The Relationship between Burrow Opening Dimensions and Biomass of Intertidal Macroinvertebrates by Feeding Mode (Surface Deposit Feeders vs. Suspension Feeders)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Measurement
2.3. Data Analysis
3. Results
3.1. Macrophthalmus japonicus De Haan, 1835
3.2. Uca arcuata (De Haan, 1835)
3.3. Uca lactea lactea (De Haan, 1835)
3.4. Ocypode stimpsoni Ortmann, 1897
3.5. Urechis unicinctus (Drasche, 1880)
3.6. Upogebia major (De Haan, 1841)
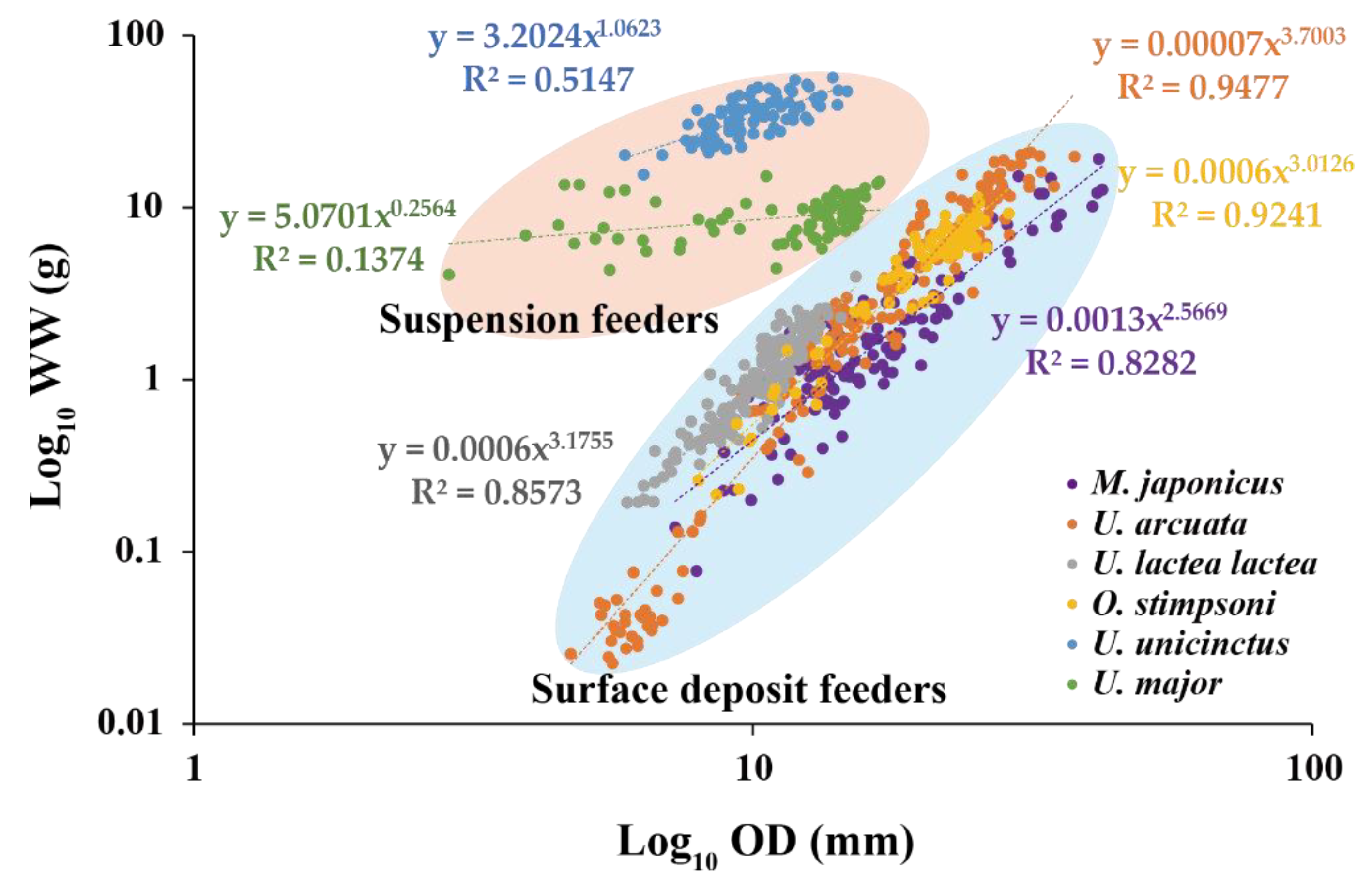
3.7. Comparison between Surface Deposit Feeders and Suspension Feeders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padedda, B.M.; Pulina, S.; Satta, C.T.; Lugliè, A.; Magni, P. Eutrophication and nutrient fluxes in Mediterranean coastal lagoons. In Encyclopedia of Water; Maurice, P., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 1–16. [Google Scholar]
- Painter, S.C.; Lapworth, D.J.; Woodward, E.M.S.; Kroeger, S.; Evans, C.D.; Mayor, D.J.; Sanders, R.J. Terrestrial dissolved organic matter distribution in the North Sea. Sci. Total Environ. 2018, 630, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.J.; Ryu, J.H. Research on the Tidal Flats with Artificial Intelligence and Drones; The library of Dreams for Future, Jisungsa Publishing Co.: Seoul, Korea, 2022; Volume 44, ISBN 978-89-7889-507-1. [Google Scholar]
- Shortis, M. Calibration techniques for accurate measurements by underwater camera systems. Sensors 2015, 15, 30810–30826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlacher, T.A.; Lucrezi, S.; Peterson, C.H.; Connolly, R.M.; Olds, A.D.; Althaus, F.; Hyndes, G.A.; Maslo, B.; Gilby, B.L.; Leon, J.X.; et al. Estimating animal populations and body sizes from burrows: Marine ecologists have their heads buried in the sand. J. Sea Res. 2016, 112, 55–64. [Google Scholar] [CrossRef]
- Kristensen, E.; Kostka, J.E. Macrofaunal burrows and irrigation in marine sediment: Microbiological and biogeochemical interactions. In Interaction between Macro- and Microorganisms in Marine Sediments; Kristensen, E., Haese, R.R., Kostka, J.E., Eds.; American Geophysical Union: Washington, DC, USA, 2005; Volume 60, pp. 125–157. [Google Scholar]
- Koo, B.J. Burrows of Macroinvertebrates in the Korean Tidal Flats; KIOST: Ansan, Korea, 2017; ISBN 978-89-444-9060-6. [Google Scholar]
- Leme, M.H.A. A comparative analysis of the population biology of the mangrove crabs Aratus pisonii and Sesarma rectum (Brachyura, Grapsidae) from the north coast of Sao Paulo State, Brazil. J. Crustac. Biol. 2002, 22, 553–557. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.J.; Kwon, K.K.; Hyun, J.H. Effect of environmental conditions on variation in the sediment-water interface created by complex macrofaunal burrows on a tidal flat. J. Sea Res. 2007, 58, 302–312. [Google Scholar] [CrossRef]
- Butler, S.; Bird, F.L. Estimating density of intertidal ghost shrimps using counts of burrow openings. Is the method reliable? Hydrobiologia 2007, 589, 303–314. [Google Scholar] [CrossRef]
- Campbell, L.; Wood, L.; Gerwing, A.M.A.; Allen, S.; Sizmur, T.; Rogers, M.; Gray, O.; Drewes, M.; Juanes, F.; Gerwing, T.G. A rapid, non-invasive population assessment technique for marine burrowing macrofauna inhabiting soft sediments. Estuar. Coast. Shelf Sci. 2019, 227, 106343. [Google Scholar] [CrossRef]
- Moh, H.H.; Chong, V.C.; Sasekumar, A. Distribution and burrow morphology of three sympatric species of Thalassina mud lobsters in relation to environmental parameters on a Malayan mangrove shore. J. Sea Res. 2015, 95, 75–83. [Google Scholar] [CrossRef]
- Edgwa, R.; Shama, S.; Nadaoka, K.; Mackenzie, R. Burrow dynamics of crabs in subtropical estuarine mangrove forest. Estuar. Coast. Shelf Sci. 2021, 252, 107244. [Google Scholar]
- Min, W.W.; Kathiresan, K. Burrow morphologies, crab characteristics and soil properties in different seasons across intertidal areas of a restored mangrove forest. J. Sea Res. 2021, 177, 102111. [Google Scholar] [CrossRef]
- He, W.; Fang, J.; Zhang, Y.; Yan, Y.; Zhang, Z.; Huang, J.; Fang, J.; Mao, Y.; Jiang, Z.; Wang, J. Factors affecting burrow architecture of the polychaete Perinereis aibuhitensis. Front. Mar. Sci. 2022, 9, 893539. [Google Scholar] [CrossRef]
- Koo, B.J.; Kwon, K.K.; Hyun, J.H. The sediment-water interface increment due to the complex burrows of macrofauna in a tidal flat. Ocean Sci. J. 2005, 40, 221–227. [Google Scholar] [CrossRef]
- Nickell, L.A.; Atkinson, R.J.A. Functional morphology of burrows and trophic modes of three thalassinidean shrimp species, and a new approach to the classification of thalassinidean burrow morphology. Mar. Ecol. Prog. Ser. 1995, 128, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, E.T. Contribuição para o Conhecimento da Biologia do Caranguejo Fantasma (Ocypode cursor L.) na ilha de Boavista (Cabo Verde): Densidade Edistribuição Espacial. Relatório de estágio Do Curso de Licenciatura Em Biologia Marinha e Pescas; DECM; Universidade de Cabo Verde: Mindelo, Cape Verde, 2012. [Google Scholar]
- Wolcott, T.G. Ecological role of ghost crabs, Ocypode quadrata (Fab.) on an ocean beach: Scavengers or predators? J. Exp. Mar. Biol. Ecol. 1978, 31, 67–82. [Google Scholar] [CrossRef]
- Mouton, E.C., Jr.; Felder, D.L. Burrow distributions and population estimates for the fiddler crabs Uca spinicarpa and Uca longisignalis in a gulf of Mexico salt marsh. Estuaries 1996, 19, 51–61. [Google Scholar] [CrossRef]
- Skov, M.W.; Hartnoll, R.G. Comparative suitability of binocular observation, burrow counting and excavation for the quantification of the mangrove fiddler crab Uca annulipes (H. Milne Edwards). Hydrobiologia 2001, 449, 201–212. [Google Scholar] [CrossRef]
- Yong, A.Y.P.; Lim, S.S.L.; Kaenphet, A.; Tantichodok, P. Evidence of precision engineering in the excavation of Ocypode ceratophthalmus burrows on the west and east coasts of Thailand. Crustaceana 2011, 84, 749–761. [Google Scholar] [CrossRef]
- Lim, S.S.L.; Yong, A.Y.P. Rapid population recovery of Ocypode ceratophthalmus (Pallas, 1772) (Crustacea: Brachyura: Ocypodidae) after an oil spill in Singapore. Raffles Bull. Zool. 2015, 63, 270–278. [Google Scholar]
- Candisani, L.C.; Sumida, P.Y.G.; Pires-Vanin, A.M.S. Burrow morphology and mating behavior of the thalassinidean shrimp Upogebia noronhensis. J. Mar. Biol. Ass. UK 2001, 81, 799–803. [Google Scholar] [CrossRef]
- Kinoshita, K. Burrow structure of the mud shrimp Upogebia major (Decapoda: Thalassinidea: Upogebiidae). J. Crustacean Biol. 2002, 22, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Carrel, J.E. Ecology of Two Burrowing Wolf Spiders (Araneae: Lycosidae) Syntopic in Florida Scrub: Burrow/Body Size Relationships and Habitat Preferences. J. Kans. Entomol. Soc. 2003, 76, 16–30. [Google Scholar]
- Sample, S.; Albrecht, M. Determination of the burrow shapes of Cardisoma guanhumi on Vieques, Puerto Rico. J. Coast. Life Med. 2016, 4, 94–97. [Google Scholar] [CrossRef]
- Koo, B.J.; Seo, J. Filtration rates of the manila clam, Ruditapes philippinarum, in tidal flats with different hydrographic regimes. PLoS ONE 2020, 15, e0228873. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.O.; Kim, H.C.; Koh, C.H.; Ryu, J.; Son, S.; Kim, Y.H.; Khim, J.S. Development of temperature-based algorithms for the estimation of microphytobenthic primary production in a tidal flat: A case study in Daebu mudflat, Korea. Environ. Pollut. 2018, 241, 115–123. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, I.S.; Choi, M.; Choi, H.G. Distibutions of organic matter and trace metals in sediment around a tidal-flat oyster Crassostrea gigas farming area on the Taean peninsula, Korea. Korean J. Fish. Aquat. Sci. 2014, 47, 1014–1025. [Google Scholar]
- Henmi, Y. The description of wandering behavior and its occurrence varying in different tidal areas in Macrophthalmus japonicus (De Haan) (Crustacea: Ocypodidae). J. Exp. Mar. Biol. Ecol. 1984, 84, 211–224. [Google Scholar] [CrossRef]
- Koo, B.J.; Kim, S.H.; Hyun, J.H. Feeding behavior of the ocypodid crab Macrophthalmus japonicus and its effects on oxygen-penetration depth and organic-matter removal in intertidal sediments. Estuar. Coast. Shelf Sci. 2019, 228, 106366. [Google Scholar] [CrossRef]
- Sharifian, S.; Malekzadeh, V.; Kamrani, E.; Safaie, M. Population structure and morphometric variation in the sand-bubbler crab Scopimera crabricauda (Brachyura: Dotillidae). Anim. Biol. 2017, 67, 319–330. [Google Scholar] [CrossRef]
- Li, N.; Song, S.L.; Tang, Y.Z.; Wang, B.G. Reproductive biology of Urechis unicinctus along the coast of Shandong province. Acta Zool. Sin. 1997, 43, 433–435. [Google Scholar]
- Abe, H.; Sato-Okoshi, W.; Tanake, M.; Okoshi, K.; Teramoto, W.; Kondoh, T.; Nishitani, G.; Endo, Y. Swimming behavior of the spoon worm Urechis unicinctus (Annelida, Echiura). Zoology 2014, 117, 216–223. [Google Scholar] [CrossRef]
- Kornienko, E.S. Burrowing shrimp of the infraorders Gebiidea and Axiidea (Crustacea: Decapoda). Russ. J. Mar. Biol. 2013, 39, 1–14. [Google Scholar] [CrossRef]
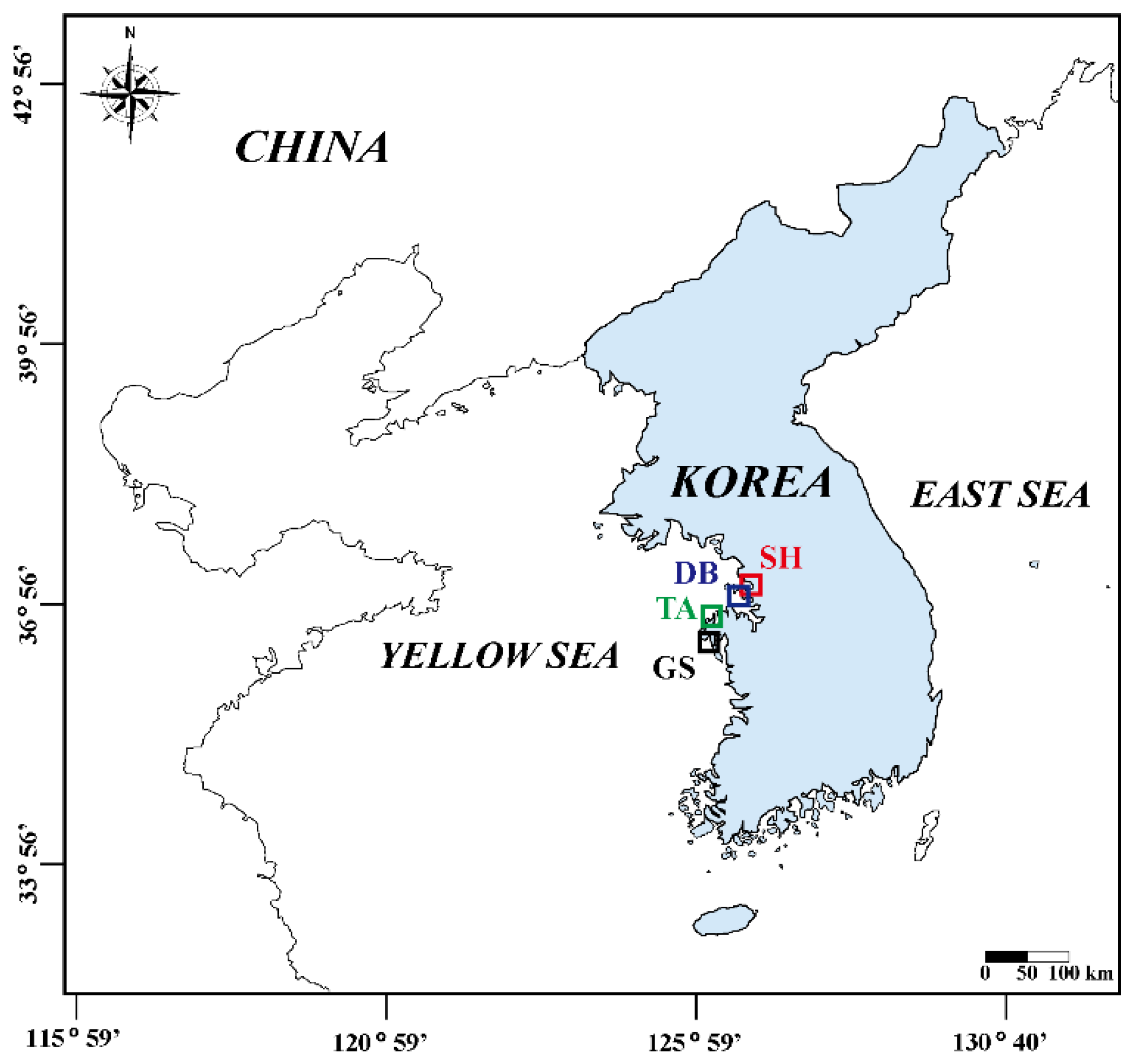

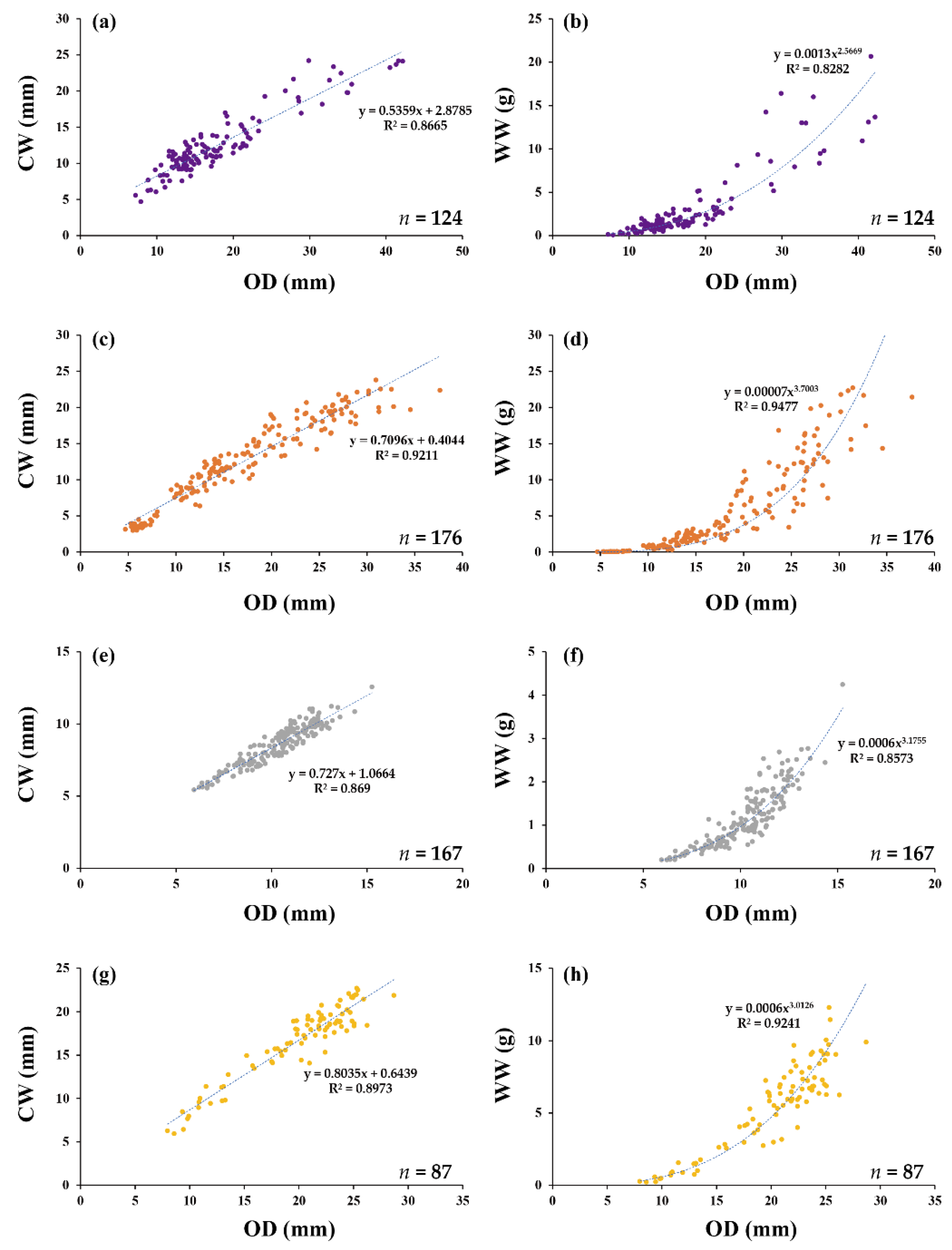
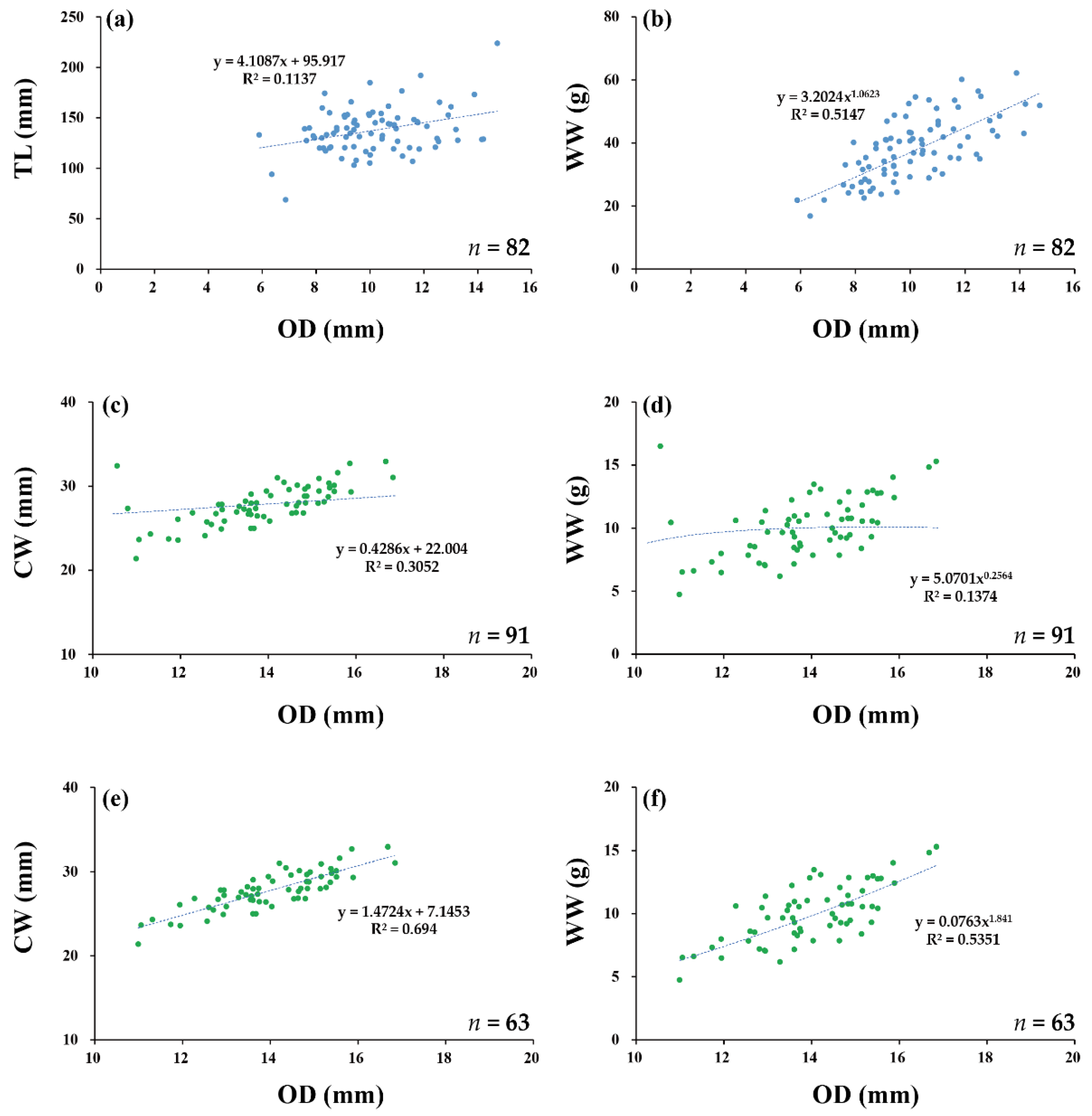

| Species (Family) | FM | SA | NS | Inhabitant Collection (Month) | Measurements | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mar. | Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | BD | Inhabitant | ||||
| M. japonicus (Ocy.) | SDF | SH | 124 | OD | CL, CW, WW, DW, AFDW | |||||||||
| U. arcuata (Ocy.) | SDF | DB | 176 | OD | CL, CW, WW, DW, AFDW | |||||||||
| U. lactea lactea (Ocy.) | SDF | DB | 167 | OD | CL, CW, WW, DW, AFDW | |||||||||
| O. stimpsoni (Ocy.) | SDF | TA | 87 | OD | CL, CW, WW, DW, AFDW | |||||||||
| U. unicinctus (Ure.) | SF | TA | 82 | OD | TL, WW, DW, AFDW | |||||||||
| U. major (Upo.) | SF | GS | 91 | OD | CW, TL, WW, DW, AFDW | |||||||||
| Species | NS | BD | Inhabitant | |||||
|---|---|---|---|---|---|---|---|---|
| OD (mm) | CL (mm) | CW (mm) | TL (mm) | WW (g) | DW (g) | AFDW (g) | ||
| M. japonicus | 124 | 17.90 ± 7.46 | 18.26 ± 6.55 | 12.47 ± 4.30 | nm | 3.03 ± 3.87 | 0.82 ± 1.14 | 0.39 ± 0.52 |
| U. arcuata | 176 | 17.11 ± 7.95 | 20.04 ± 9.55 | 12.55 ± 5.88 | nm | 5.35 ± 6.02 | 1.44 ± 1.64 | 0.68 ± 0.74 |
| U. lactea | 167 | 10.26 ± 1.79 | 13.02 ± 2.24 | 8.52 ± 1.40 | nm | 1.19 ± 0.69 | 0.35 ± 0.22 | 0.17 ± 0.10 |
| O. stimpsoni | 87 | 19.93 ± 4.98 | 19.42 ± 4.90 | 16.66 ± 4.23 | nm | 5.50 ± 2.95 | 1.38 ± 0.84 | 0.85 ± 0.55 |
| U. unicinctus | 82 | 10.12 ± 1.85 | nm | nm | 137.50 ± 22.52 | 38.11 ± 9.95 | 5.78 ± 2.29 | 3.03 ± 1.78 |
| U. major | 91 | 11.86 ± 3.64 | nm | 27.13 ± 2.86 | 75.92 ± 6.96 | 9.78 ± 2.61 | 2.00 ± 0.55 | 1.26 ± 0.39 |
| Species | NS | OD -CL | OD -CW | OD -TL | CL -WW | TL -WW | CW -WW | CL -DW | TL -DW | CW -DW | OD -WW | OD -DW | OD -AFDW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. japonicus | 124 | 0.84 *** | 0.87 *** | nd | 0.99 *** | nd | 0.99 *** | 0.94 *** | nd | 0.95 *** | 0.83 *** | 0.80 *** | 0.80 *** |
| U. arcuata | 176 | 0.92 *** | 0.92 *** | nd | 0.99 *** | nd | 0.99 *** | 0.97 *** | nd | 0.97 *** | 0.94 *** | 0.91 *** | 0.92 *** |
| U. lactea lactea | 167 | 0.91 *** | 0.92 *** | nd | 0.92 *** | nd | 0.94 *** | 0.77 *** | nd | 0.80 *** | 0.86 *** | 0.71 *** | 0.79 *** |
| O. stimpsoni | 87 | 0.94 *** | 0.90 *** | nd | 0.97 *** | nd | 0.98 *** | 0.94 *** | nd | 0.96 *** | 0.92 *** | 0.90 *** | 0.88 *** |
| U. unicinctus | 82 | nd | nd | 0.11 ** | nd | 0.19 *** | nd | nd | 0.10 ** | nd | 0.51 *** | 0.49 *** | 0.28 |
| U. major | 91 | nd | 0.31 *** | 0.12 *** | nd | 0.68 *** | 0.69 *** | nd | 0.36 *** | 0.37 *** | 0.14 *** | 0.11 ** | 0.06 * |
| Type | NS | Sample Collection | Measurements | Regression Coefficient (R2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| OD (mm) | WW (g) | DW (g) | AFDW (g) | OD-WW | OD-DW | OD-AFDW | |||
| Normal openings | 63 | March to July and November | 13.99 ± 1.30 | 10.00 ± 2.28 | 2.04 ± 0.49 | 1.28 ± 0.37 | 0.54 *** | 0.14 *** | 0.05 |
| Abnormal openings | 28 | August to October | 6.77 ± 2.14 | 9.30 ± 3.15 | 1.94 ± 0.66 | 1.22 ± 0.43 | 0.11 | 0.30 ** | 0.24 * |
| p value | <0.05 | 0.05< | 0.05< | 0.05< | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, B.J.; Seo, J.; Jang, M.S. The Relationship between Burrow Opening Dimensions and Biomass of Intertidal Macroinvertebrates by Feeding Mode (Surface Deposit Feeders vs. Suspension Feeders). Animals 2022, 12, 2878. https://doi.org/10.3390/ani12202878
Koo BJ, Seo J, Jang MS. The Relationship between Burrow Opening Dimensions and Biomass of Intertidal Macroinvertebrates by Feeding Mode (Surface Deposit Feeders vs. Suspension Feeders). Animals. 2022; 12(20):2878. https://doi.org/10.3390/ani12202878
Chicago/Turabian StyleKoo, Bon Joo, Jaehwan Seo, and Min Seong Jang. 2022. "The Relationship between Burrow Opening Dimensions and Biomass of Intertidal Macroinvertebrates by Feeding Mode (Surface Deposit Feeders vs. Suspension Feeders)" Animals 12, no. 20: 2878. https://doi.org/10.3390/ani12202878






