang, X.; Guo, S.; Guo, X.; Yan, P. Effect of Lipids in Yak Muscle under Different Feeding Systems on Meat Quality Based on Untargeted Lipidomics. Animals 2022, 12, 2814. https://doi.org/10.3390/ani12202814
Xiong L, Pei J, Wang X, Guo S, Guo X, Yan P. Effect of Lipids in Yak Muscle under Different Feeding Systems on Meat Quality Based on Untargeted Lipidomics. Animals. 2022; 12(20):2814. https://doi.org/10.3390/ani12202814
Chicago/Turabian StyleXiong, Lin, Jie Pei, Xingdong Wang, Shaoke Guo, Xian Guo, and Ping Yan. 2022. "Effect of Lipids in Yak Muscle under Different Feeding Systems on Meat Quality Based on Untargeted Lipidomics" Animals 12, no. 20: 2814. https://doi.org/10.3390/ani12202814
APA StyleXiong, L., Pei, J., Wang, X., Guo, S., Guo, X., & Yan, P. (2022). Effect of Lipids in Yak Muscle under Different Feeding Systems on Meat Quality Based on Untargeted Lipidomics. Animals, 12(20), 2814. https://doi.org/10.3390/ani12202814






