L-Tryptophan Differentially Regulated Glucose and Amino Acid Transporters in the Small Intestine of Rat Challenged with Lipopolysaccharide
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Experimental Design and Sample Collection
2.3. Ussing Chamber Analysis
2.4. Quantitative RT-PCR
2.5. Statistical Analysis
3. Results
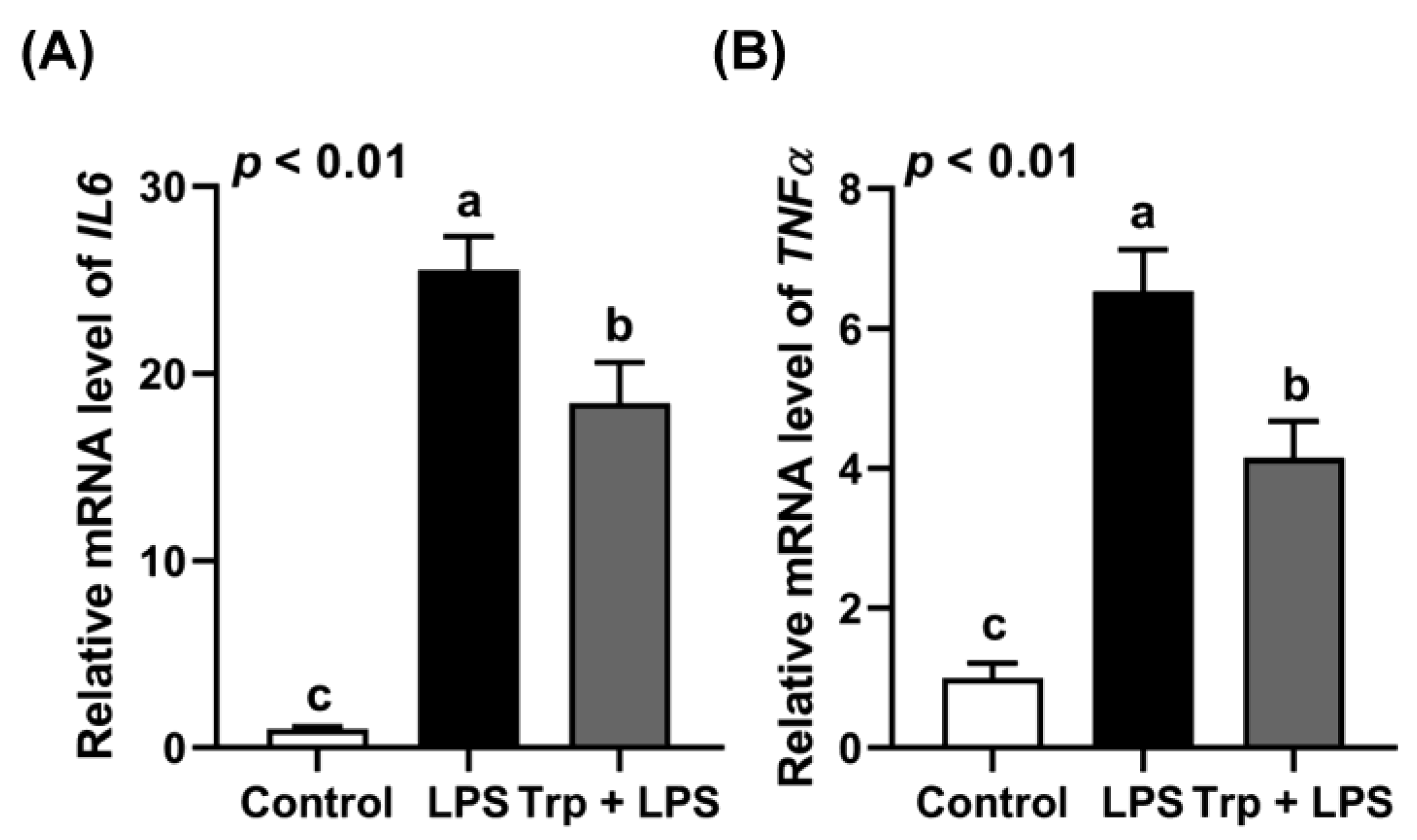
3.1. Animal Growth and Expression of Inflammatory Cytokines in the Jejunum
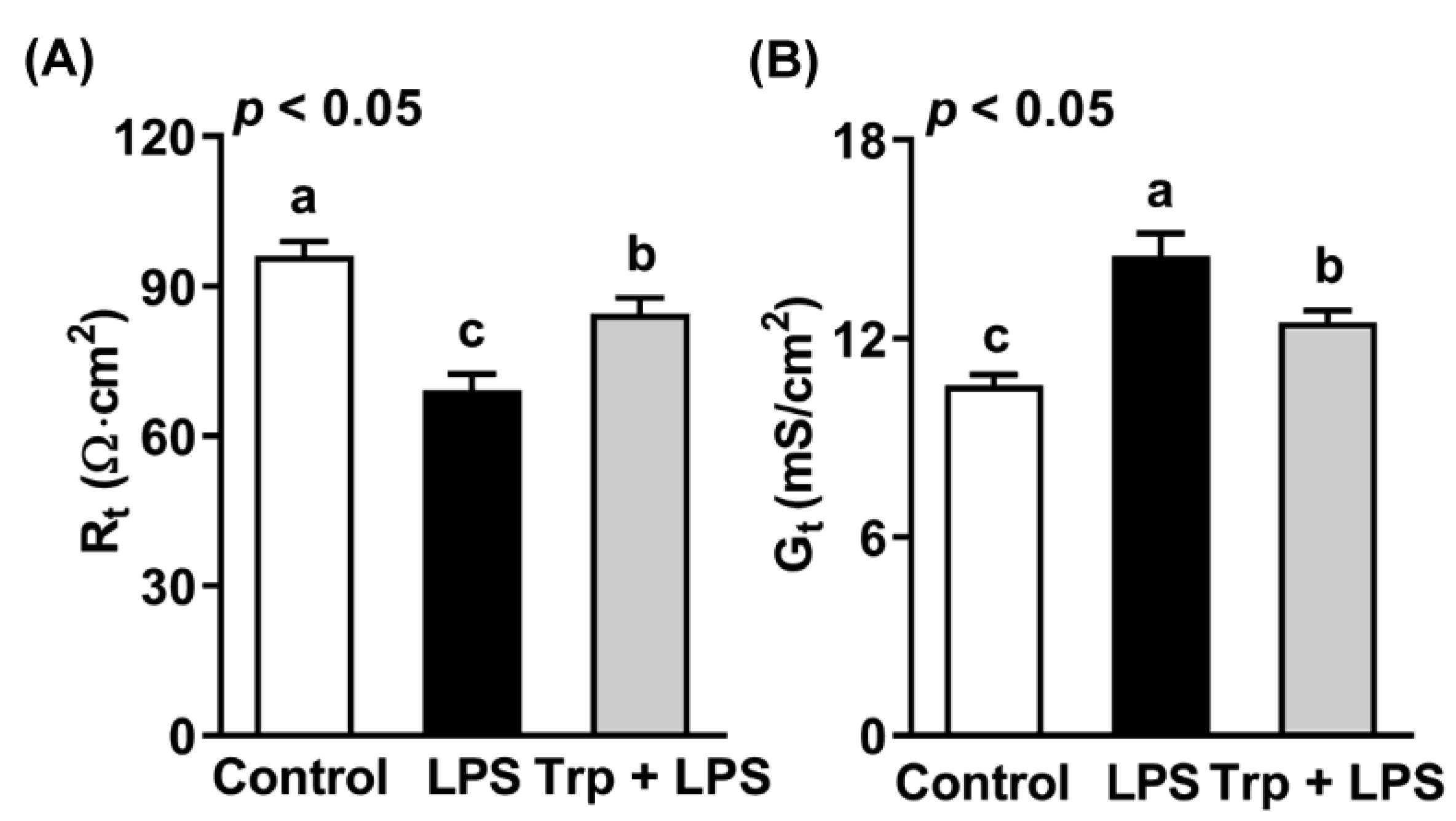
3.2. Effects of Trp and LPS on Transepithelial Resistance and Conductance of Rat Jejunum
3.3. Effects of Trp on LPS-Induced Changes in Short-Circuit Current of the Rat Jejunum in Response to Glucose and AAs
3.4. Effects of Trp and LPS on Gene Expression of Glucose and Amino Acid Transporters in Rat Jejunum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Yin, Y.; Tu, Q.; Yang, H. Glucose and amino acid in enterocyte: Absorption, metabolism and maturation. Front. Biosci. 2018, 23, 1721–1739. [Google Scholar] [CrossRef]
- Ye, J.L.; Gao, C.Q.; Li, X.G.; Jin, C.L.; Wang, D.; Shu, G.; Wang, W.C.; Kong, X.F.; Yao, K.; Yan, H.C.; et al. EAAT3 promotes amino acid transport and proliferation of porcine intestinal epithelial cells. Oncotarget 2016, 7, 38681–38692. [Google Scholar] [CrossRef]
- Ferraris, R.P. Dietary and developmental regulation of intestinal sugar transport. Biochem. J. 2001, 360, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Important roles of amino acids in immune responses. Br. J. Nutr. 2022, 127, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, J.; Tan, B.; Wang, J.; Kong, X.; Guan, G.; Li, F.; Yin, Y. Characterization and regulation of the amino acid transporter SNAT2 in the small intestine of piglets. PLoS ONE 2015, 10, e0128207. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Li, B.; Hou, Y.; Wang, L.; Zhao, D.; Chen, H.; Wu, T.; Zhou, Y.; Ding, B.; Wu, G. Dietary supplementation with an amino acid blend enhances intestinal function in piglets. Amino. Acids. 2018, 50, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Wu, Z.; Ji, Y.; Wang, B.; Dai, Z.; Wu, G. L-Glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J. Nutr. 2015, 145, 2258–2264. [Google Scholar] [CrossRef]
- Jando, J.; Camargo, S.M.R.; Herzog, B.; Verrey, F. Expression and regulation of the neutral amino acid transporter B0AT1 in rat small intestine. PLoS ONE 2017, 12, e0184845. [Google Scholar] [CrossRef]
- Morales, A.; Gomez, T.; Villalobos, Y.D.; Bernal, H.; Htoo, J.K.; Gonzalez-Vega, J.C.; Espinoza, S.; Yanez, J.; Cervantes, M. Dietary protein-bound or free amino acids differently affect intestinal morphology, gene expression of amino acid transporters, and serum amino acids of pigs exposed to heat stress. J. Anim. Sci. 2020, 98, skaa056. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, D.; Yi, D.; Wu, M.; Chen, H.; Wu, T.; Zhou, J.; Li, P.; Hou, Y.; Wu, G. Microarray analysis reveals the inhibition of intestinal expression of nutrient transporters in piglets infected with porcine epidemic diarrhea virus. Sci. Rep. 2019, 9, 19798. [Google Scholar] [CrossRef] [PubMed]
- Abad, B.; Mesonero, J.E.; Salvador, M.T.; Garcia Herrera, J.; Rodriguez-Yoldi, M.J. The administration of lipopolysaccharide, in vivo, induces alteration in L-leucine intestinal absorption. Life Sci. 2001, 70, 615–628. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.P.; Xiong, K.N. Effects of epidermal growth factor on glutamine and glucose absorption by IPEC-J2 cells challenged by lipopolysaccharide using the ussing chamber system. Pak. J. Zool. 2021, 53, 417–422. [Google Scholar] [CrossRef]
- Tang, X.P.; Xiong, K.N. Epidermal growth factor activates EGFR/AMPK signalling to up-regulate the expression of SGLT1 and GLUT2 to promote intestinal glucose absorption in lipopolysaccharide challenged IPEC-J2 cells and piglets. Ital. J. Anim. Sci. 2022, 21, 943–954. [Google Scholar] [CrossRef]
- Schiering, C.; Wincent, E.; Metidji, A.; Iseppon, A.; Li, Y.; Potocnik, A.J.; Omenetti, S.; Henderson, C.J.; Wolf, C.R.; Nebert, D.W.; et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 2017, 542, 242–245. [Google Scholar] [CrossRef]
- Liang, H.; Dai, Z.; Liu, N.; Ji, Y.; Chen, J.; Zhang, Y.; Yang, Y.; Li, J.; Wu, Z.; Wu, G. Dietary L-tryptophan modulates the structural and functional composition of the intestinal microbiome in weaned piglets. Front. Microbiol. 2018, 9, 1736. [Google Scholar] [CrossRef]
- Liang, H.W.; Dai, Z.L.; Kou, J.; Sun, K.J.; Chen, J.Q.; Yang, Y.; Wu, G.Y.; Wu, Z.L. Dietary L-tryptophan supplementation enhances the intestinal mucosal barrier function in weaned piglets: Implication of tryptophan-metabolizing microbiota. Int. J. Mol. Sci. 2019, 20, 20. [Google Scholar] [CrossRef]
- Wang, H.; Ji, Y.; Wu, G.; Sun, K.; Sun, Y.; Li, W.; Wang, B.; He, B.; Zhang, Q.; Dai, Z.; et al. l-Tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. J. Nutr. 2015, 145, 1156–1162. [Google Scholar] [CrossRef]
- Wang, B.; Sun, S.; Liu, M.; Chen, H.; Liu, N.; Wu, Z.; Wu, G.; Dai, Z. Dietary L-tryptophan regulates colonic serotonin homeostasis in mice with dextran sodium sulfate-induced colitis. J. Nutr. 2020, 150, 1966–1976. [Google Scholar] [CrossRef]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The influence of nutritional factors on immunological outcomes. Front. Immunol. 2021, 12, 665968. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.; Texeira, T.F.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, Q.; Tan, B.; Wang, J.; Huang, B.; Li, J.; Kang, M.; Huang, K.; Deng, Q.; Yin, Y. The amino acids sensing and utilization in response to dietary aromatic amino acid supplementation in LPS-induced inflammation piglet model. Front. Nutr. 2021, 8, 819835. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, Q.; Wang, C.; Wu, H.; Jiao, L.; Hong, Q.; Hu, C. LPS challenge increased intestinal permeability, disrupted mitochondrial function and triggered mitophagy of piglets. Innate Immun. 2018, 24, 221–230. [Google Scholar] [CrossRef]
- Hollander, D.; Kaunitz, J.D. The “leaky gut”: Tight junctions but loose associations? Dig. Dis. Sci. 2020, 65, 1277–1287. [Google Scholar] [CrossRef]
- Derting, T.L.; Compton, S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus). Physiol. Biochem. Zool. 2003, 76, 744–752. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Qi, M.; Li, J.; Tan, B. Glutamine, glutamate, and aspartate differently modulate energy homeostasis of small intestine under normal or low energy status in piglets. Anim. Nutr. 2022, 8, 216–226. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Fraga, S.; Pinho, M.J.; Soares-da-Silva, P. Expression of LAT1 and LAT2 amino acid transporters in human and rat intestinal epithelial cells. Amino Acids. 2005, 29, 229–233. [Google Scholar] [CrossRef]
- Weintraut, M.L.; Kim, S.; Dalloul, R.A.; Wong, E.A. Expression of small intestinal nutrient transporters in embryonic and posthatch turkeys. Poult. Sci. 2016, 95, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.X.; Ottestad-Hansen, S.; Holmseth, S.; Hassel, B.; Danbolt, N.C.; Zhou, Y. Expression of Glutamate Transporters in Mouse Liver, Kidney, and Intestine. J. Histochem. Cytochem. 2018, 66, 189–202. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, G. Oxidation of amino acids, glucose, and fatty acids as metabolic fuels in enterocytes of developing pigs. Amino Acids 2022, 54, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Camargo, S.M.R.; Vuille-Dit-Bille, R.N.; Meier, C.F.; Verrey, F. ACE2 and gut amino acid transport. Clin. Sci. 2020, 134, 2823–2833. [Google Scholar] [CrossRef]
- Scott, N.A.; Lawson, M.A.E.; Hodgetts, R.J.; Le Gall, G.; Hall, L.J.; Mann, E.R. Macrophage metabolism in the intestine is compartment specific and regulated by the microbiota. Immunology 2022, 166, 138–152. [Google Scholar] [CrossRef]
| Treatments | Trp Intake from Drinking Water (mg/d) | Trp Intake from Feed (mg/d) | Total Daily Trp Intake (mg/d) | Trp Intake, % of Control | |
|---|---|---|---|---|---|
| Feed | Total | ||||
| Control | 0.00 ± 0.00 | 45.2 ± 3.00 | 45.2 ± 3.00 | 100 | 100 |
| Trp | 25.06 ± 1.98 | 47.9 ± 2.23 | 73.0 ± 2.79 | 106 | 162 |
| Genes | Primer Sequence (5′ → 3′) | Product Size, bp | |
|---|---|---|---|
| IL6 | Forward | CAAGAGACTTCCAGCCAGTTG | 106 |
| Reverse | TGGGTGGTATCCTCTGTGAAG | ||
| TNFα | Forward | GACTCTGACCCCCATTACTCTG | 151 |
| Reverse | GTCTCGTGTGTTTCTGAGCATC | ||
| ATB0,+ (Slc6a14) | Forward | TATCCGGAAGCACTAGCTCAA | 82 |
| Reverse | CCAGACCCAATGTTAAAAGCA | ||
| ATP1A1 | Forward | TAAAGTGCATCGAGGTCTGCT | 121 |
| Reverse | TTTGGGTTCTTGTGAATGGAG | ||
| ATP1A2 | Forward | GGGAACATGCAGAATCAGTGT | 112 |
| Reverse | CCAGCTTCCTGTCTCAATCTG | ||
| B0AT1 (Slc6a19) | Forward | GGACATATCTCATCGCCTTCA | 162 |
| Reverse | CGATGTCCTTGTTGAACCTGT | ||
| EAAT3 (Slc1a1) | Forward | GGGCTTCTCGGTAGACAAATC | 151 |
| Reverse | TTGTGTCCTTCTCGCTCCTTA | ||
| GLUT2 (Slc2a2) | Forward | CTCATCATTGCTGGAAGAAGC | 133 |
| Reverse | CAAGAGCCAGTTGGTGAAGAG | ||
| LAT2 (Slc7a8) | Forward | CACATTTGGTGGAGTCAACG | 105 |
| Reverse | TTCACGTGGATCATGGCTAA | ||
| SGLT1 (Slc5a1) | Forward | CCAACTTTGGTTTCTGATGGA | 84 |
| Reverse | GTCTCGGAATATGTGGAACGA | ||
| SNAT2 (Slc38a2) | Forward | TCCTTCCGTCTGCTTTCTACA | 78 |
| Reverse | AACACAGAGCCCCAATCTTTT | ||
| y+LAT1 (Slc7a7) | Forward | TCAGCTTTACCTACGCTGGAA | 101 |
| Reverse | CCACCAGGAAGATGGTACAGA | ||
| GAPDH | Forward | CTGTGACTTCAACAGCAACTCC | 123 |
| Reverse | ACCCTGTTGCTGTAGCCATATT |
| Items | Treatment | p-Value | |
|---|---|---|---|
| Control | Trp | ||
| Body weight (g) | |||
| d 0 | 218 ± 7.7 | 222 ± 6.8 | 0.70 |
| d 7 | 248 ± 8.1 | 255 ± 6.7 | 0.51 |
| Average daily weight gain (g/day) | |||
| d 0–7 | 19.1 ± 1.3 | 21.6 ± 1.6 | 0.24 |
| Average daily feed intake (g/day) | |||
| 23.8 ± 2.18 | 25.2 ± 1.61 | 0.85 | |
| Nutrient Added | Control | LPS | Trp + LPS | p-Value |
|---|---|---|---|---|
| Glucose | 0.97 ± 0.10 a | 0.35 ± 0.04 b | 0.84 ± 0.10 a | p < 0.05 |
| Arg | 1.29 ± 0.15 a | 0.36 ± 0.06 b | 1.06 ± 0.13 a | p < 0.05 |
| Gln | 0.91 ± 0.14 a | 0.35 ± 0.08 b | 0.90 ± 0.17 a | p < 0.05 |
| Glu | 0.57 ± 0.09 a | 0.14 ± 0.06 b | 0.40 ± 0.04 a | p < 0.05 |
| Gly | 0.31 ± 0.04 a | 0.10 ± 0.03 b | 0.20 ± 0.06 ab | p < 0.05 |
| His | 0.58 ± 0.04 a | 0.23 ± 0.13 b | 0.47 ± 0.07 ab | p < 0.05 |
| Leu | 0.44 ± 0.02 a | 0.14 ± 0.02 b | 0.39 ± 0.06 a | p < 0.05 |
| Lys | 0.55 ± 0.16 a | 0.12 ± 0.07 b | 0.44 ± 0.06 ab | p < 0.05 |
| Tau | 0.44 ± 0.12 a | 0.11 ± 0.04 b | 0.34 ± 0.05 ab | p < 0.05 |
| Thr | 0.88 ± 0.10 a | 0.10 ± 0.03 c | 0.55 ± 0.09 b | p < 0.05 |
| Trp | 0.84 ± 0.14 a | 0.31 ± 0.08 b | 0.73 ± 0.12 a | p < 0.05 |
| Genes (Fold of Control) | Control | LPS | Trp + LPS | p-Value |
|---|---|---|---|---|
| GLUT2 | 1.00 ± 0.13 | 1.49 ± 0.22 | 0.92 ± 0.19 | 0.16 |
| SGLT1 | 1.00 ± 0.07 a | 0.38 ± 0.10 b | 0.94 ± 0.14 a | p < 0.05 |
| SNAT2 | 1.00 ± 0.11 ab | 0.56 ± 0.09 b | 1.39 ± 0.17 a | p < 0.05 |
| LAT2 | 1.00 ± 0.10 ab | 0.51 ± 0.06 b | 1.22 ± 0.17 a | p < 0.05 |
| B0AT1 | 1.00 ± 0.20 | 0.90 ± 0.19 | 1.20 ± 0.24 | 0.63 |
| EAAT3 | 1.00 ± 0.27 c | 13.3 ± 1.8 b | 347 ± 7.2 a | p < 0.01 |
| y+LAT2 | 1.00 ± 0.18 b | 3.34 ± 0.26 a | 2.63 ± 0.25 a | p < 0.01 |
| ATB0,+ | 1.00 ± 0.13 b | 23.9 ± 3.0 a | 17.4 ± 1.9 a | p < 0.05 |
| ATP1A1 | 1.00 ± 0.07 | 1.28 ± 0.10 | 1.18 ± 0.16 | 0.48 |
| ATP1A2 | 1.00 ± 0.16 a | 0.07 ± 0.05 c | 0.40 ± 0.05 b | p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Jiang, L.; Wu, Z.; Dai, Z. L-Tryptophan Differentially Regulated Glucose and Amino Acid Transporters in the Small Intestine of Rat Challenged with Lipopolysaccharide. Animals 2022, 12, 3045. https://doi.org/10.3390/ani12213045
Wang B, Jiang L, Wu Z, Dai Z. L-Tryptophan Differentially Regulated Glucose and Amino Acid Transporters in the Small Intestine of Rat Challenged with Lipopolysaccharide. Animals. 2022; 12(21):3045. https://doi.org/10.3390/ani12213045
Chicago/Turabian StyleWang, Bin, Lili Jiang, Zhenlong Wu, and Zhaolai Dai. 2022. "L-Tryptophan Differentially Regulated Glucose and Amino Acid Transporters in the Small Intestine of Rat Challenged with Lipopolysaccharide" Animals 12, no. 21: 3045. https://doi.org/10.3390/ani12213045
APA StyleWang, B., Jiang, L., Wu, Z., & Dai, Z. (2022). L-Tryptophan Differentially Regulated Glucose and Amino Acid Transporters in the Small Intestine of Rat Challenged with Lipopolysaccharide. Animals, 12(21), 3045. https://doi.org/10.3390/ani12213045





