The Impacts of Female Access during Rearing on the Reproductive Behavior and Physiology of Pekin Drakes, and Flock Fertility
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Behavioral Analysis
2.3. Blood Sampling and Hormone Analysis
2.4. Egg Production Data
2.5. Statistical Analysis
3. Results
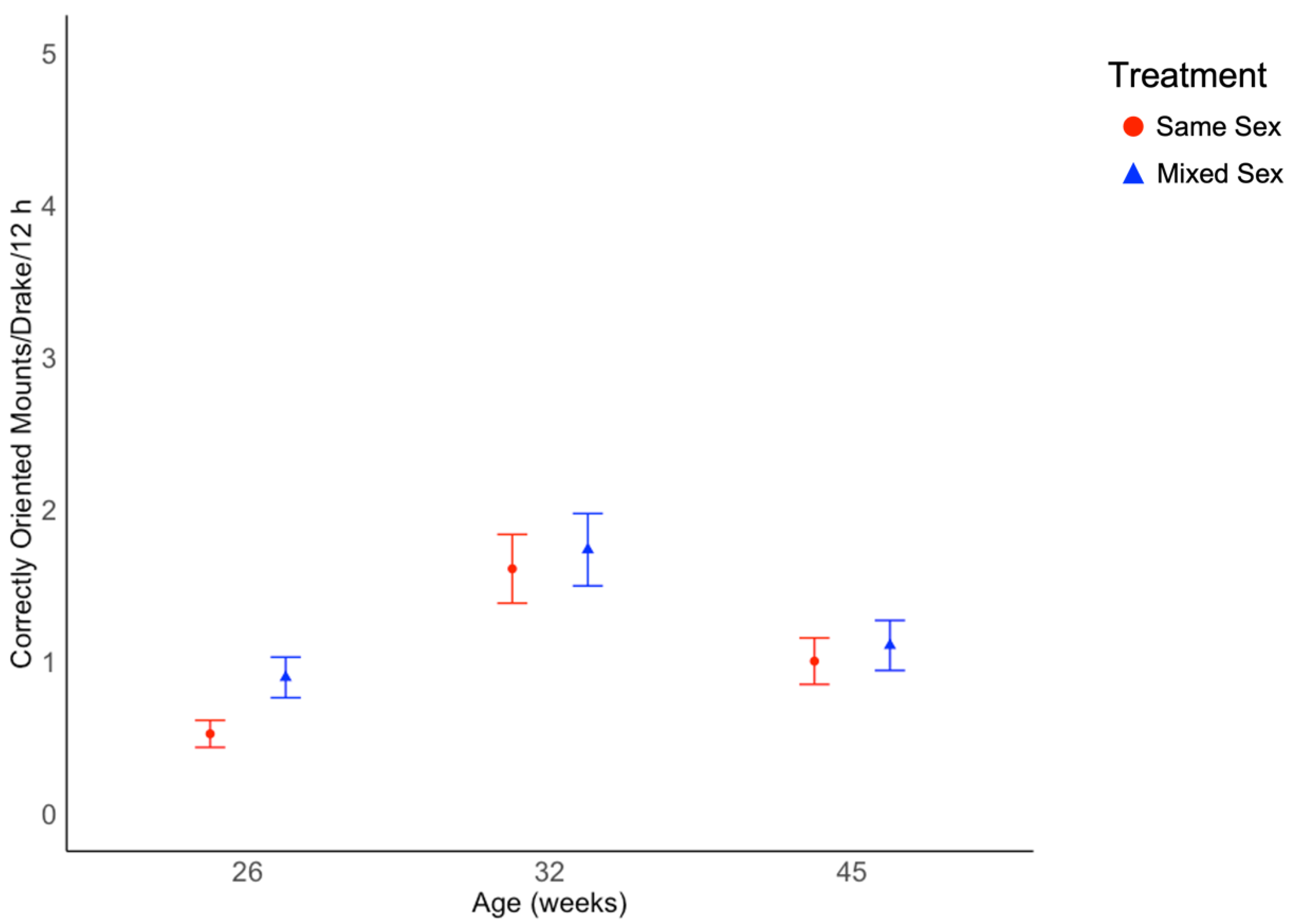
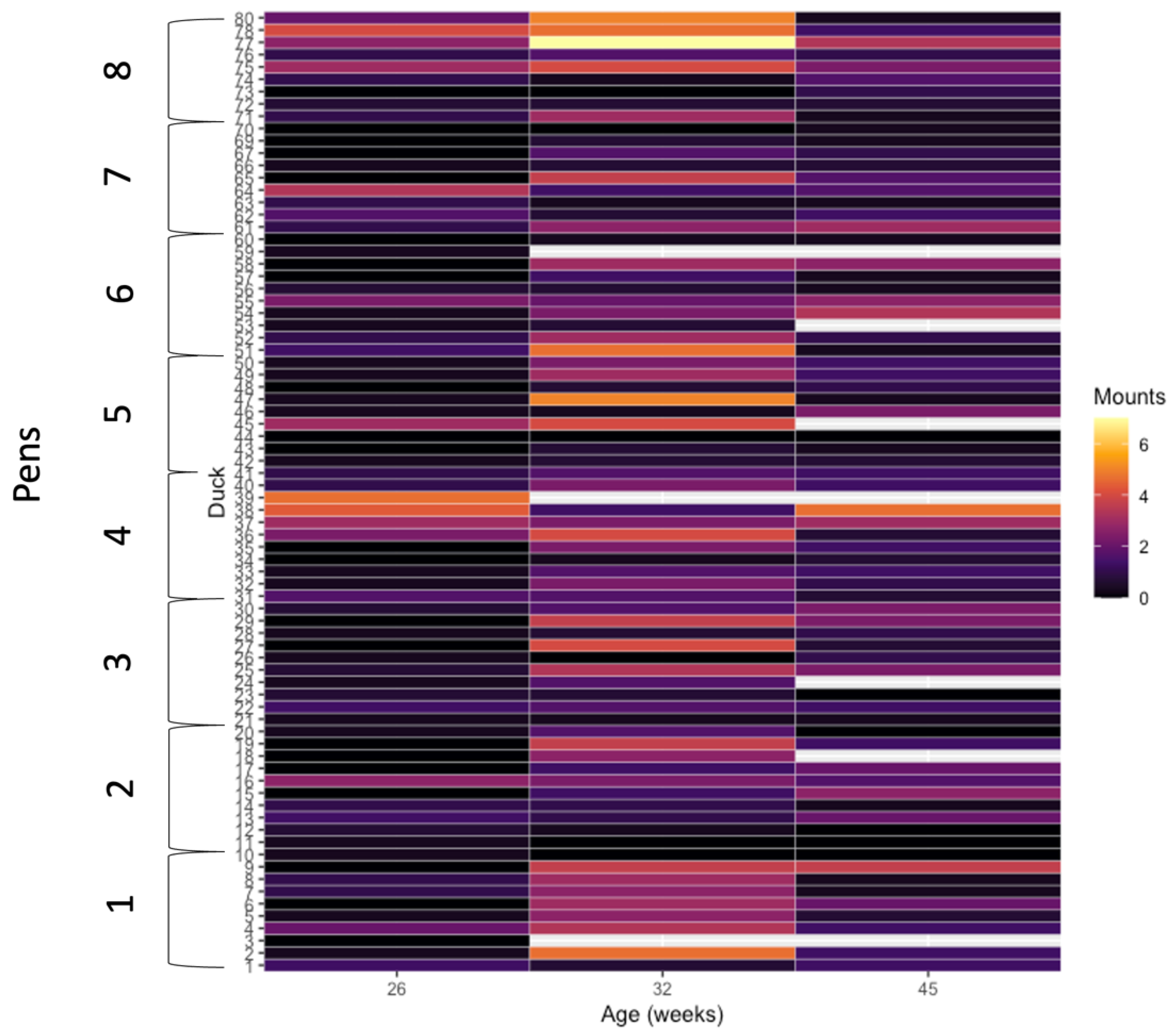
3.1. Behavioral Data
3.2. Hormone Data
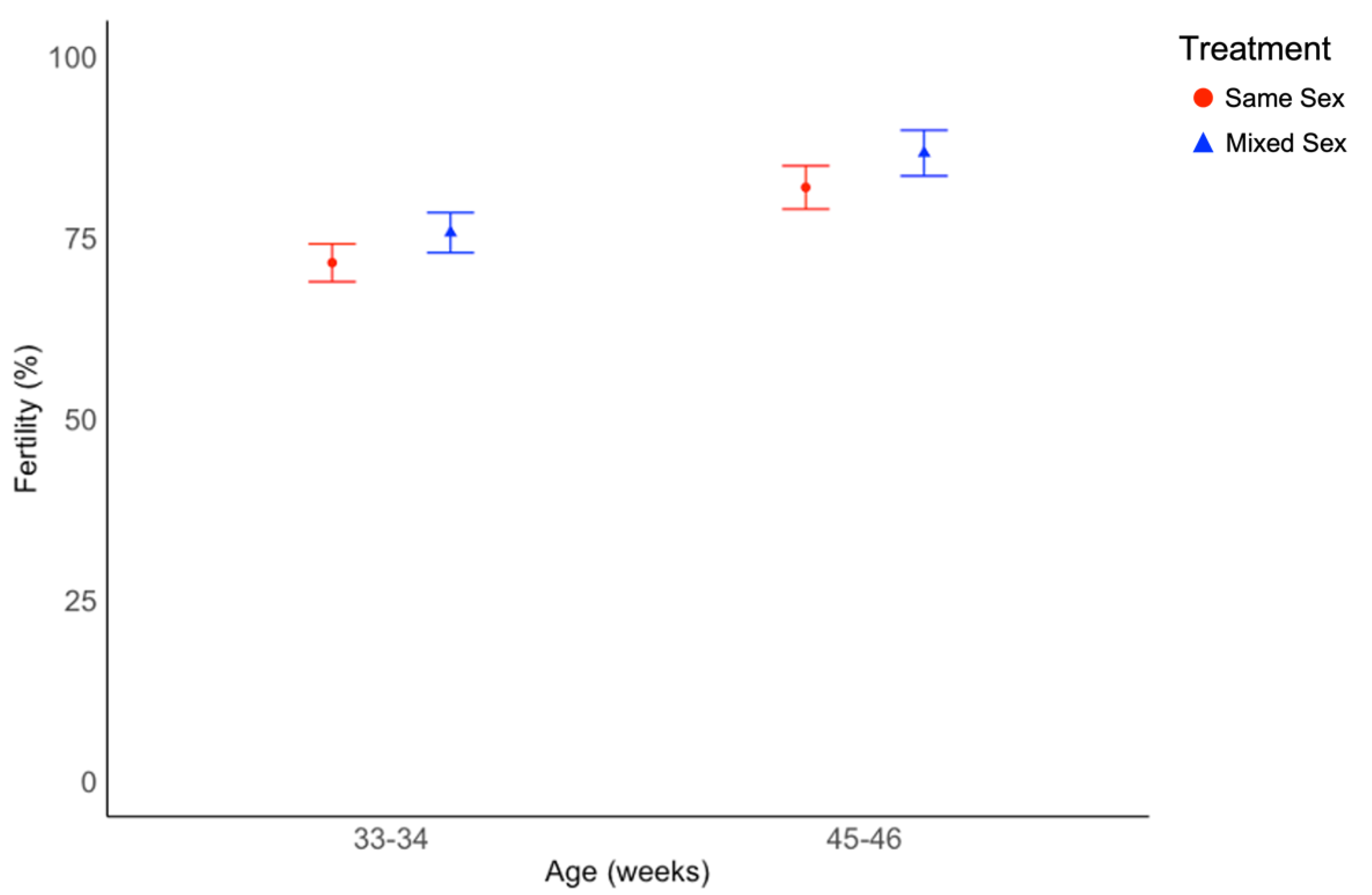
3.3. Fertility Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherry, P.; Morris, T.R. Domestic Duck Production: Science and Practice; CABI: Wallingford, UK, 2008; ISBN 9780851990545. [Google Scholar]
- Naguib, M.; Flörcke, C.; van Oers, K. Effects of Social Conditions during Early Development on Stress Response and Personality Traits in Great Tits (Parus Major). Dev. Psychobiol. 2011, 53, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.L.; Zanette, L.; Fairfull, R.W. Early Exposure to Females Affects Interactions between Male White Leghorn Chickens. Appl. Anim. Behav. Sci. 1993, 36, 29–38. [Google Scholar] [CrossRef]
- Balthazart, J. Behavioural and Physiological Effects of Testosterone Propionate and Cyproterone Acetate in Immature Male Domestic Ducks, Anas Platyrhynchos. Z. Tierpsychol. 1978, 47, 410–421. [Google Scholar] [CrossRef]
- Burns, J.T.; Cheng, K.M.; McKinney, F. Forced Copulation in Captive Mallards. I. Fertilization of Eggs. Auk 1980, 97, 875–879. [Google Scholar]
- Donham, R.S. Annual Cycle of Plasma Luteinizing Hormone and Sex Hormones in Male and Female Mallards (Anas Platyrhynchos). Biol. Reprod. 1979, 21, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Penfold, L.M.; Wildt, D.E.; Herzog, T.L.; Lynch, W.; Ware, L.; Derrickson, S.E.; Monfort, S.L. Seasonal Patterns of LH, Testosterone and Semen Quality in the Northern Pintail Duck (Anas Acuta). Reprod. Fertil. Dev. 2000, 12, 229–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ball, G.F.; Balthazart, J. Hormonal Regulation of Brain Circuits Mediating Male Sexual Behavior in Birds. Physiol. Behav. 2004, 83, 329–346. [Google Scholar] [CrossRef]
- Balthazart, J. Daily Variations of Behavioural Activities and of Plasma Testosterone Levels in the Domestic Duck Anas Platyrhynchos. J. Zool. 1976, 180, 155–173. [Google Scholar] [CrossRef]
- Balthazart, J. Hormonal Correlates of Behavior. In Avian Biology; Farner, D.S., King, J.R., Parkes, K.C., Eds.; National Academic Press: Washington, DC, USA, 1983; pp. 221–365. [Google Scholar]
- Balthazart, J.; Hendrick, J. Annual Variation in Reproductive Behavior, Testosterone, and Plasma FSH Levels in the Rouen Duck, Anas Platyrhynchos. Gen. Comp. Endocrinol. 1976, 28, 171–183. [Google Scholar] [CrossRef]
- Balthazart, J.; Hendrick, J.C. Relationships between the Daily Variations of Social Behavior and of Plasma FSH, LH and Testosterone Levels in the Domestic Duck Anas Platyrhynchos L. Behav. Process. 1979, 4, 107–128. [Google Scholar] [CrossRef]
- Balthazart, J.; Stevens, M. Effects of Testosterone Propionate on the Social Behaviour of Groups of Male Comestic Ducklings Anas Platyrhynchos L. Anim. Behav. 1975, 23, 926–931. [Google Scholar] [CrossRef]
- Harding, C. Hormonal Influences on Avian Aggressive Behavior. In Hormones and Aggressive Behavior; Svare, B.B., Ed.; Springer: Boston, MA, USA, 1983; pp. 435–476. [Google Scholar]
- Wingfield, J.C.; Ball, G.F.; Dufty, A.M.; Hegner, R.E.; Ramenofsky, M. Testosterone and Aggression in Birds. Am. Sci. 1987, 75, 602–608. [Google Scholar]
- Wingfield, J.C.; Hegner, R.E.; Dufty, A.M.; Gregory, F. The “Challenge Hypothesis”: Theoretical Implications for Patterns of Testosterone Secretion, Mating Systems, and Breeding Strategies. Am. Nat. 1990, 136, 829–846. [Google Scholar] [CrossRef]
- Pinxten, R.; de Ridder, E.; Eens, M. Female Presence Affects Male Behavior and Testosterone Levels in the European Starling (Sturnus Vulgaris). Horm. Behav. 2003, 44, 103–109. [Google Scholar] [CrossRef]
- Van Wyk, B.; Fraley, G. Ontogeny of Opn4, Opn5, Gnrh and Gnih Mrna Expression in the Posthatch Male and Female Pekin Duck (Anas Platyrhynchos Domesticus) Suggests Opn4 May Have Additional Functions beyond Reproduction. Animals 2021, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Love, O.P.; Williams, T.D. Plasticity in the Adrenocortical Response of a Free-Living Vertebrate: The Role of Pre- and Post-Natal Developmental Stress. Horm. Behav. 2008, 54, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Steenweg, R.J. Carryover Effects of Winter and Pre-Breeding Conditions on Reproduction in Northern Common Eiders Somateria Mollissima Borealis Nesting in Arctic Canada. Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 2020. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R Package Version 0.2.2; 2022. Available online: https://cran.r-project.org/package=DHARMa (accessed on 9 February 2022).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Klint, T.; Edsman, L.; Holmberg, K.; Silverin, B. Hormonal Correlates of Male Attractiveness during Mate Selection in the Mallard Duck (Anas Platyrhynchos). Horm. Behav. 1989, 23, 83–91. [Google Scholar] [CrossRef]
- Bateson, P.P.G. The Characteristics and Context of Imprinting. Biol. Rev. 1966, 41, 177–220. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, J.J. Mechanisms of Avian Imprinting: A Review. Biol. Rev. 1991, 66, 303–345. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, J.J.; Bateson, P. The Importance of Being First: A Primacy Effect in Filial Imprinting. Anim. Behav. 1990, 40, 472–483. [Google Scholar] [CrossRef]
- Oetting, S.; Prove, E.; Bischof, H.J. Sexual Imprinting as a 2-Stage Process-Mechanisms of Information-Storage and Stabilization. Anim. Behav. 1995, 50, 393–403. [Google Scholar] [CrossRef]
- Klopfer, P.H.; Gottlieb, G. Imprinting and Behavioral Polymorphism: Auditory and Visual Imprinting in Domestic Ducks (Anas Platyrhynchos) and the Involvement of the Critical Period. J. Comp. Physiol. Psychol. 1962, 55, 126–130. [Google Scholar] [CrossRef]
- Barnard, C.J. Animal Behaviour: Mechanism, Development, Function and Evolution; Pearson Education: Essex, UK, 2004; ISBN 0130899364. [Google Scholar]
- Bolhuis, J.J.; Giraldeau, L.A. The Behavior of Animals: Mechanisms, Function, and Evolution; Blackwell Publishing: Malden, MA, USA, 2005. [Google Scholar]
- Rodríguez-Manzo, G.; Canseco-Alba, A. A Role for Learning and Memory in the Expression of an Innate Behavior: The Case of Copulatory Behavior. The Case of Copulatory Behavior. In Identification of Neural Markers Accompanying Memory; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–147. ISBN 9780124081390. [Google Scholar]
- Chen, X.; Shafer, D.; Sifri, M.; Lilburn, M.; Karcher, D.; Cherry, P.; Wakenell, P.; Fraley, S.; Turk, M.; Fraley, G.S. Centennial Review: History and Husbandry Recommendations for Raising Pekin Ducks in Research or Commercial Production. Poult. Sci. 2021, 100, 101241. [Google Scholar] [CrossRef]
- Assenmacher, I.; Astier, H.; Daniel, J.Y.; Jallageas, M. Experimental Studies on the Annual Cycles of Thyroid and Adrenocortical Functions in Relation to the Reproductive Cycle of Drakes. J. Physiol. 1975, 70, 507–520. [Google Scholar]
- Jallageas, M.; Tamisier, A.; Assenmacher, I. A Comparative Study of the Annual Cycles in Sexual and Thyroid Function in Male Peking Ducks (Anas Platyrhynchos) and Teal (Anas Crecca). Gen. Comp. Endocrinol. 1978, 36, 201–210. [Google Scholar] [CrossRef]
- NOAA/ESRL Global Monitoring Laboratory. Available online: https://gml.noaa.gov/grad/solcalc/ (accessed on 2 April 2022).
- Porter, L.; Porter, A.; Potter, H.; Alenciks, E.; Fraley, S.M.; Fraley, G.S. Low Light Intensity in Pekin Duck Breeder Barns Has a Greater Impact on the Fertility of Drakes than Hens. Poult. Sci 2018, 97, 4262–4271. [Google Scholar] [CrossRef]
- Garnier, D.H. Variations de La Testosterone Du Plasma Peripherique Chez Le Canard Pekin Au Tours Du Cycle Annuel. Comptes Rendus L’académie Des Sci. 1971, 272, 1665–1668. [Google Scholar]
- Ouyang, Q.; Bao, D.; Lu, Y.; Hu, J.; Hu, B.; Lan, C.; Hu, S.; He, H.; Liu, H.; Li, L.; et al. A Comparative Study of Libido in Drakes: From Phenotypes to Molecules. Poult. Sci. 2021, 10, 101503. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.L.R.; Clark, C.J.; Prum, R.O. Explosive Eversion and Functional Morphology of the Duck Penis Supports Sexual Conflict in Waterfowl Genitalia. Proc. R. Soc. B Biol. Sci. 2010, 277, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.L.R. Bird with Penises: Copulation Mechanics and Behavior, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 4, ISBN 9780128132517. [Google Scholar]
- Davis, E.S. Female Choice and the Benefits of Mate Guarding by Male Mallards. Anim. Behav. 2002, 64, 619–628. [Google Scholar] [CrossRef]
- Miller, D.B. Social Displays of Mallard Ducks (Anas Platyrhynchos): Effects of Domestication. J. Comp. Physiol. Psychol. 1977, 91, 221–232. [Google Scholar] [CrossRef]
- Kempenaers, B.; Peters, A.; Foerster, K. Sources of Individual Variation in Plasma Testosterone Levels. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.E.; Millar, R.P. Seasonal Changes of Sexual and Territorial Behaviour and Plasma Testosterone Levels in Male Lesser Sheathbills (Chionis Minor). Z. Tierpsychol. 1980, 52, 397–406. [Google Scholar] [CrossRef]
- Denk, A.G.; Holzmann, A.; Peters, A.; Vermeirssen, E.L.M.; Kempenaers, B. Paternity in Mallards: Effects of Sperm Quality and Female Sperm Selection for Inbreeding Avoidance. Behav. Ecol. 2005, 16, 825–833. [Google Scholar] [CrossRef]
- Brennan, P.L.R.; Prum, R.O. The Limits of Sexual Conflict in the Narrow Sense: New Insights from Waterfowl Biology. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2324–2338. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Hurtado, C.B.; Toro, D.M.; Alagawany, M.; Abdelfattah, E.M.; Elnesr, S.S. Fertility and Hatchability in Duck Eggs. Worlds Poult. Sci. J. 2019, 75, 599–608. [Google Scholar] [CrossRef]
- Yakubu, A. Characterisation of the Local Muscovy Duck in Nigeria and Its Potential for Egg and Meat Production. Worlds Poult. Sci. J. 2013, 69, 931–938. [Google Scholar] [CrossRef]
- Brommer, J.E.; Rattiste, K. “Hidden” Reproductive Conflict between Mates in a Wild Bird Population. Evolution 2008, 62, 2326–2333. [Google Scholar] [CrossRef]
- Applegate, T.J.; Harper, D.; Lilburn, M.S. Effect of Hen Production Age on Egg Composition and Embryo Development in Commercial Pekin Ducks. Poult. Sci. 1998, 77, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Buhr, R.J. Incubation Relative Humidity Effects on Allantoic Fluid Volume and Hatchability. Poult. Sci. 1995, 74, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Farghly, M.F.A.; El-Hack, M.E.A.; Alagawany, M.; Saadeldin, I.M.; Swelum, A.A. Wet Feed and Cold Water as Heat Stress Modulators in Growing Muscovy Ducklings. Poult. Sci 2018, 97, 1588–1594. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broadus, L.J.; Lee, B.; Makagon, M.M. The Impacts of Female Access during Rearing on the Reproductive Behavior and Physiology of Pekin Drakes, and Flock Fertility. Animals 2022, 12, 2979. https://doi.org/10.3390/ani12212979
Broadus LJ, Lee B, Makagon MM. The Impacts of Female Access during Rearing on the Reproductive Behavior and Physiology of Pekin Drakes, and Flock Fertility. Animals. 2022; 12(21):2979. https://doi.org/10.3390/ani12212979
Chicago/Turabian StyleBroadus, Lindsey J., Brian Lee, and Maja M. Makagon. 2022. "The Impacts of Female Access during Rearing on the Reproductive Behavior and Physiology of Pekin Drakes, and Flock Fertility" Animals 12, no. 21: 2979. https://doi.org/10.3390/ani12212979
APA StyleBroadus, L. J., Lee, B., & Makagon, M. M. (2022). The Impacts of Female Access during Rearing on the Reproductive Behavior and Physiology of Pekin Drakes, and Flock Fertility. Animals, 12(21), 2979. https://doi.org/10.3390/ani12212979




