Condensed and Hydrolyzable Tannins for Reducing Methane and Nitrous Oxide Emissions in Dairy Manure—A Laboratory Incubation Study †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Fresh Dairy Manure Substrate Preparation
2.3. Gas Production Analysis
2.4. Sampling and DNA Extraction from In Vitro Dairy Cattle Manure
2.5. Manure Chemical Analysis
2.6. Statistical Analyses
3. Results and Discussions
3.1. Ambient Temperature, Chemical Composition of Dairy Cattle Manure
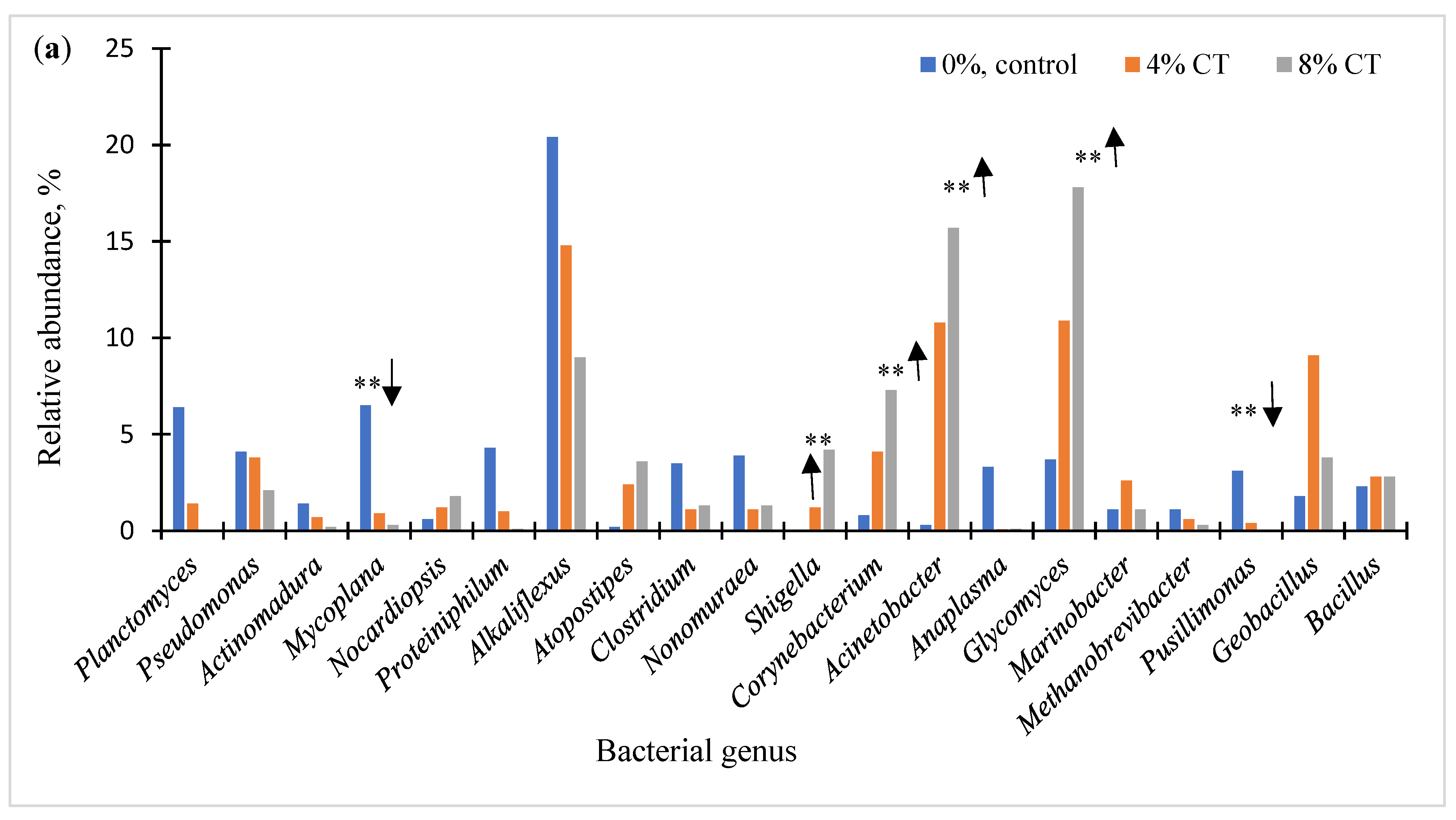
3.2. Microbial Communities of Dairy Manure
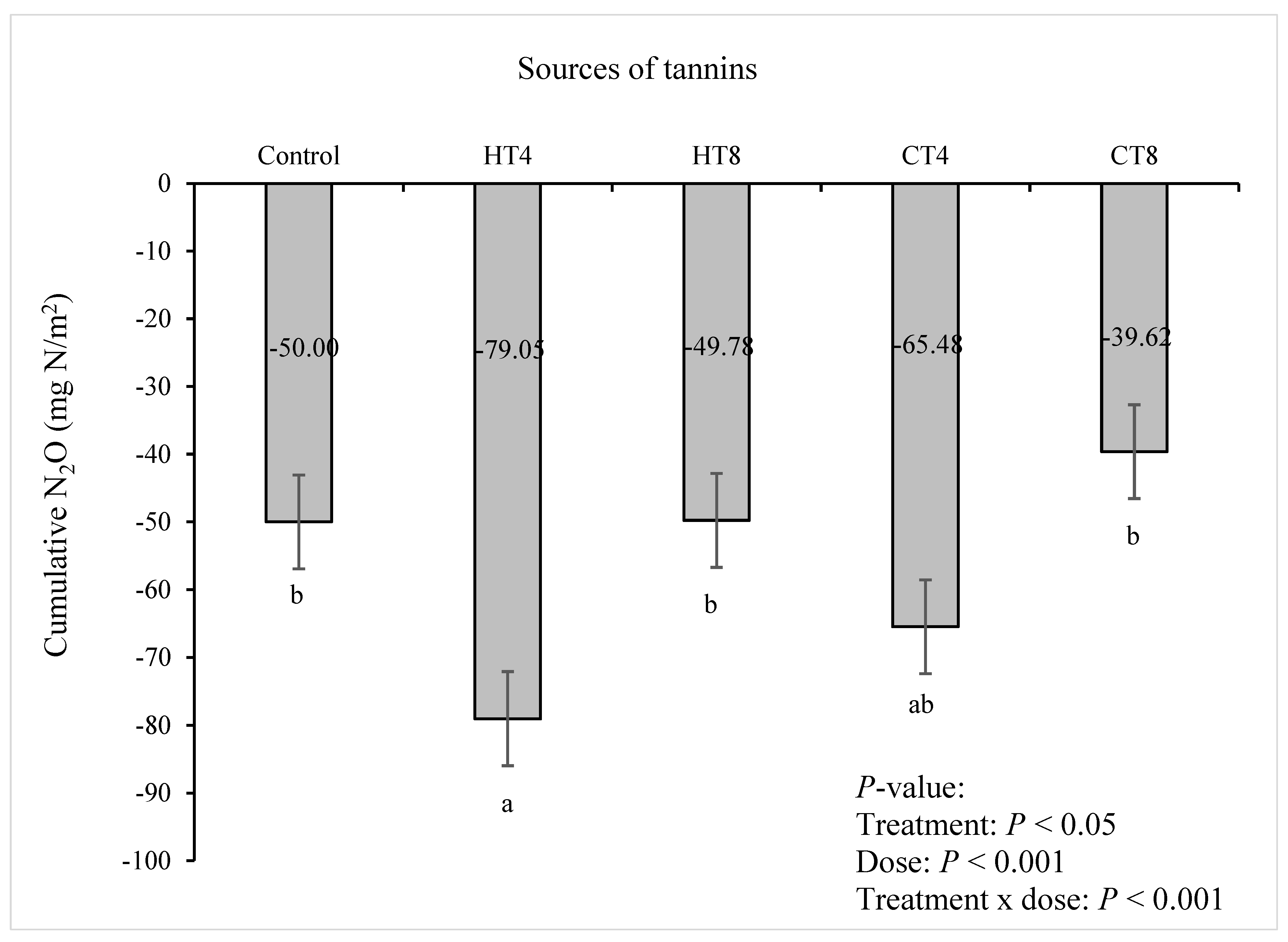
3.3. Rates of Emissions and Cumulative Production of Methane (CH4) and Nitrous Oxide (N2O)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Mara, F.P. The significance of livestock as a contributor to global greenhouse gas emissions today and in the near future. Anim. Feed Sci. Technol. 2011, 166–167, 7–15. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s long shadow-Environmental issues and options. In Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2006; pp. 10–390. [Google Scholar]
- IPCC (International Panel on Climate Change). The physical science basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 996–997. [Google Scholar]
- EPA (Environmental Protection Agency). Inventory of U.S. Greenhouse Gas Emissions and Sinks. 2020. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks (accessed on 10 September 2021).
- IPCC (International Panel for Climate Change). Climate change 2014: Synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the IPCC; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Min, B.; Barry, T.; Attwood, G.; McNabb, W. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Fulford, J.D.; Puchala, R. Effect of feed additives on in vitro and in vivo rumen characteristics and frothy bloat dynamics in steers grazing wheat pasture. Anim. Feed Sci. Technol. 2005, 123–124, 615–629. [Google Scholar] [CrossRef]
- Kebreab, E.; Feng, X. Strategies to Reduce Methane Emissions from Enteric and Lagoon Sources; California Air Resources Board Project (#17RD018); University of California: Davis, CA, USA, 2021; pp. 1–92. [Google Scholar]
- Waterman, P.G.; Mole, S. Analysis of phenolic. In Plant Metabolites; Blackwell Scientific Publications: London, UK, 1994; p. 238. [Google Scholar]
- Ayres, M.P.; Clausen, T.P.; Maclean, S.E.J.; Redman, A.M.; Reichardt, P.B. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 1997, 78, 1696–1712. [Google Scholar] [CrossRef]
- MacKenzie, M.D.; DeLuca, T.H. Charcoal and shrubs modify soil processes in ponderosa pine forests of western Montana. Plant Soil 2006, 287, 257–266. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Puchala, R.; Min, B.R.; Goetsch, A.L.; Sahlu, T. The effect of a condensed tannin-containing forage on methane emission by goats1. J. Anim. Sci. 2005, 83, 182–186. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S. Comparative aspects of plant tannins on digestive physiology, nutrition and microbial community changes in sheep and goats: A review. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1181–1193. [Google Scholar] [CrossRef]
- Śliwiński, B.; Kreuzer, M.; Sutter, F.; Machmüller, A.; Wettstein, H.-R. Performance, body nitrogen conversion and nitrogen emission from manure of dairy cows fed diets supplemented with different plant extracts. J. Anim. Feed Sci. 2004, 13, 73–91. [Google Scholar] [CrossRef]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Aust. J. Agric. Res. 2005, 56, 961–970. [Google Scholar] [CrossRef]
- Powell, J.; Wu, Z.; Satter, L. Dairy diet effects on phosphorus cycles of cropland. J. Soil Water Conserv. 2001, 56, 22–26. [Google Scholar]
- Min, B.R.; Solaiman, S.; Shange, R.; Eun, J.-S. Gastrointestinal Bacterial and Methanogenic Archaea Diversity Dynamics Associated with Condensed Tannin-Containing Pine Bark Diet in Goats Using 16S rDNA Amplicon Pyrosequencing. Int. J. Microbiol. 2014, 2014, 141909. [Google Scholar] [CrossRef]
- Min, B.R.; Wright, C.; Ho, P.; Eun, J.S.; Gurung, N.; Shange, R. The effect of phytochemical tannins-containing diet on rumen fermentation characteristics and microbial diversity dynamics in goats using 16S rDNA amplicon pyrosequencing. Agric. Food Anal. Bacterial. 2014, 4, 195–211. [Google Scholar]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Jordan, G.; Predotova, M.; Ingold, M.; Goenster, S.; Dietz, H.; Joergensen, R.G.; Buerkert, A. Effects of activated charcoal and tannin added to compost and to soil on carbon dioxide, nitrous oxide and ammonia volatilization. J. Plant Nutr. Soil Sci. 2015, 178, 218–228. [Google Scholar] [CrossRef]
- Smith, A.; Mackie, R.I. Effect of Condensed Tannins on Bacterial Diversity and Metabolic Activity in the Rat Gastrointestinal Tract. Appl. Environ. Microbiol. 2004, 70, 1104–1115. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Anderson, R.C.; Callaway, T. Effect of Tannins on the In Vitro Growth of Escherichia coli O157:H7 and In Vivo Growth of Generic Escherichia coli Excreted from Steers. J. Food Prot. 2007, 70, 543–550. [Google Scholar] [CrossRef]
- Callaway, T.R.; Dowd, S.E.; Edrington, T.S.; Anderson, R.C.; Krueger, N.; Bauer, N.; Kononoff, P.J.; Nisbet, D.J. Evaluation of bacterial diversity in the rumen and feces of cattle fed different levels of dried distillers grains plus solubles using bacterial tag-encoded FLX amplicon pyrosequencing. J. Anim. Sci. 2010, 88, 3977–3983. [Google Scholar] [CrossRef]
- Waldrip, H.; Parker, D.; Miller, S.; Miller, D.; Casey, K.; Todd, R.; Min, B.; Spiehs, M.; Woodbury, B. Nitrous Oxide from Beef Cattle Manure: Effects of Temperature, Water Addition and Manure Properties on Denitrification and Nitrification. Atmosphere 2020, 11, 1056. [Google Scholar] [CrossRef]
- Dowd, S.E.; Sun, Y.; Wolcott, R.D.; Domingo, A.; Carroll, J.A. Bacterial Tag–Encoded FLX Amplicon Pyrosequencing (bTEFAP) for Microbiome Studies: Bacterial Diversity in the Ileum of Newly Weaned Salmonella-Infected Pigs. Foodborne Pathog. Dis. 2008, 5, 459–472. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis: Part 3-Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Meeker, E.W.; Wagner, E.C. Titration of Ammonia in Presence of Boric Acid. Ind. Eng. Chem. Anal. Ed. 1933, 5, 396–398. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Inventory of U.S. Greenhouse Gas Emissions And Sinks: 1990–2008; USEPA: Washington, DC, USA, 2010. Available online: https://www.epa.gov/climatechange/emissions/usinventoryreport.html (accessed on 27 September 2020).
- U.S. Environmental Protection Agency (USEPA). Methods for Chemical Analysis of Water and Wastes; Publ. 600/4-79-020, rev. Mar. 1983; Environmental Monitoring and Support Lab., U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1979.
- Black, C.A. Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- NBS (National Bureau of Standards). Standard Reference Material Catalog, 1986–1987, Special Publication 260; Seward, R.W., Ed.; U.S. Department of Commerce National Bureau of Standards: Gaithersburg, MD, USA, 1986; pp. 1–154, NBS Special Publication 260.
- Parker, D.; Casey, K.; Todd, R.W.; Waldrip, H.M.; Marek, G.M.; Auvermann, B.W.; Marek, T.H.; Webb, K.; Willis, W.M.; Pemberton, B.; et al. Improved Chamber Systems for Rapid, Real-Time Nitrous Oxide Emissions from Manure and Soil. Trans. ASABE 2017, 60, 1235–1258. [Google Scholar] [CrossRef]
- Parker, D.B.; Waldrip, H.M.; Casey, K.; Todd, R.W.; Willis, W.M.; Webb, K. Temporal Nitrous Oxide Emissions from Beef Cattle Feedlot Manure after a Simulated Rainfall Event. J. Environ. Qual. 2017, 46, 733–740. [Google Scholar] [CrossRef]
- The SAS system for Windows; Release 9.4.; SAS Institute: Cary, NC, USA, 2013.
- Joanisse, G.D.; Bradley, R.L.; Preston, C.M.; Munson, A.D. Soil enzyme inhibition by condensed litter tannins may drive ecosystem structure and processes: The case of Kalmia angustifolia. New Phytol. 2007, 175, 535–546. [Google Scholar] [CrossRef]
- Mutabaruka, R.; Hairiah, K.; Cadisch, G. Microbial degradation of hydrolysable and condensed tannin polyphenol–protein complexes in soils from different land-use histories. Soil Biol. Biochem. 2007, 39, 1479–1492. [Google Scholar] [CrossRef]
- Komolong, M.; Barber, D.; McNeill, D. Post-ruminal protein supply and N retention of weaner sheep fed on a basal diet of lucerne hay (Medicago sativa) with increasing levels of quebracho tannins. Anim. Feed Sci. Technol. 2001, 92, 59–72. [Google Scholar] [CrossRef]
- Al-Dobaib, S.N. Effect of different levels of Quebracho tannin on nitrogen utilization and growth performance of Najdi sheep fed alfalfa (Medicago sativa) hay as a sole diet. Anim. Sci. J. 2009, 80, 532–541. [Google Scholar] [CrossRef]
- Hao, X.; Benke, M.B. Nitrogen transformation and losses during composting and mitigation strategies. In Dynamic Soil, Dynamic Plant 2 (Special issue 1); Agriculture and Agri-Food Canada, Lethbridge Research and Development Center: Lethbridge, AB, Canada, 2008; pp. 10–18. [Google Scholar]
- García-Amado, M.A.; Godoy-Vitorino, F.; Piceno, Y.M.; Tom, L.M.; Andersen, G.L.; Herrera, E.A.; Domínguez-Bello, M.G. Bacterial Diversity in the Cecum of the World’s Largest Living Rodent (Hydrochoerus hydrochaeris). Microb. Ecol. 2011, 63, 719–725. [Google Scholar] [CrossRef]
- Barker, C.J.; Gillett, A.; Polkinghorne, A.; Timms, P. Investigation of the koala (Phascolarctos cinereus) hindgut microbiome via 16S pyrosequencing. Veter-Microbiol. 2013, 167, 554–564. [Google Scholar] [CrossRef]
- Liu, X.-L.; Hao, Y.-Q.; Jin, L.; Xu, Z.-J.; McAllister, T.A.; Wang, Y. Anti-Escherichia coli O157:H7 Properties of Purple Prairie Clover and Sainfoin Condensed Tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.J.; Blackall, L.L.; Klieve, A.V. Rumen bacterial diversity with and without mulga (Acacia anuera) tannins. In Tannins in Livestock and Human Nutrition, Proceedings of the an International Workshop, Adelaide, Australia, 31 May–2 June, 1999; Brooker, J.D., Ed.; ACIAR Proceedings No. 92; Australian Centre for International Agricultural Research: Canberra, Australia, 2000. [Google Scholar]
- Okubo, T.; Ishihara, N.; Oura, A.; Serit, M.; Kim, M.; Yamamoto, T.; Mitsuoka, T. In vivo effects of tea polyphenol intake on human intestinal microflora and metabolism. Biosci. Biotechnol. Biochem. 1992, 56, 588–591. [Google Scholar] [CrossRef]
- Hara, H.; Orita, N.; Hatano, S.; Ichikawa, H.; Hara, Y.; Matsumoto, N.; Kimura, Y.; Terada, A.; Mitsuoka, T. Effect of Tea Polyphenols on Fecal Flora Metabolic Products of Pigs. J. Veter-Med Sci. 1995, 57, 45–49. [Google Scholar] [CrossRef]
- Hara, Y. Influence of tea catechins on the digestive tract. J. Cell Biochem. 1997, 27, 52–58. [Google Scholar] [CrossRef]
- Brooker, J.; O’Donovan, L.; Skene, I.; Clarke, K.; Blackall, L.; Muslera, P. Streptococcus caprinus sp.nov., a tannin-resistant ruminal bacterium from feral goats. Lett. Appl. Microbiol. 1994, 18, 313–318. [Google Scholar] [CrossRef]
- Nelson, K.E.; Thonney, M.L.; Woolston, T.K.; Zinder, S.H.; Pell, A.N. Phenotypic and Phylogenetic Characterization of Ruminal Tannin-Tolerant Bacteria. Appl. Environ. Microbiol. 1998, 64, 3824–3830. [Google Scholar] [CrossRef]
- Wiryawan, K.G.; Tangendjaja, B.; Suryahadi, A. Tannin degrading bacteria from Indonesian ruminants. In Tannins in Livestock and Human Nutrition, Proceedings of the an International Workshop, Adelaide, Australia, 31 May–2 June, 1999; Brooker, J.D., Ed.; ACIAR Proceedings No. 92; Australian Centre for International Agricultural Research: Adelaide, Australia, 2000; pp. 123–126. [Google Scholar]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Molan, A.L.; Attwood, G.T.; Min, B.R.; McNabb, W.C. The effect of condensed tannins fromLotus pedunculatusandLotus corniculatuson the growth of proteolytic rumen bacteria in vitro and their possible mode of action. Can. J. Microbiol. 2001, 47, 626–633. [Google Scholar] [CrossRef]
- Min, B.R.; Attwood, G.T.; McNabb, W.C.; Molan, A.L.; Barry, T.N. The effect of condensed tannins from Lotus corniculatus on the proteolytic activities and growth of rumen bacteria. Anim. Feed Sci. Technol. 2005, 121, 45–58. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Banso, A.; Adeyemo, S.O. Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. Afr. J. Biotechnol. 2010, 6, 1785–1787. Available online: http://www.academicjournals.org/AJB (accessed on 20 March 2020). [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Yakoob, R.; Pradeep, B. Bifidobacterium sp as Probiotic Agent—Roles and Applications. J. Pure Appl. Microbiol. 2019, 13, 1407–1417. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry 2010, 71, 1198–1222. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Waldrip, H.M.; Parker, D.; Todd, R.W.; Brauer, D. Dietary mitigation of enteric methane emissions from ruminants: A review of plant tannin mitigation options. Anim. Nutr. 2020, 6, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef]
- Haslam, E. Plant polyphenols. In Vegetable Tannins Revisited; Haslam, E., Ed.; Cambridge University Press: Cambridge, UK, 1989; pp. 14–130. [Google Scholar]
- Haslam, E. Gallic acid derivatives and hydrolysable tannins. In Natural Products of Woody Plants; Rowe, J.W., Ed.; Springer: Berlin, Germany, 1989; pp. 399–438. [Google Scholar]
- Engström, M.T.; Arvola, J.; Nenonen, S.; Virtanen, V.T.J.; Leppä, M.M.; Tähtinen, P.; Salminen, J.-P. Structural Features of Hydrolyzable Tannins Determine Their Ability to Form Insoluble Complexes with Bovine Serum Albumin. J. Agric. Food Chem. 2019, 67, 6798–6808. [Google Scholar] [CrossRef]
- Puljula, E.; Walton, G.E.; Woodward, M.J.; Karonen, M. Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef]
- Paul, J.W.; Beauchamp, E.G.; Zhang, X. Nitrous and nitric oxide emissions during nitrification and denitrification from manure-amended soil in the laboratory. Can. J. Soil Sci. 1993, 73, 539–553. [Google Scholar] [CrossRef]
- Møller, H.; Sommer, S.; Ahring, B. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 2004, 26, 485–495. [Google Scholar] [CrossRef]
- Tisdale, S.L.; Nelson, W.L.; Beaton, J.D.; Havlin, J.L. Soil acidity and basicity. In Soil Fertility and Fertilizers, 5th ed.; Tisdale, S.L., Nelson, W.L., Beaton, J.D., Havlin, J.L., Eds.; Macmillian Publishing: New York, NY, USA, 1993; pp. 10–150. [Google Scholar]
- Montes, F.; Meinen, R.; Dell, C.; Rotz, A.; Hristov, A.N.; Oh, J.; Waghorn, G.; Gerber, P.J.; Henderson, B.; Makkar, H.P.S.; et al. Mitigation of methane and nitrous oxide emissions from animal operations: II. A review of manure management mitigation options1. J. Anim. Sci. 2013, 91, 5070–5094. [Google Scholar] [CrossRef]
- Maeda, K.; Toyoda, S.; Shimojima, R.; Osada, T.; Hanajima, D.; Morioka, R.; Yoshida, N. Source of Nitrous Oxide Emissions during the Cow Manure Composting Process as Revealed by Isotopomer Analysis of and amoA Abundance in Betaproteobacterial Ammonia-Oxidizing Bacteria. Appl. Environ. Microbiol. 2010, 76, 1555–1562. [Google Scholar] [CrossRef]
- Whitehead, T.R.; Spence, C.; Cotta, M. Inhibition of hydrogen sulfide, methane, and total gas production and sulfate-reducing bacteria in in vitro swine manure by tannins, with focus on condensed quebracho tannins. Appl. Microbiol. Biotechnol. 2012, 97, 8403–8409. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, M. Tannins: Their adverse role in ruminant nutrition. J. Agric. Food Chem. 1984, 32, 447–453. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. Sci. Food Agric. 2012, 93, 332–339. [Google Scholar] [CrossRef]
- Pellikaan, W.F.; Stringano, E.; Leenaars, J.; Bongers, D.J.; Schuppen, S.V.; Plant, J.; Mueller-Harvey, I. Evaluating effects of tannins on extent and rate of in vitro gas and CH4 production using an automated pressure evaluation system (APES). Anim. Feed Sci. Technol. 2011, 166–167, 377–390. [Google Scholar] [CrossRef]
- Jones, G.A.; McAllister, T.A.; Muir, A.D.; Cheng, K.-J. Effects of Sainfoin (Onobrychis viciifolia Scop.) Condensed Tannins on Growth and Proteolysis by Four Strains of Ruminal Bacteria. Appl. Environ. Microbiol. 1994, 60, 1374–1378. [Google Scholar] [CrossRef]
- Field, J.; Kortekaas, S.; Lettinga, G. The tannin theory of methanogenic toxicity. Biol. Wastes 1989, 29, 241–262. [Google Scholar] [CrossRef]
- Cieslak, A.; Zmora, P.; Pers-Kamczyc, E.; Szumacher-Strabel, M. Effects of tannins source (Vaccinium vitis idaea L.) on rumen microbial fermentation in vivo. Anim. Feed Sci. Technol. 2012, 176, 102–106. [Google Scholar] [CrossRef]
- Vasta, V.; Makkar, H.P.S.; Mele, M.; Priolo, A. Ruminal biohydrogenation as affected by tanninsin vitro. Br. J. Nutr. 2008, 102, 82–92. [Google Scholar] [CrossRef]
- Bae, H.D.; McAllister, T.A.; Yanke, J.; Cheng, K.-J.; Muir, A.D. Effects of Condensed Tannins on Endoglucanase Activity and Filter Paper Digestion by Fibrobacter succinogenes S85. Appl. Environ. Microbiol. 1993, 59, 2132–2138. [Google Scholar] [CrossRef]
- Mole, S.; Waterman, P.G. Stimulatory effects of tannins and cholic acid on tryptic hydrolysis of proteins: Ecological implications. J. Chem. Ecol. 1985, 11, 1323–1332. [Google Scholar] [CrossRef]
- Fillingham, M.A.; VanderZaag, A.C.; Burtt, S.; Balde, H.; Ngwabie, N.M.; Smith, W.; Hakami, A.; Wagner-Riddle, C.; Bittman, S.; MacDonald, D. Greenhouse gas and ammonia emissions from production of compost bedding on a dairy farm. Waste Manag. 2017, 70, 45–52. [Google Scholar] [CrossRef]
- Aboagye, I.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet1,2. J. Anim. Sci. 2018. [CrossRef]
- Deaville, E.R.; Green, R.; Mueller-Harvey, I.; Willoughby, A.I.; Frazier, R. Hydrolyzable Tannin Structures Influence Relative Globular and Random Coil Protein Binding Strengths. J. Agric. Food Chem. 2007, 55, 4554–4561. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Aguerre, M.; Wattiaux, M.; Powell, J. Emissions of ammonia, nitrous oxide, methane, and carbon dioxide during storage of dairy cow manure as affected by dietary forage-to-concentrate ratio and crust formation. J. Dairy Sci. 2012, 95, 7409–7416. [Google Scholar] [CrossRef]
- Bergstermann, A.; Cárdenas, L.; Bol, R.; Gilliam, L.; Goulding, K.; Meijide, A.; Scholefield, D.; Vallejo, A.; Well, R. Effect of antecedent soil moisture conditions on emissions and isotopologue distribution of N2O during denitrification. Soil Biol. Biochem. 2011, 43, 240–250. [Google Scholar] [CrossRef]
- Hwang, S.; Hanaki, K. Effects of oxygen concentration and moisture content of refuse on nitrification, denitrification and nitrous oxide production. Bioresour. Technol. 2000, 71, 159–165. [Google Scholar] [CrossRef]
- Khalil, K.; Mary, B.; Renault, P. Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biol. Biochem. 2004, 36, 687–699. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Parker, D.B.; Casey, K.; Waldrip, H.M.; Min, B.R.; Woodbury, B.L.; Spiehs, M.J.; Willis, W. Nitrous Oxide Emissions from an Open-Lot Beef Cattle Feedyard in Texas. Trans. ASABE 2019, 62, 1173–1183. [Google Scholar] [CrossRef]
- Sepperer, T.; Tondi, G.; Petutschnigg, A.; Young, T.M.; Steiner, K. Mitigation of Ammonia Emissions from Cattle Manure Slurry by Tannins and Tannin-Based Polymers. Biomolecules 2020, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Chotte, J.-L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Norris, C.E.; Preston, C.M.; Hogg, K.E.; Titus, B.D. The Influence of Condensed Tannin Structure on Rate of Microbial Mineralization and Reactivity to Chemical Assays. J. Chem. Ecol. 2011, 37, 311–319. [Google Scholar] [CrossRef]
- Northup, R. Footprint in the Landscape: Sustainability Through Plant and Soil Science. In Proceedings of the 2009 International Annual Meetings of ASA-CSSA-SSSA, 1–5 November 2009. [Google Scholar]
- Barnard, R.; Leadley, P.W.; Hungate, B.A. Global change, nitrification and denitrification: A review. Glob. Biogeochem. Cycles 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]





| Quebracho CT | Chestnut HT | ||||
|---|---|---|---|---|---|
| Item | Control | 4% CT | 8% CT | 4% HT | 8% HT |
| Chemical composition at day 0 | |||||
| Moisture, % | 62.7 | 61.9 | 61.9 | 62.3 | 59.8 |
| Total solids, as-is basis | 37.3 | 38.1 | 38.1 | 37.7 | 40.2 |
| ------------------------- % DM ------------------------------ | |||||
| Ash, % DM | 7.8 | 6.9 | 7.1 | 7.5 | 7.5 |
| Organic matter, % DM | 29.5 | 31.2 | 31.0 | 30.2 | 32.8 |
| Total N, % DM | 9.4 | 8.5 | 8.4 | 9.1 | 8.5 |
| Organic-N, % DM | 9.2 | 8.5 | 8.3 | 9.1 | 8.5 |
| C/N ratio | 18.3 | 21.3 | 21.4 | 19.2 | 22.2 |
| Ammonium–N, mg/kg | 0.02 | 0.03 | 0.06 | 0.06 | 0.03 |
| Nitrate-N, mg/kg | 10.9 | 10.9 | 10.0 | 10.8 | 10.9 |
| Nitrite–N, mg/kg | 1.0 | 1.0 | 1.0 | 0.9 | 0.9 |
| Nitrate-N + Nitrite-N, mg/kg | 11.9 | 11.9 | 11.0 | 11.7 | 10.9 |
| pH | 8.8 | 8.5 | 8.5 | 7.8 | 7.1 |
| Chemical composition at day 14 | ------------------------- % DM ---------------------------- | ||||
| Moisture, % | 29.5 | 23.6 | 26.4 | 30.7 | 31.8 |
| Total N, % DM | 1.8 | 1.7 | 1.7 | 1.9 | 1.6 |
| Organic-N, % DM | 1.8 | 1.7 | 1.6 | 1.8 | 1.6 |
| Ammonium-N, mg/kg | 0.19 | 0.42 | 0.72 | 0.25 | 0.25 |
| Nitrate-N, mg/kg | 0.63 | 0.73 | 0.65 | 0.71 | 0.71 |
| Nitrite-N, mg/kg | 1.0 | 1.0 | 1.0 | 0.9 | 0.9 |
| Nitrate-N + Nitrite-N, mg/kg | 1.63 | 1.73 | 1.65 | 1.61 | 1.61 |
| N loss or retention | ------------------------- % ------------------------------- | ||||
| Total N | −80.9 | −80.0 | −79.8 | −79.1 | −81.2 |
| Organic-N | −80.4 | −80.0 | −80.7 | −80.2 | −81.1 |
| Ammonium-N | 89.4 | 92.9 | 91.7 | 76.0 | 88.0 |
| Nitrate-N | −94.2 | −93.3 | −95.7 | −93.4 | −98.7 |
| Nitrite-N | 0 | 0 | 0 | 0 | 0 |
| Nitrate-N + Nitrite-N | −86.3 | −85.5 | −85.0 | −86.2 | −85.2 |
| Item | Bacterial Phylum, % | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | CT, % | HT, % | |||||||
| 0.0 | 4.0 | 8.0 | 4.0 | 8.0 | SEM | Tannins | Dose | Interaction | |
| Bacteroidetes | 28.8 a | 18.9 ab | 10.5 b | 30.4 a | 9.4 b | 4.78 | 0.04 | 0.03 | 0.13 |
| Firmicutes | 14.1 | 24.9 | 27.0 | 17.6 | 28.4 | 5.54 | 0.12 | 0.36 | 0.60 |
| Proteobacteria | 32.8 | 29.0 | 28.4 | 21.1 | 23.6 | 4.30 | 0.09 | 0.86 | 0.93 |
| Actinobacteria | 13.7 b | 23.6 ab | 32.6 a | 22.2 ab | 33.6 a | 4.09 | 0.01 | 0.05 | 0.35 |
| Planctomycetes | 7.0 | 1.4 | 0.1 | 6.5 | 1.0 | 3.45 | 0.23 | 0.43 | 0.72 |
| Euryarchaeota | 1.1 | 0.6 | 0.4 | 0.5 | 1.5 | 0.76 | 0.67 | 0.67 | 0.71 |
| Chloroflexi | 1.3 | 0.8 | 0.4 | 1.0 | 1.0 | 0.57 | 0.50 | 0.80 | 0.94 |
| Item Phylum/Species | Bacterial Species, % | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | CT, % | HT, % | |||||||
| 0.0 | 4.0 | 8.0 | 4.0 | 8.0 | SEM | Tannins | Dose | Interaction | |
| Firmicutes, % | |||||||||
| Bacillus spp. | 4.3 | 5.1 | 4.9 | 8.2 | 1.8 | 3.19 | 0.96 | 0.41 | 0.54 |
| Clostridium spp. | 3.4 a | 1.0 b | 1.2 b | 1.1 b | 2.6 a | 0.47 | 0.01 | 0.17 | 0.25 |
| Bacteriodetes, % | |||||||||
| Proteiniphilum spp. | 4.3 a | 1.1 b | 0.1 b | 6.4 a | 1.5 b | 0.86 | 0.001 | 0.01 | 0.03 |
| Alkaliflexus sp. | 20.4 a | 14.8 a | 9.0 b | 20.6 a | 4.2 b | 4.15 | 0.11 | 0.05 | 0.17 |
| Proteobacteria, % | |||||||||
| Pusillimonas noertemannii | 2.0 a | 0.09 b | 0.01 b | 0.1 b | 0.04 b | 0.54 | 0.01 | 0.92 | 0.99 |
| Pseudomonas tuomuerense | 2.1 | 0.3 | 0.2 | 1.0 | 1.9 | 0.76 | 0.08 | 0.66 | 0.78 |
| Shigella sonnei | 0.05 b | 1.2 ab | 4.2 a | 0.1 ab | 2.4 a | 0.86 | 0.03 | 0.02 | 0.23 |
| Idiomarina indica | 2.5 a | 0.7 b | 0.01 b | 0.06 b | 0.02 b | 0.15 | 0.001 | 0.08 | 0.09 |
| Actinobacteria, % | |||||||||
| Nonomuraea sp. | 3.8 a | 0.3 b | 0.05 b | 5.4 a | 0.7 b | 0.66 | 0.001 | 0.01 | 0.01 |
| Glycomyces sp. | 3.5 | 5.4 | 5.9 | 4.4 | 3.6 | 1.88 | 0.40 | 0.74 | 0.94 |
|
Bifidobacterium choerinum | 0.02 b | 0.5 ab | 1.0 a | 0.04 b | 0.6 ab | 0.32 | 0.05 | 0.19 | 0.63 |
| Tenericutes, % | |||||||||
| Mycoplana spp. | 6.5 a | 0.9 b | 0.3 b | 3.4 a | 0.8 b | 2.23 | 0.05 | 0.56 | 0.83 |
| Corynebacterium sp. | 0.5 c | 2.0 b | 5.0 a | 0.5 c | 2.9 b | 1.19 | 0.05 | 0.12 | 0.53 |
| Planctomycetes, % | |||||||||
| Planctomyces spp. | 6.5 | 1.4 | 0.03 | 6.4 | 0.9 | 3.23 | 0.25 | 0.40 | 0.68 |
| Euryarchaeota (Archaea), % | |||||||||
| Methanobrevibacter sp. | 1.1 | 0.4 | 0.4 | 0.5 | 0.3 | 0.57 | 0.42 | 0.88 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, B.R.; Willis, W.; Casey, K.; Castleberry, L.; Waldrip, H.; Parker, D. Condensed and Hydrolyzable Tannins for Reducing Methane and Nitrous Oxide Emissions in Dairy Manure—A Laboratory Incubation Study. Animals 2022, 12, 2876. https://doi.org/10.3390/ani12202876
Min BR, Willis W, Casey K, Castleberry L, Waldrip H, Parker D. Condensed and Hydrolyzable Tannins for Reducing Methane and Nitrous Oxide Emissions in Dairy Manure—A Laboratory Incubation Study. Animals. 2022; 12(20):2876. https://doi.org/10.3390/ani12202876
Chicago/Turabian StyleMin, Byeng Ryel, Will Willis, Kenneth Casey, Lana Castleberry, Heidi Waldrip, and David Parker. 2022. "Condensed and Hydrolyzable Tannins for Reducing Methane and Nitrous Oxide Emissions in Dairy Manure—A Laboratory Incubation Study" Animals 12, no. 20: 2876. https://doi.org/10.3390/ani12202876
APA StyleMin, B. R., Willis, W., Casey, K., Castleberry, L., Waldrip, H., & Parker, D. (2022). Condensed and Hydrolyzable Tannins for Reducing Methane and Nitrous Oxide Emissions in Dairy Manure—A Laboratory Incubation Study. Animals, 12(20), 2876. https://doi.org/10.3390/ani12202876





