Advances, Implications, and Limitations of Low-Crude-Protein Diets in Pig Production
Abstract
Simple Summary
Abstract
1. Introduction
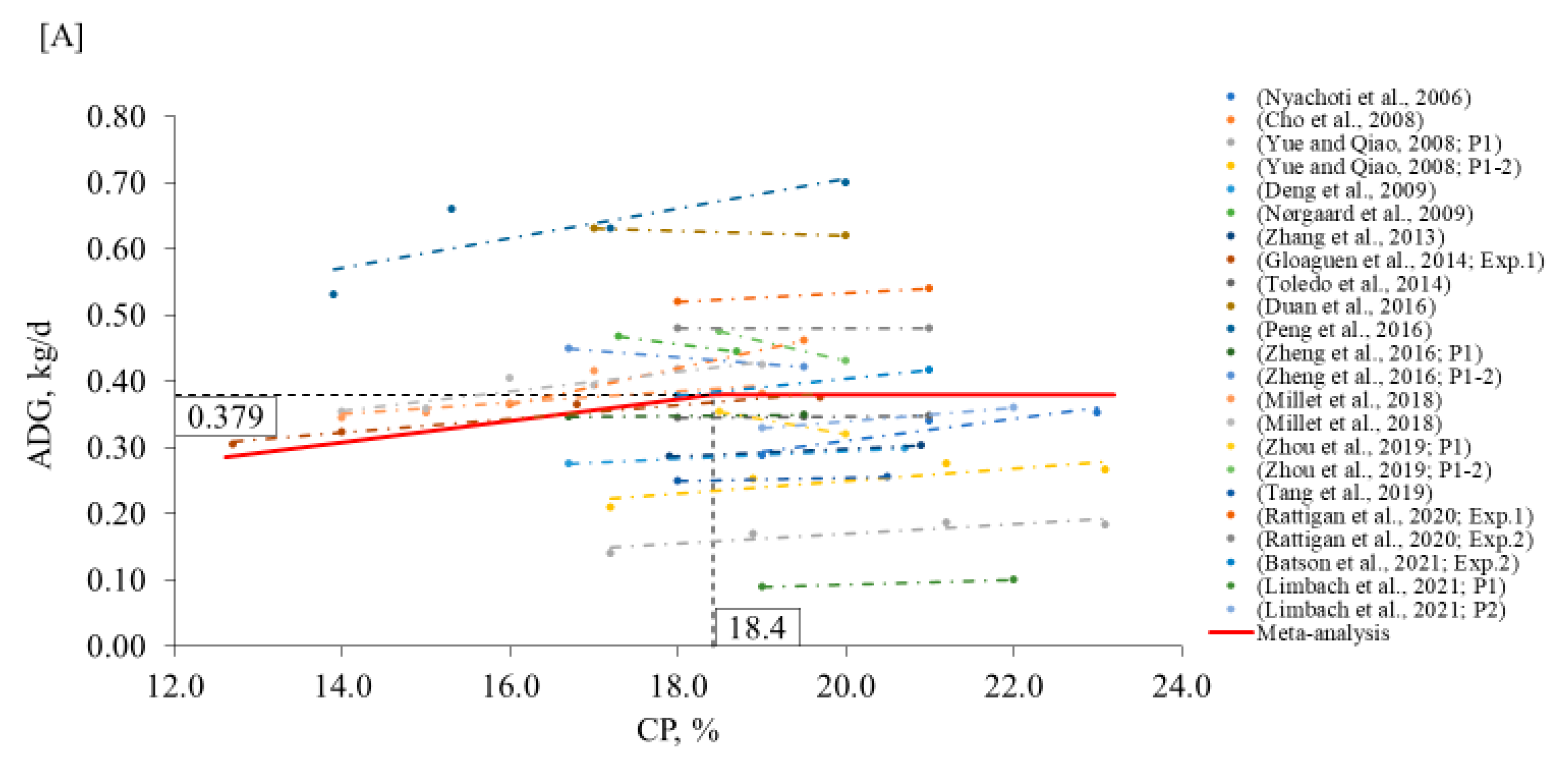
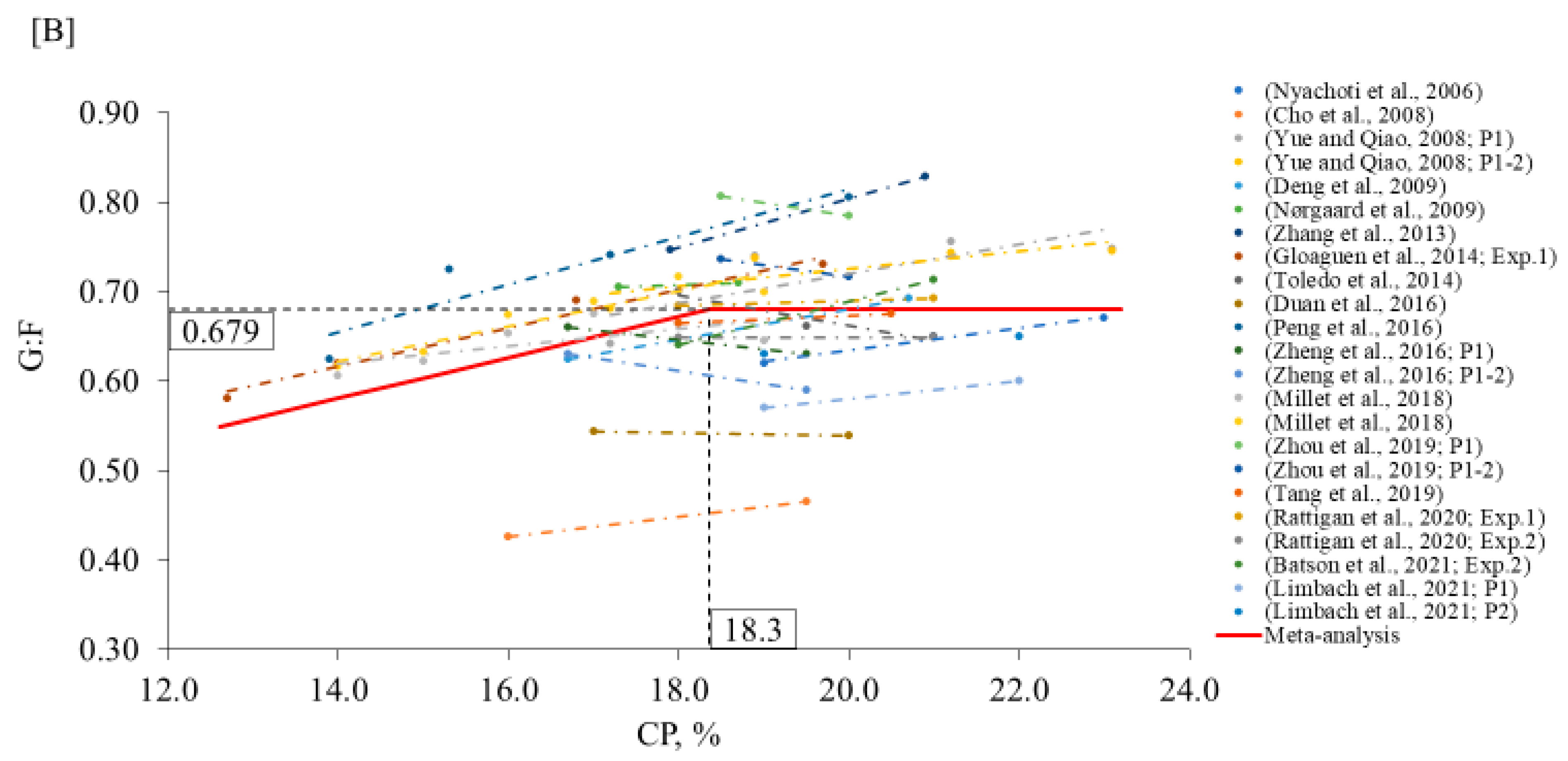
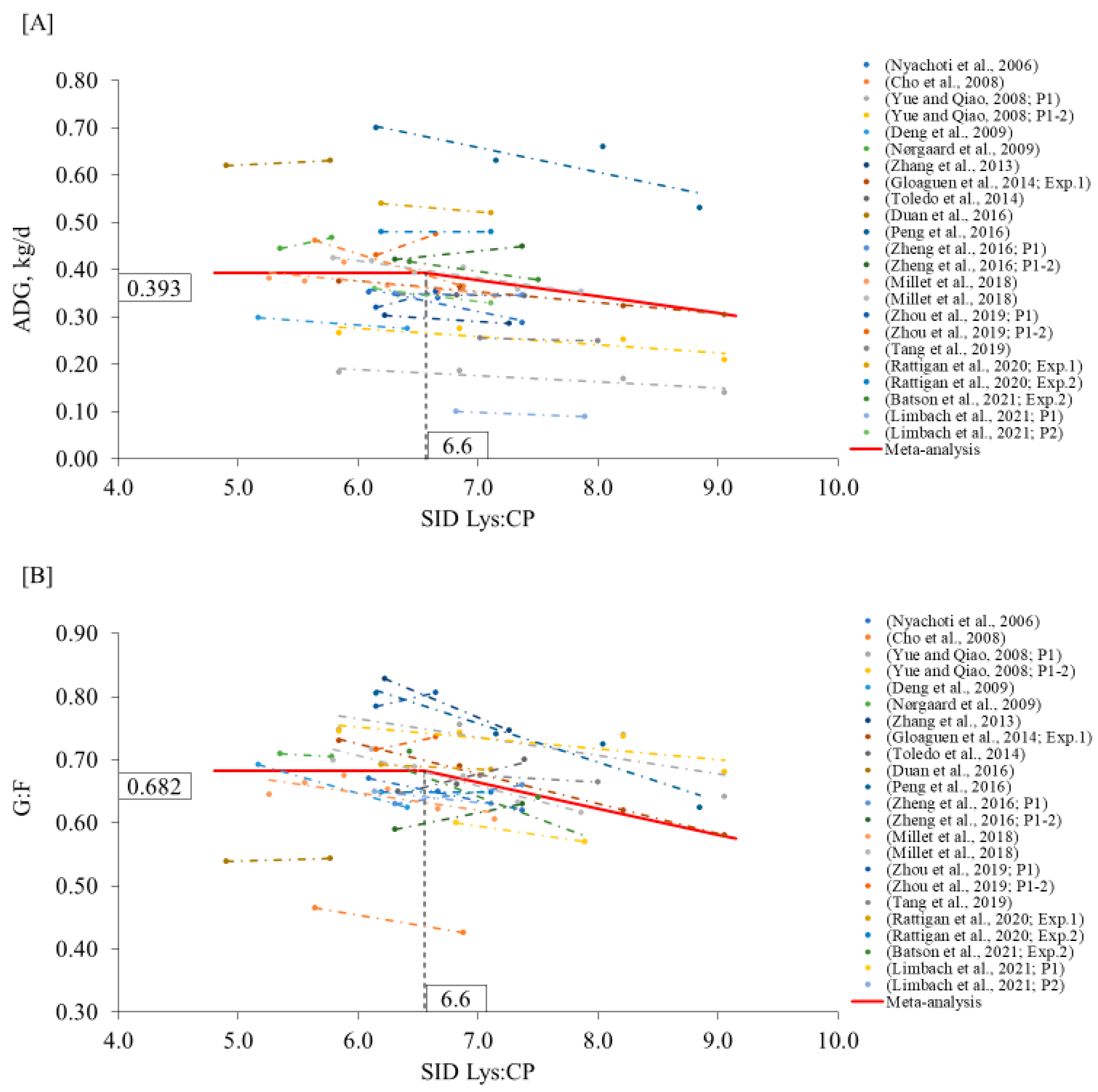
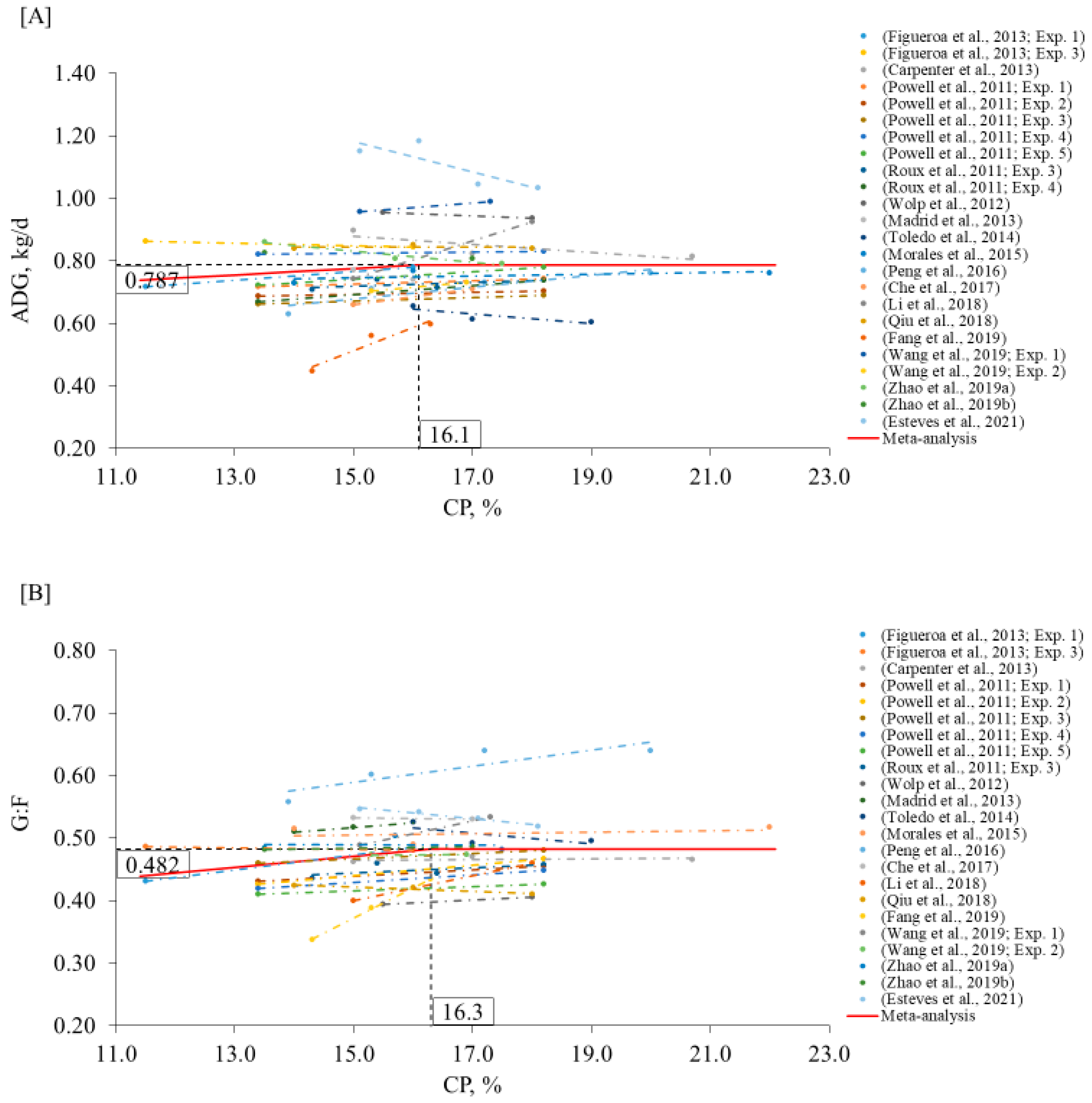
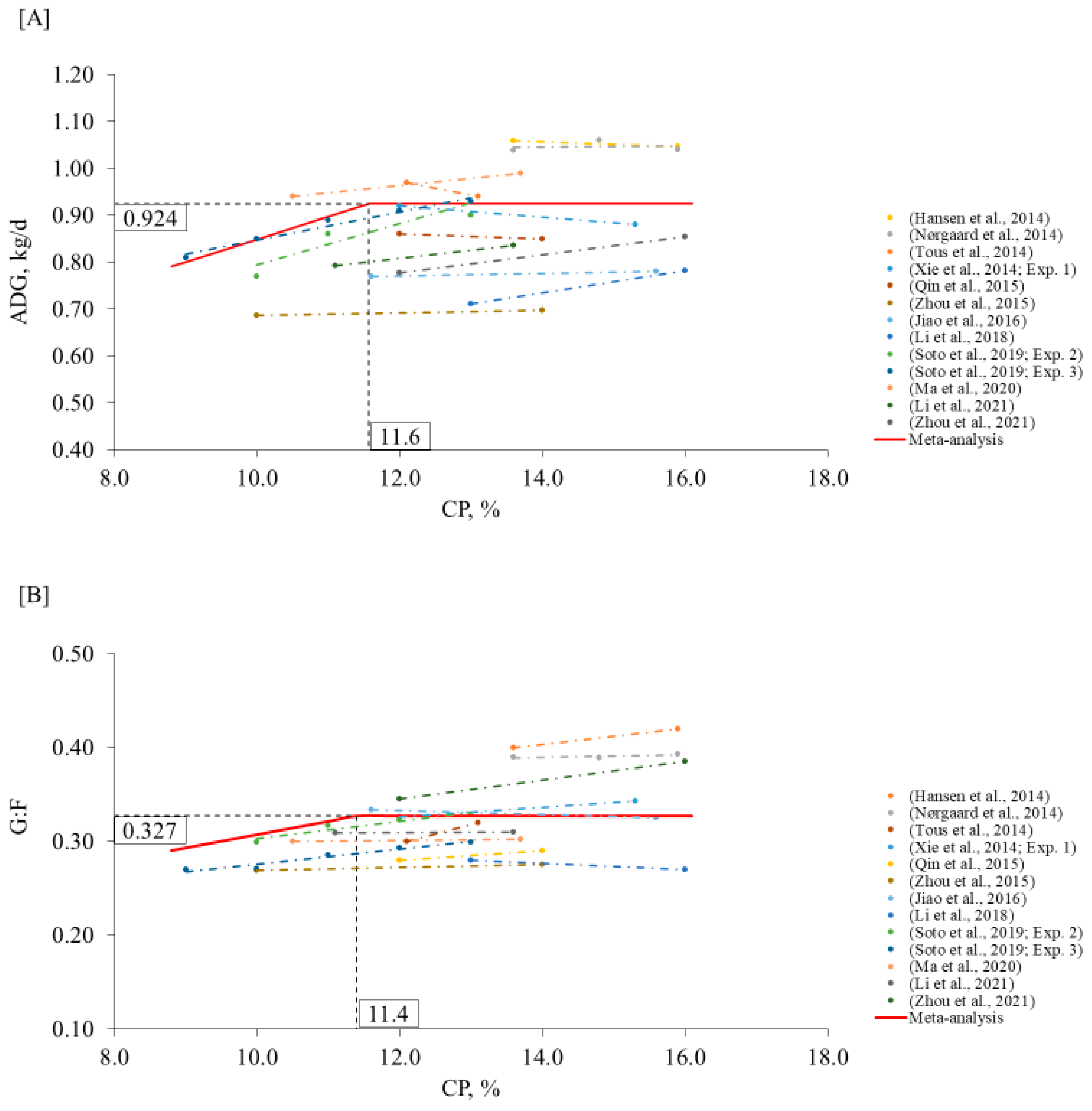
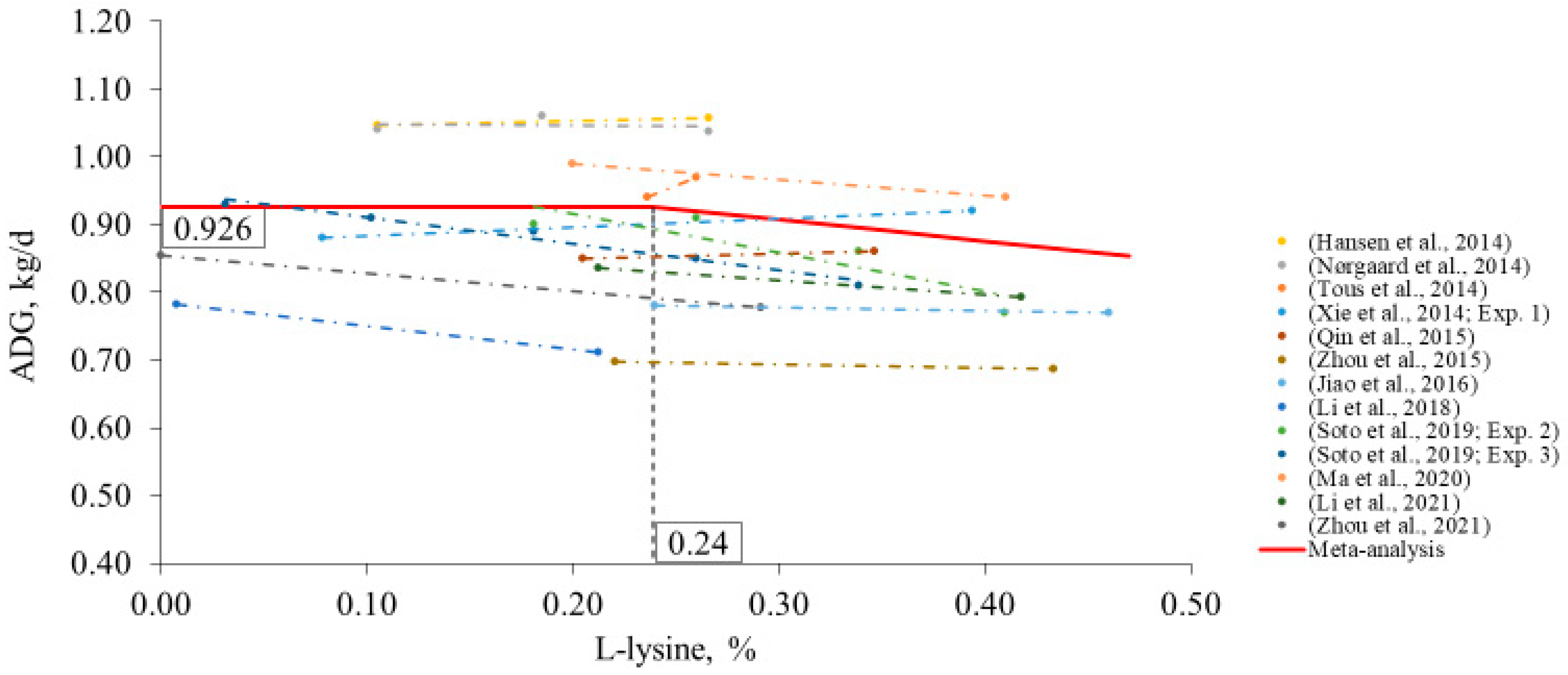
2. Meta-Analysis
3. Low-CP Formulations
3.1. Nursery Phase
3.2. Growing Phase
3.3. Finishing Phase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Research Council. NRC Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- Kim, S.W.; Hansen, J.A. Diet Formulation and Feeding Programs. In Sustainable Swine Nutrition; Chiba, L.I., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2022; pp. 217–227. ISBN 978-1-119-58389-9. [Google Scholar]
- Rostagno, H.S.S.; Albino, L.F.T.; Donzele, J.L.; Gomes, P.C.; de Oliveira, R.F.; Lopes, D.C.; Ferreira, A.S.; Barreto, S.L.T.; Euclides, R. Brazilian Tables for Poultry and Swine, 4th ed.; UFV, Universidade Federal de Viçosa, Departamento de Zootecnia: Viçosa, Brazil, 2017; ISBN 9788581791203. [Google Scholar]
- van Milgen, J.; Dourmad, J.-Y. Concept and application of ideal protein for pigs. J. Anim. Sci. Biotechnol. 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.K.; Baker, D.H. Ideal amino acid pattern for 10-kilogram pigs. J. Anim. Sci. 1992, 70, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Millet, S.; Aluwé, M.; De Boever, J.; De Witte, B.; Douidah, L.; Van den Broeke, A.; Leen, F.; De Cuyper, C.; Ampe, B.; De Campeneere, S. The effect of crude protein reduction on performance and nitrogen metabolism in piglets (four to nine weeks of age) fed two dietary lysine levels. J. Anim. Sci. 2018, 96, 3824–3836. [Google Scholar] [CrossRef]
- Figueroa, J.L.; Lewis, A.J.; Miller, P.S.; Fischer, R.L.; Diedrichsen, R.M. Growth, carcass traits, and plasma amino acid concentrations of gilts fed low-protein diets supplemented with amino acids including histidine, isoleucine, and valine. J. Anim. Sci. 2003, 81, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Che, L.Q.; Peng, X.; Hu, L.; Wu, C.; Xu, Q.; Fang, Z.F.; Lin, Y.; Xu, S.Y.; Li, J.; Feng, B.; et al. The addition of protein-bound amino acids in low-protein diets improves the metabolic and immunological characteristics in fifteen- to thirty-five-kg pigs. J. Anim. Sci. 2017, 95, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Limbach, J.R.; Espinosa, C.D.; Perez-Calvo, E.; Stein, H.H. Effect of dietary crude protein level on growth performance, blood characteristics, and indicators of intestinal health in weanling pigs. J. Anim. Sci. 2021, 99, skab166. [Google Scholar] [CrossRef] [PubMed]
- Rochell, S.J.; Alexander, L.S.; Rocha, G.C.; Van Alstine, W.G.; Boyd, R.D.; Pettigrew, J.E.; Dilger, R.N. Effects of dietary soybean meal concentration on growth and immune response of pigs infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2015, 93, 2987–2997. [Google Scholar] [CrossRef]
- Peng, X.; Hu, L.; Liu, Y.; Yan, C.; Fang, Z.F.; Lin, Y.; Xu, S.Y.; Li, J.; Wu, C.M.; Chen, D.W.; et al. Effects of low-protein diets supplemented with indispensable amino acids on growth performance, intestinal morphology and immunological parameters in 13 to 35 kg pigs. Animal 2016, 10, 1812–1820. [Google Scholar] [CrossRef]
- Soto, J.A.; Tokach, M.D.; Dritz, S.S.; Woodworth, J.C.; Derouchey, J.M.; Goodband, R.D.; Wu, F. Optimal dietary standardized ileal digestible lysine and crude protein concentration for growth and carcass performance in finishing pigs weighing greater than 100 kg. J. Anim. Sci. 2019, 97, 1701–1711. [Google Scholar] [CrossRef]
- Carpenter, D.A.; O’Mara, F.P.; O’Doherty, J.V. The effect of dietary crude protein concentration on growth performance, carcass composition and nitrogen excretion in entire grower-finisher pigs. Irish J. Agric. Food Res. 2004, 43, 227–236. [Google Scholar]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. Effects of varying levels of dietary protein and net energy on growth performance, nitrogen balance and faecal characteristics of growingfinishing pigs. Rev. Bras. Zootec. 2019, 48, e20180021. [Google Scholar] [CrossRef]
- Wang, H.; Long, W.; Chadwick, D.; Velthof, G.L.; Oenema, O.; Ma, W.; Wang, J.; Qin, W.; Hou, Y.; Zhang, F. Can dietary manipulations improve the productivity of pigs with lower environmental and economic cost? A global meta-analysis. Agric. Ecosyst. Environ. 2020, 289, 106748. [Google Scholar] [CrossRef]
- Moughan, P.J.; Fuller, M.F. Modelling amino acid metabolism and the estimation of amino acid requirements. In Amino Acids in Animal Nutrition; CABI Publishing: Wallingford, UK, 2003; pp. 187–202. [Google Scholar]
- Qin, C.; Huang, P.; Qiu, K.; Sun, W.; Xu, L.; Zhang, X.; Yin, J. Influences of dietary protein sources and crude protein levels on intracellular free amino acid profile in the longissimus dorsi muscle of finishing gilts. J. Anim. Sci. Biotechnol. 2015, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Esteves, L.A.C.; Monteiro, A.N.T.R.; Sitanaka, N.Y.; Oliveira, P.C.; Castilha, L.D.; Paula, V.R.C.; Pozza, P.C. The reduction of crude protein with the supplementation of amino acids in the diet reduces the environmental impact of growing pigs production evaluated through life cycle assessment. Sustainability 2021, 13, 4815. [Google Scholar] [CrossRef]
- Batson, K.L.; Calderón, H.I.; Tokach, M.D.; Woodworth, J.C.; Goodband, R.D.; Dritz, S.S.; DeRouchey, J.M. Effects of feeding diets containing low crude protein and coarse wheat bran as alternatives to zinc oxide in nursery pig diets. J. Anim. Sci. 2021, 99, skab090. [Google Scholar] [CrossRef]
- St-Pierre, N.R. Invited review. Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 2001, 84, 741–755. [Google Scholar] [CrossRef]
- Jang, K.B.; Kim, S.W. Role of milk carbohydrates in intestinal health of nursery pigs: A review. J. Anim. Sci. Biotechnol. 2022, 13, 6. [Google Scholar] [CrossRef]
- Quigley, J.D.; Dennis, T.S.; Suarez-Mena, F.X.; Hill, T.M.; Aragona, K.M. Meta-analysis of effects of age on intestinal digestibility of liquid feeds in young calves. JDS Commun. 2021, 2, 114–117. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Omogbenigun, F.O.; Rademacher, M.; Blank, G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 2006, 84, 125–134. [Google Scholar] [CrossRef]
- Deng, D.; Yao, K.; Chu, W.; Li, T.; Huang, R.; Yin, Y.; Liu, Z.; Zhang, J.; Wu, G. Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low-protein diet. J. Nutr. Biochem. 2009, 20, 544–552. [Google Scholar] [CrossRef]
- Spring, S.; Premathilake, H.; DeSilva, U.; Shili, C.; Carter, S.; Pezeshki, A. Low Protein-High Carbohydrate Diets Alter Energy Balance, Gut Microbiota Composition and Blood Metabolomics Profile in Young Pigs. Sci. Rep. 2020, 10, 3318. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, J.V.; Fernández, J.A. Isoleucine and valine supplementation of crude protein-reduced diets for pigs aged 5–8 weeks. Anim. Feed Sci. Technol. 2009, 154, 248–253. [Google Scholar] [CrossRef]
- Zheng, L.; Wei, H.; Cheng, C.; Xiang, Q.; Pang, J.; Peng, J. Supplementation of branched-chain amino acids to a reduced-protein diet improves growth performance in piglets: Involvement of increased feed intake and direct muscle growth-promoting effect. Br. J. Nutr. 2016, 115, 2236–2245. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Zheng, C.; Wang, L.; Zhu, C.; Yang, X.; Wen, X.; Ma, X. Effects of protein sources and levels in antibiotic-free diets on diarrhea, intestinal morphology, and expression of tight junctions in weaned piglets. Anim. Nutr. 2015, 1, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Duan, Y.; Li, F.; Li, Y.; Guo, Q. Effects of supplementation with branched—chain amino acids to low—protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids 2016, 48, 2131–2144. [Google Scholar] [CrossRef]
- Rattigan, R.; Sweeney, T.; Maher, S.; Ryan, M.T.; Thornton, K.; O’Doherty, J.V. Effects of reducing dietary crude protein concentration and supplementation with either laminarin or zinc oxide on the growth performance and intestinal health of newly weaned pigs. Anim. Feed Sci. Technol. 2020, 270, 114693. [Google Scholar] [CrossRef]
- Zhao, Y.; Weaver, A.C.; Fellner, V.; Payne, R.L.; Kim, S.W. Amino acid fortified diets for weanling pigs replacing fish meal and whey protein concentrate: Effects on growth, immune status, and gut health. J. Anim. Sci. Biotechnol. 2014, 5, 57. [Google Scholar] [CrossRef]
- Cervantes-Pahm, S.K.; Stein, H.H. Ileal digestibility of amino acids in conventional, fermented, and enzyme-treated soybean meal and in soy protein isolate, fish meal, and casein fed to weanling pigs. J. Anim. Sci. 2010, 88, 2674–2683. [Google Scholar] [CrossRef]
- Deng, Z.; Duarte, M.E.; Jang, K.B.; Kim, S.W. Soy protein concentrate replacing animal protein supplements and its impacts on intestinal immune status, intestinal oxidative stress status, nutrient digestibility, mucosa-associated microbiota, and growth performance of nursery pigs. J. Anim. Sci. 2022, 100, skac255. [Google Scholar] [CrossRef]
- Duarte, M.E.; Kim, S.W. Intestinal microbiota and its interaction to intestinal health in nursery pigs. Anim. Nutr. 2022, 8, 169–184. [Google Scholar] [CrossRef]
- Yue, L.Y.; Qiao, S.Y. Effects of low-protein diets supplemented with crystalline amino acids on performance and intestinal development in piglets over the first 2 weeks after weaning. Livest. Sci. 2008, 115, 144–152. [Google Scholar] [CrossRef]
- Gloaguen, M.; Le Floc’h, N.; Corrent, E.; Primot, Y.; van Milgen, J. The use of free amino acids allows formulating very low crude protein diets for piglets. J. Anim. Sci. 2014, 92, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qiao, S.; Ren, M.; Zeng, X.; Ma, X.; Wu, Z.; Thacker, P.; Wu, G. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 2013, 45, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Mateo, R.D.; Yin, Y.-L.; Wu, G. Functional amino acids and fatty acids for enhancing production performance of sows and piglets. Asian-Australas. J. Anim. Sci. 2006, 20, 295–306. [Google Scholar] [CrossRef]
- Kim, S.W.; Chen, H.; Parnsen, W. Regulatory Role of Amino Acids in Pigs Fed on Protein-restricted Diets. Curr. Protein Pept. Sci. 2019, 20, 132–138. [Google Scholar] [CrossRef]
- Wu, G.; Knabe, D.A.; Kim, S.W. Arginine Nutrition in Neonatal Pigs. J. Nutr. 2004, 134, 2783S–2790S. [Google Scholar] [CrossRef]
- Kim, S.W.; McPherson, R.L.; Wu, G. Dietary Arginine Supplementation Enhances the Growth of Milk-Fed Young Pigs. J. Nutr. 2004, 134, 625–630. [Google Scholar] [CrossRef]
- Zhan, Z.; Ou, D.; Piao, X.; Kim, S.W.; Liu, Y.; Wang, J. Dietary arginine supplementation affects microvascular development in the small intestine of early-weaned pigs. J. Nutr. 2008, 138, 1304–1309. [Google Scholar] [CrossRef]
- da Silva Gomes, M.; Valente Júnior, D.T.; de Oliveira Silva, F.C.; Cunha Júnior, R.L.; Ribeiro Junior, V.; Saraiva, A.; Rocha, G.C. Effects of glutamine and glutamate on nursery piglets fed diets with different digestible lysine content. Semin. Ciências Agrárias 2021, 42, 3919–3930. [Google Scholar] [CrossRef]
- Valini, G.A.C.; Duarte, M.S.; Calderano, A.A.; Teixeira, L.M.; Rodrigues, G.A.; Fernandes, K.M.; Veroneze, R.; Serão, N.V.L.; Mantovani, H.C.; Rocha, G.C. Dietary nucleotide supplementation as an alternative to in-feed antibiotics in weaned piglets. Animal 2021, 15, 100021. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Qiu, Y.; Xiong, Y.; Xiao, H.; Yi, H.; Wen, X.; Lin, Z.; Wang, Z.; Yang, X.; et al. Effects of protein restriction and subsequent realimentation on body composition, gut microbiota and metabolite profiles in weaned piglets. Animals 2021, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Lynegaard, J.C.; Kjeldsen, N.J.; Bache, J.K.; Weber, N.R.; Hansen, C.F.; Nielsen, J.P.; Amdi, C. Low protein diets without medicinal zinc oxide for weaned pigs reduced diarrhoea treatments and average daily gain. Animal 2021, 15, 100075. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Guo, Q.; Wen, C.; Wang, W.; Li, Y.; Tan, B.; Li, F.; Yin, Y. Free Amino Acid Profile and Expression of Genes Implicated in Protein Metabolism in Skeletal Muscle of Growing Pigs Fed Low-Protein Diets Supplemented with Branched-Chain Amino Acids. J. Agric. Food Chem. 2016, 64, 9390–9400. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Valini, G.A.; de Souza Duarte, M.; de Amorim Rodrigues, G.; Veroneze, R.; Saraiva, A.; Hausman, G.; Rocha, G.C. Guanidinoacetic acid supplementation on growth performance and molecular mechanisms of lean mass gain in nursery pigs. Cienc. Rural 2020, 50, e20190948. [Google Scholar] [CrossRef]
- Cho, J.H.; Chen, Y.J.; Min, B.J.; Yoo, J.S.; Wang, Y.; Kim, I.H. Effects of reducing dietary crude protein on growth performance, odor gas emission from manure and blood urea nitrogen and IGF-1 concentrations of serum in nursery pigs. Anim. Sci. J. 2008, 79, 453–459. [Google Scholar] [CrossRef]
- Toledo, J.B.; Furlan, A.C.; Pozza, P.C.; Piano, L.M.; Carvalho, P.L.O.; Peñuela-Sierra, L.M.; Huepa, L.M.D. Effect of the reduction of the crude protein content of diets supplemented with essential amino acids on the performance of piglets weighing 6–15 kg. Livest. Sci. 2014, 168, 94–101. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, D.; Mao, X.; He, J.; Yu, J.; Zheng, P.; Luo, J.; Gao, J.; Htoo, J.K.; Yu, B. Evaluation of standardized ileal digestible lysine requirement for 8–20 kg pigs fed low crude protein diets. Anim. Sci. J. 2019, 90, 237–246. [Google Scholar] [CrossRef]
- Tang, W.; Qian, Y.; Yu, B.; Zhang, T.; Gao, J.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; Luo, J.; et al. Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets. J. Anim. Sci. 2019, 97, 2125–2138. [Google Scholar] [CrossRef]
- Liu, H.; Wu, L.; Han, H.; Li, Y.; Wang, L.; Yin, J.; Fan, W.; Bai, M.; Yao, J.; Huang, X.; et al. Reduced dietary nitrogen with a high Lys:CP ratio restricted dietary N excretion without negatively affecting weaned piglets. Anim. Nutr. 2019, 5, 115–123. [Google Scholar] [CrossRef]
- PIC. PIC Nutrition and Feeding Guidelines; PIC (Pig Improvement Company), Ed.; PIC.: Hendersonville, TN, USA, 2022. [Google Scholar]
- Madrid, J.; Martínez, S.; López, C.; Orengo, J.; López, M.J.; Hernández, F. Effects of low protein diets on growth performance, carcass traits and ammonia emission of barrows and gilts. Anim. Prod. Sci. 2013, 53, 146–153. [Google Scholar] [CrossRef]
- Qiu, K.; Zhang, X.; Jiao, N.; Xu, D.; Huang, C.; Wang, Y.; Yin, J. Dietary protein level affects nutrient digestibility and ileal microbiota structure in growing pigs. Anim. Sci. J. 2018, 89, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.; Bidner, T.D.; Payne, R.L.; Southern, L.L. Growth performance of 20- to 50-kilogram pigs fed low-crude-protein diets supplemented with histidine, cystine, glycine, glutamic acid, or arginine. J. Anim. Sci. 2011, 89, 3643–3650. [Google Scholar] [CrossRef]
- Li, Y.; Yin, J.; Han, H.; Liu, G.; Deng, D.; Kim, S.W.; Wu, G.; Li, T.; Yin, Y. Metabolic and Proteomic Responses to Long-Term Protein Restriction in a Pig Model. J. Agric. Food Chem. 2018, 66, 12571–12579. [Google Scholar] [CrossRef] [PubMed]
- Roux, M.L.; Donsbough, A.L.; Waguespack, A.M.; Powell, S.; Bidner, T.D.; Payne, R.L.; Southern, L.L. Maximizing the use of supplemental amino acids in corn-soybean meal diets for 20- to 45-kilogram pigs. J. Anim. Sci. 2011, 89, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Sotak-Peper, K.M.; Gonzalez-Vega, J.C.; Stein, H.H. Concentrations of digestible, metabolizable, and net energy in soybean meal produced in different areas of the United States and fed to pigs. J. Anim. Sci. 2015, 93, 5694. [Google Scholar] [CrossRef]
- Lopez, D.A.; Lagos, L.V.; Stein, H.H. Digestible and metabolizable energy in soybean meal sourced from different countries and fed to pigs. Anim. Feed Sci. Technol. 2020, 268, 114600. [Google Scholar] [CrossRef]
- Wolp, R.C.; Rodrigues, N.E.B.; Zangeronimo, M.G.; Cantarelli, V.S.; Fialho, E.T.; Philomeno, R.; Alvarenga, R.R.; Rocha, L.F. Soybean oil and crude protein levels for growing pigs kept under heat stress conditions. Livest. Sci. 2012, 147, 148–153. [Google Scholar] [CrossRef]
- Toledo, J.B.; Furlan, A.C.; Pozza, P.C.; Carraro, J.; Moresco, G.; Ferreira, S.L.; Gallego, A.G. Reduction of the crude protein content of diets supplemented with essential amino acids for piglets weighing 15 to 30 kilograms. Rev. Bras. Zootec. 2014, 43, 301–309. [Google Scholar] [CrossRef][Green Version]
- Morales, A.; Buenabad, L.; Castillo, G.; Arce, N.; Araiza, B.A.; Htoo, J.K.; Cervantes, M. Low-protein amino acid-supplemented diets for growing pigs: Effect on expression of amino acid transporters, serum concentration, performance, and carcass composition. J. Anim. Sci. 2015, 93, 2154–2164. [Google Scholar] [CrossRef]
- Fang, L.H.; Jin, Y.H.; Do, S.H.; Hong, J.S.; Kim, B.O.; Han, T.H.; Kim, Y.Y. Effects of dietary energy and crude protein levels on growth performance, blood profiles, and carcass traits in growing-finishing pigs. J. Anim. Sci. Technol. 2019, 61, 204–215. [Google Scholar] [CrossRef]
- Wang, Y.M.; Yu, H.T.; Zhou, J.Y.; Zeng, X.F.; Wang, G.; Cai, S.; Huang, S.; Zhu, Z.P.; Tan, J.J.; Johnston, L.J.; et al. Effects of feeding growing-finishing pigs with low crude protein diets on growth performance, carcass characteristics, meat quality and nutrient digestibility in different areas of China. Anim. Feed Sci. Technol. 2019, 256, 114256. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; et al. Effect of different dietary protein levels and amino acids supplementation patterns on growth performance, carcass characteristics and nitrogen excretion in growing-finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.Y.; Zhang, G.J.; Zhang, F.R.; Zhang, S.H.; Zeng, X.F.; Thacker, P.A.; Qiao, S.Y. Estimation of the optimal ratio of standardized ileal digestible tryptophan to lysine for finishing barrows fed low protein diets supplemented with crystalline amino acids. Czech J. Anim. Sci. 2014, 59, 26–34. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, L.; Li, J.; Luo, Y.; Zhang, B.; Xing, S.; Zhu, Y.; Sun, H.; Gao, F.; Zhou, G. Effects of dietary crude protein levels and cysteamine supplementation on protein synthetic and degradative signaling in skeletal muscle of finishing pigs. PLoS ONE 2015, 10, e0139393. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, J.V.; Hansen, M.J.; Soumeh, E.A.; Adamsen, A.P.S.; Poulsen, H.D. Effect of protein level on performance, nitrogen utilisation and carcass composition in finisher pigs. Acta Agric. Scand. A Anim. Sci. 2014, 64, 123–129. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, F.N.; Duan, Y.H.; Guo, Q.P.; Wen, C.Y.; Wang, W.L.; Huang, X.G.; Yin, Y.L. Low-protein diet improves meat quality of growing and finishing pigs through changing lipid metabolism, fiber characteristics, and free amino acid profile of the muscle. J. Anim. Sci. 2018, 96, 3221–3232. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Zhang, L.; Kong, X.; Li, F. Serine-to-glycine ratios in low-protein diets regulate intramuscular fat by affecting lipid metabolism and myofiber type transition in the skeletal muscle of growing-finishing pigs. Anim. Nutr. 2021, 7, 384–392. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef]
- Gardner, M.L.G.; Wood, D. Transport of peptides across the gastrointestinal tract. Biochem. Soc. Trans. 1989, 17, 934–937. [Google Scholar] [CrossRef]
- Sun, X.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J. Funct. Foods 2020, 64, 103680. [Google Scholar] [CrossRef]
- Nagasawa, M.; Murakami, T.; Sato, M.; Takahata, Y.; Morimatsu, F.; Furuse, M. Dietary animal proteins alter monoamine metabolism in the brain. Anim. Sci. J. 2012, 83, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Le Dinh, P.; van der Peet-Schwering, C.M.C.; Ogink, N.W.M.; Aarnink, A.J.A. Correction: Le Dinh et al. Effect of Diet Composition on Excreta Composition and Ammonia Emissions from Growing-Finishing Pigs. Animals 2022, 12, 229. Animals 2022, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.J.; Nørgaard, J.V.; Adamsen, A.P.S.; Poulsen, H.D. Effect of reduced crude protein on ammonia, methane, and chemical odorants emitted from pig houses. Livest. Sci. 2014, 169, 118–124. [Google Scholar] [CrossRef]
- Tous, N.; Lizardo, R.; Vilà, B.; Gispert, M.; Font-i-Furnols, M.; Esteve-Garcia, E. Effect of reducing dietary protein and lysine on growth performance, carcass characteristics, intramuscular fat, and fatty acid profile of finishing barrows. J. Anim. Sci. 2014, 92, 129–140. [Google Scholar] [CrossRef]
- Jiao, X.; Ma, W.; Chen, Y.; Li, Z. Effects of amino acids supplementation in low crude protein diets on growth performance, carcass traits and serum parameters in finishing gilts. Anim. Sci. J. 2016, 87, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Mao, P.; Guo, L.; Qiao, S. Crystalline amino acids supplementation improves the performance and carcass traits in late-finishing gilts fed low-protein diets. Anim. Sci. J. 2020, 91, e13317. [Google Scholar] [CrossRef]
- Li, P.L.; Wu, F.; Wang, Y.M.; Wang, C.P.; Zhou, J.Y.; Zeng, X.F.; Qiao, S.Y. Combination of non-protein nitrogen and N-carbamylglutamate supplementation improves growth performance, nutrient digestibility and carcass characteristics in finishing pigs fed low protein diets. Anim. Feed Sci. Technol. 2021, 273, 4753. [Google Scholar] [CrossRef]

| BW, kg | CP Level, % | SID Lys, % | Supplemental L-lysine 1, % | Additional Amino Acids 2 | Reference 3 | |
|---|---|---|---|---|---|---|
| Control | Lower | |||||
| 6–12 | 23.0 | 19.0 | 1.40 | 0.67 | Ile | Nyachoti et al. [23] |
| 13–19 | 19.5 | 16.0 | 1.10 | 0.46 | - | Cho et al. [49] |
| 6–8 | 23.1 | 17.2 | 1.30 | 0.36 | Val, Ile, His, Phe | Yue and Qiao [35] (P1) |
| 6–10 | 23.1 | 17.2 | 1.30 | 0.36 | Val, Ile, His, Phe | Yue and Qiao [35] (P1–2) |
| 8–11 | 20.7 | 16.7 | 1.07 | 0.22 | Val, Ile, Leu | Deng et al. [24] |
| 9–21 | 18.7 | 17.3 | 1.00 | 0.39 | Val, Ile | Nørgaard et al. [26] |
| 8–11 * | 20.9 | 17.9 | 1.30 | 0.65 | Val, Ile, Leu | Zhang et al. [37] |
| 12–16 | 19.7 | 12.7 | 1.15 | 0.82 | Val, Ile, His, Phe, Leu | Gloaguen et al. [36] (Exp.1) |
| 6–16 | 21.0 | 18.0 | 1.33 | 0.31 | Val, Ile | Toledo et al. [50] |
| 9–36 | 20.0 | 17.0 | 0.98 * | 0.63 | Val, Ile, Leu | Duan et al. [29] |
| 13–18 | 20.0 | 13.9 | 1.23 | 0.70 | Val, Ile, His, Phe, Leu | Peng et al. [11] |
| 8–9 | 19.5 | 16.7 | 1.23 | 0.38 | Val, Ile, Leu | Zheng et al. [27] (P1) |
| 8–18 | 19.5 | 16.7 | 1.23 | 0.38 | Val, Ile, Leu | Zheng et al. [27] (P2) |
| 8–21 * | 19.0 | 14.0 | 1.00 | 0.65 | Val, Ile, His, Phe, Leu, Tyr | Millet et al. [6] |
| 8–22 * | 19.0 | 14.0 | 1.10 | 0.80 | Val, Ile, His, Phe, Leu, Tyr | Millet et al. [6] |
| 8–13 | 20.0 | 18.5 | 1.23 | 0.41 | Val, Ile | Zhou et al. [51] (P1) |
| 8–21 | 20.0 | 18.5 | 1.23 | 0.41 | Val, Ile | Zhou et al. [51] (P1–2) |
| 7–11 | 20.5 | 18.0 | 1.43 | 0.55 | Val | Tang et al. [52] |
| 6–25 * | 21.0 | 18.0 | 1.29 | 0.49 | Val | Rattigan et al. [30] (Exp.1) |
| 7–24 * | 21.0 | 18.0 | 1.29 | 0.49 | Val | Rattigan et al. [30] (Exp.2) |
| 6–12 | 21.0 | 18.0 | 1.40 | 0.58 | Val, Ile, His | Batson et al. [19] (Exp.2) |
| 5–7 | 22.0 | 19.0 | 1.50 * | 0.45 | Ile, His, Phe | Limbach et al. [9] (P1) |
| 5–12 | 22.0 | 19.0 | 1.35 * | 0.40 | His, Phe | Limbach et al. [9] (P2) |
| BW, kg | CP Level, % | SID Lys, % | Supplemental L-lysine 1, % | Additional Amino Acids 2 | Reference 3 | |
|---|---|---|---|---|---|---|
| Control | Lower | |||||
| 20–46 * | 16.0 | 12.0 | 0.77 * | 0.45 | Val, Ile | Figueroa et al. [7] (Exp. 1) |
| 21–46 * | 16.0 | 12.0 | 0.77 * | 0.45 | Val, Ile | Figueroa et al. [7] (Exp. 3) |
| 44–65 * | 20.7 | 15.0 | 1.03 * | 0.49 | - | Carpenter et al. [13] |
| 19–39 | 18.2 | 13.4 | 0.83 | 0.33 | Val, Ile | Powell et al. [57] (Exp. 1) |
| 21–40 | 18.2 | 13.4 | 0.83 | 0.33 | Val, Ile | Powell et al. [57] (Exp. 2) |
| 19–38 | 18.2 | 13.4 | 0.83 | 0.33 | Val, Ile | Powell et al. [57] (Exp. 3) |
| 23–45 | 18.2 | 13.4 | 0.83 | 0.33 | Val, Ile | Powell et al. [57] (Exp. 4) |
| 23–44 | 18.2 | 13.4 | 0.83 | 0.33 | Val, Ile | Powell et al. [57] (Exp. 5) |
| 24–44 | 18.2 | 14.3 | 0.83 | 0.26 | - | Roux et al. [59] (Exp. 3) |
| 20–40 | 18.2 | 13.4 | 0.83 | 0.33 | Val | Roux et al. [59] (Exp. 4) |
| 37–65 * | 18.0 | 15.5 | 0.90 | 0.17 | - | Wolp et al. [62] |
| 23–59 | 16.0 | 14.0 | 0.83 | 0.46 | - | Madrid et al. [55] |
| 15–30 | 16.0 | 14.0 | 1.14 | 0.36 | Val, Ile | Toledo et al. [63] |
| 24–40 | 22.0 | 14.0 | 0.98 | 0.63 | Val, Ile, His, Phe, Leu | Morales et al. [64] |
| 14–34 | 20.0 | 13.9 | 1.23 | 0.70 | Val, Ile, His, Phe, Leu | Peng et al. [11] |
| 16–33 | 17.0 | 15.0 | 1.23 | 0.62 | Val, Ile, His, Phe, Leu | Che et al. [8] |
| 36–60 | 18.3 | 15.2 | 0.96 | 0.36 | - | Li et al. [58] |
| 30–67 | 18.0 | 14.0 | 0.98 | 0.50 | Val | Qiu et al. [56] |
| 31–42 | 16.3 | 14.3 | 0.83 * | 0.16 | - | Fang et al. [65] |
| 29–63 | 17.0 | 15.0 | 1.01 | 0.54 | Val | Wang et al. [66] (Exp. 1) |
| 25–60 | 17.0 | 15.0 | 0.98 | 0.47 | Val | Wang et al. [66] (Exp. 2) |
| 25–51 | 17.5 | 13.5 | 0.98 | 0.54 | Val, Ile, His, Phe | Zhao et al. [67] |
| 24–53 | 17.0 | 13.5 | 0.98 | 0.39 | Val, Ile, His, Phe | Zhao et al. [14] |
| 30–50 | 18.1 | 15.1 | 1.07 | 0.47 | Val, Ile | Esteves et al. [18] |
| BW, kg | CP Level, % | SID Lys, % | Supplemental L-lysine 1, % | Additional Amino Acids 2 | Reference 3 | |
|---|---|---|---|---|---|---|
| Control | Lower | |||||
| 55–102 | 15.9 | 13.6 | 0.74 | 0.27 | Val | Hansen et al. [78] |
| 54–98 | 15.9 | 13.6 | 0.74 | 0.27 | Val | Nørgaard et al. [70] |
| 62–97 | 13.1 | 12.1 | 0.65 | 0.26 | Val | Tous et al. [79] |
| 72–104 | 15.3 | 12.0 | 0.71 | 0.39 | Val, Ile | Xie et al. [68] (Exp. 1) |
| 89–114 | 14.0 | 12.0 | 0.73 | 0.35 | Val, Ile | Qin et al. [17] |
| 65–95 | 14.0 | 10.0 | 0.73 | 0.43 | Val, Ile, Phe | Zhou et al. [69] |
| 59–98 | 15.6 | 11.6 | 0.85 | 0.46 | Val, Ile | Jiao et al. [80] |
| 62–100 | 16.0 | 13.0 | 0.72 | 0.21 | - | Li et al. [71] |
| 94–118 | 13.7 | 10.5 | 0.70 | 0.41 | Val, Ile | Ma et al. [81] |
| 109–127 | 13.0 | 10.0 | 0.66 | 0.41 | Val, Ile | Soto et al. [12] (Exp. 2) |
| 112–135 | 13.0 | 9.0 | 0.55 | 0.34 | Val, Ile | Soto et al. [12] (Exp. 3) |
| 74–107 | 13.6 | 11.1 | 0.73 | 0.42 | Val | Li et al. [82] |
| 59–95 | 16.0 | 12.0 | 0.73 | 0.29 | - | Zhou et al. [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, G.C.; Duarte, M.E.; Kim, S.W. Advances, Implications, and Limitations of Low-Crude-Protein Diets in Pig Production. Animals 2022, 12, 3478. https://doi.org/10.3390/ani12243478
Rocha GC, Duarte ME, Kim SW. Advances, Implications, and Limitations of Low-Crude-Protein Diets in Pig Production. Animals. 2022; 12(24):3478. https://doi.org/10.3390/ani12243478
Chicago/Turabian StyleRocha, Gabriel Cipriano, Marcos Elias Duarte, and Sung Woo Kim. 2022. "Advances, Implications, and Limitations of Low-Crude-Protein Diets in Pig Production" Animals 12, no. 24: 3478. https://doi.org/10.3390/ani12243478
APA StyleRocha, G. C., Duarte, M. E., & Kim, S. W. (2022). Advances, Implications, and Limitations of Low-Crude-Protein Diets in Pig Production. Animals, 12(24), 3478. https://doi.org/10.3390/ani12243478





