Alpine Musk Deer (Moschus chrysogaster) Adjusts to a Human-Dominated Semi-Arid Mountain Ecosystem
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
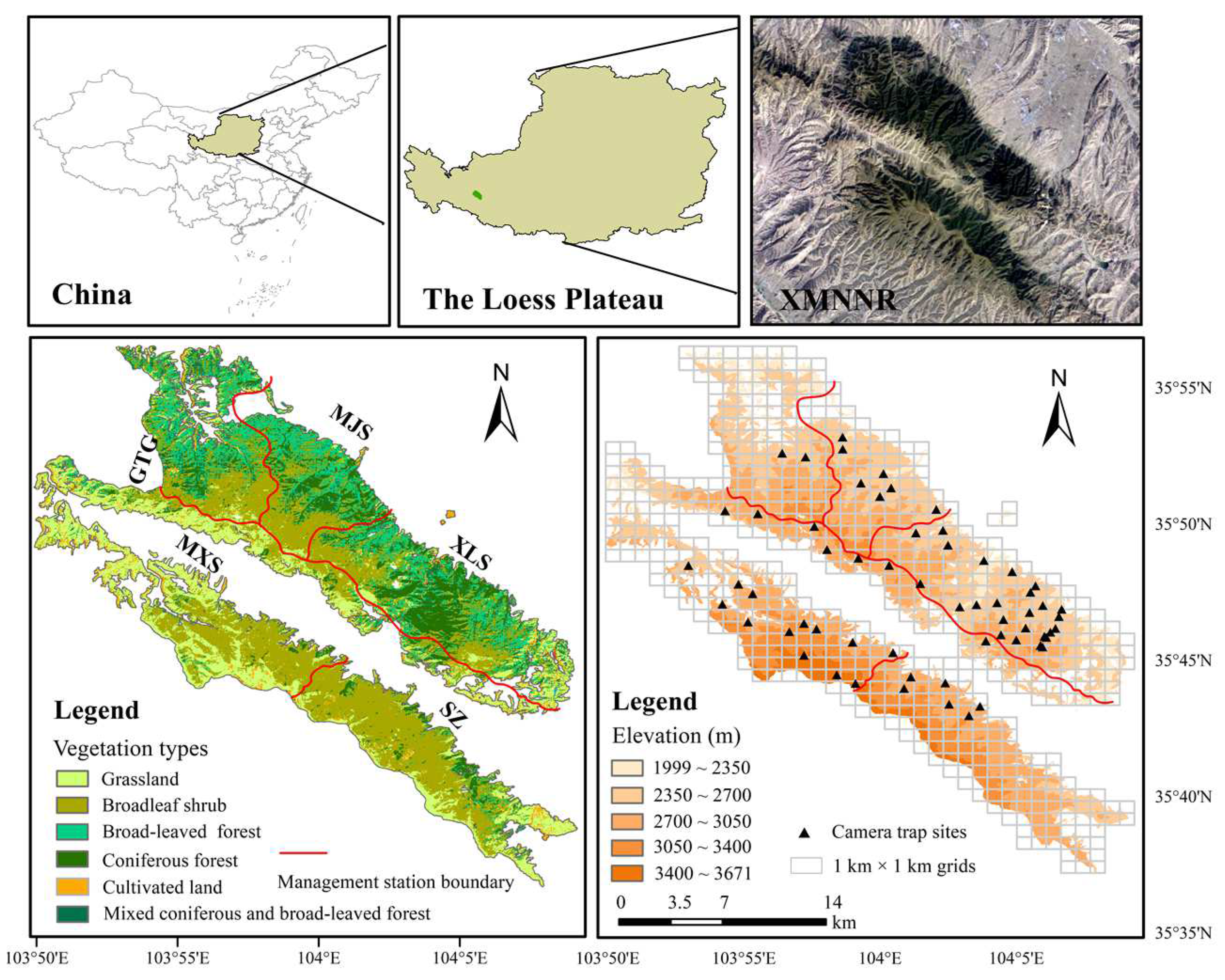
2.1. Camera Trap Survey
2.2. Potential Distribution Assessment
2.2.1. Environmental Variables Obtained and Preprocessing
2.2.2. Model Procedure
2.3. Data Analyses
3. Results
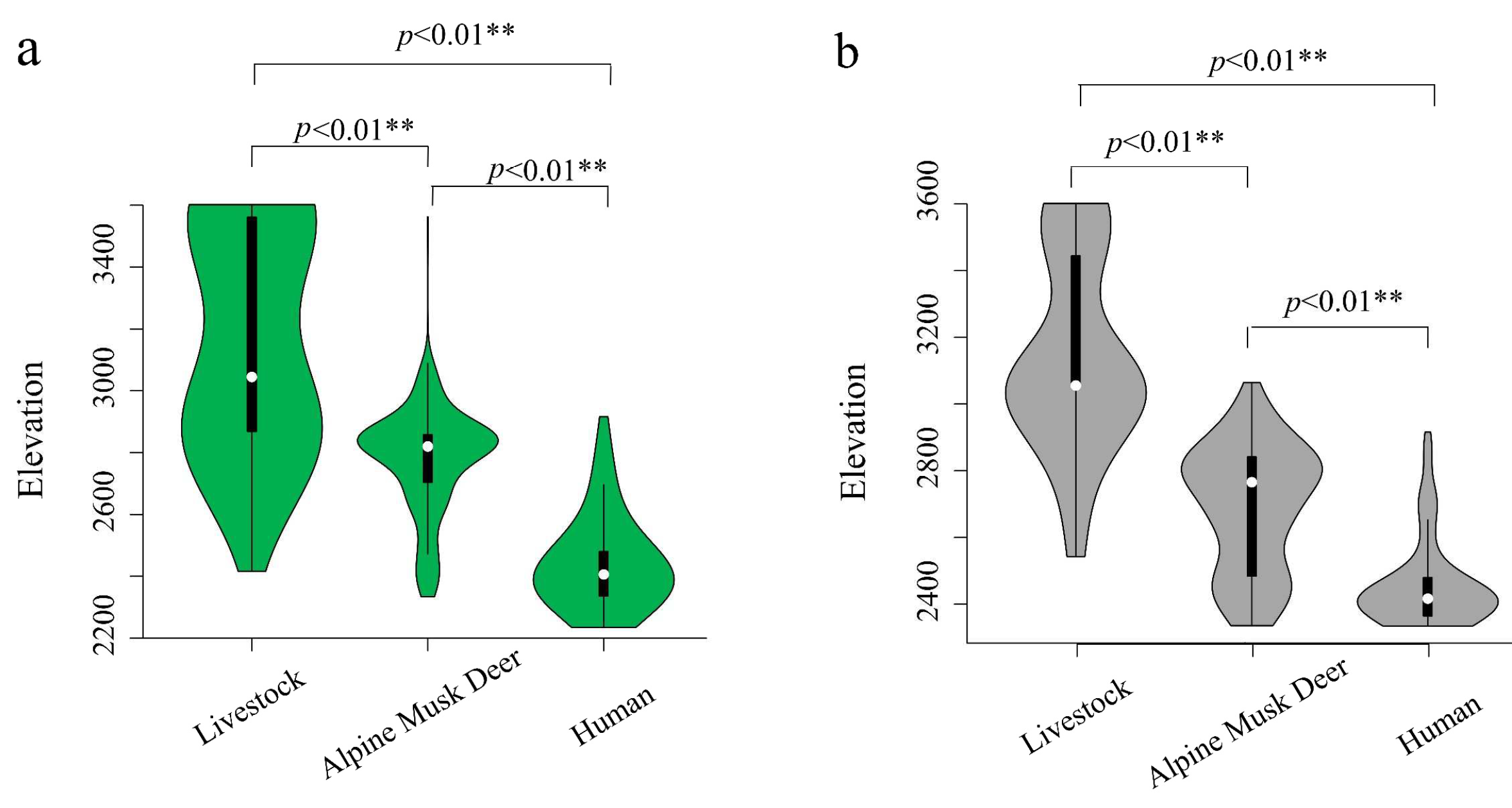
3.1. Spatial Distribution of AMD, Livestock Grazing, and Human Activities
3.2. Temporal Activity Patterns
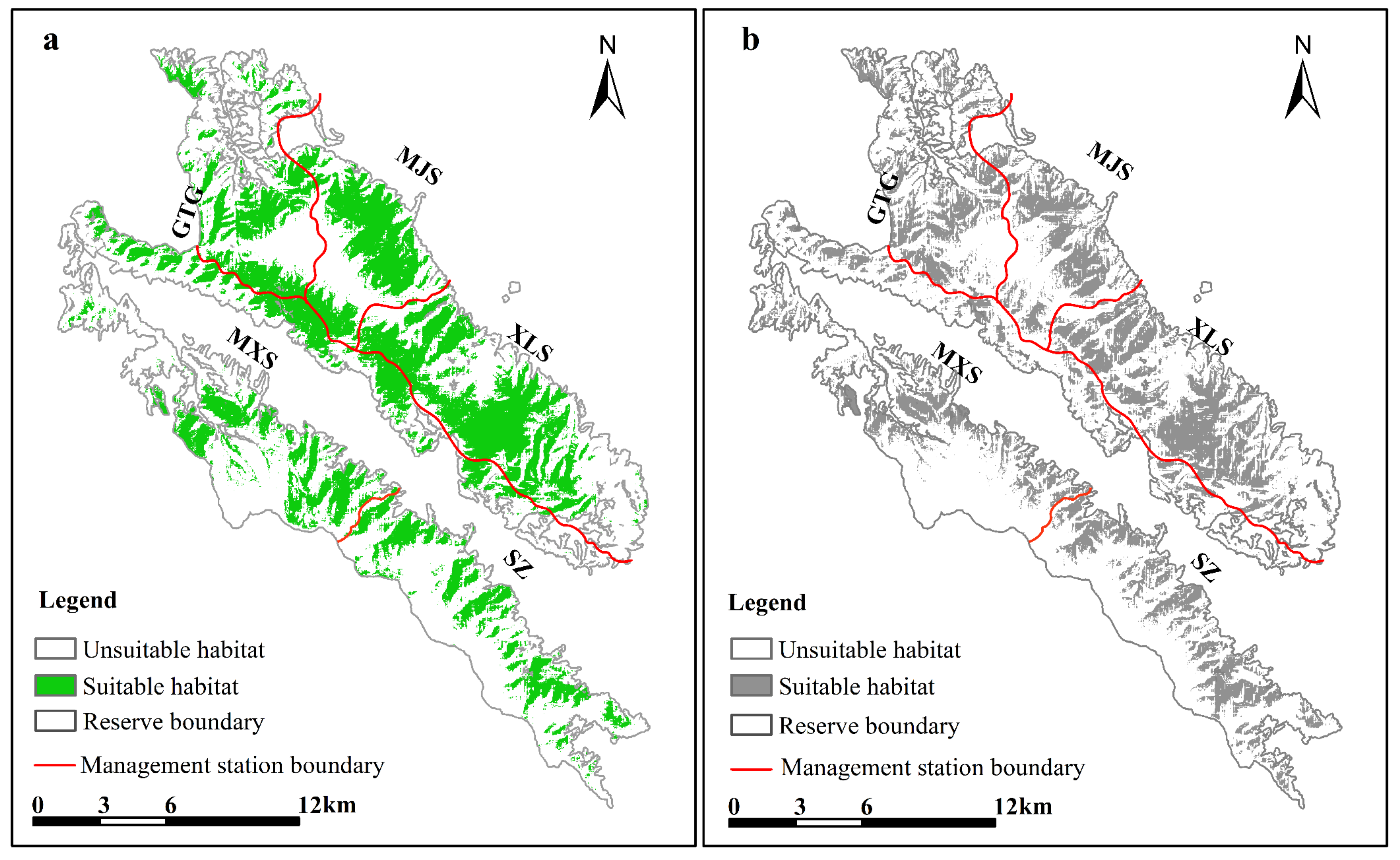
3.3. MaxENT Model Prediction Results and Habitat Suitability Evaluation
3.4. Habitat Selection Preference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Superbie, C.; Stewart, K.M.; Regan, C.E.; Johnstone, J.F.; Mcloughlin, P.D. Northern boreal caribou conservation should focus on anthropogenic disturbance, not disturbance-mediated apparent competition. Biol. Conserv. 2022, 265, 109426. [Google Scholar] [CrossRef]
- Ficetola, F.G.; Bernardi, F. Amphibians in a human-dominated landscape: The community structure is related to habitat features and isolation. Biol. Conserv. 2004, 119, 219–230. [Google Scholar] [CrossRef]
- Li, X.; Bleisch, W.V.; Jiang, X. Effects of Ethnic Settlements and Land Management Status on Species Distribution Patterns: A Case Study of Endangered Musk Deer (Moschus spp.) in Northwest Yunnan, China. PLoS ONE 2016, 11, e0155042. [Google Scholar] [CrossRef]
- Larson, C.L.; Reed, S.E.; Merenlender, A.M.; Crooks, K.R. Effects of Recreation on Animals Revealed as Widespread through a Global Systematic Review. PLoS ONE 2016, 11, e0167259. [Google Scholar] [CrossRef] [PubMed]
- Nix, J.H.; Howell, R.G.; Hall, L.K.; McMillan, B.R. The influence of periodic increases of human activity on crepuscular and nocturnal mammals: Testing the weekend effect. Behav. Process. 2018, 146, 16–21. [Google Scholar] [CrossRef]
- Blair, A.G.; Meredith, T.C. Community perception of the real impacts of human–wildlife conflict in Laikipia, Kenya: Capturing the relative significance of high-frequency, low-severity events. Oryx 2017, 52, 497–507. [Google Scholar] [CrossRef]
- Majumder, A.; Quresh, Q.; Sankar, K.; Kumar, A. Long-term monitoring of a Bengal tiger (Panthera tigris tigris) population in a human-dominated landscape of Central India. Eur. J. Wildl. Res. 2016, 63, 17. [Google Scholar] [CrossRef]
- Harris, R. Moschus chrysogaster. In The IUCN Red List of Threatened Species; 2016; p. e.T13895A61977139. Available online: http://www.sciepub.com/reference/350245 (accessed on 3 June 2022).
- Bastin, J.F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- Chang, R.Y.; Liu, B.; Yao, G.; Wang, X. Effects of soil physicochemical properties and stand age on fine root biomass and vertical distribution of plantation forests in the Loess Plateau of China. Ecol. Res. 2012, 27, 827–836. [Google Scholar] [CrossRef]
- Benayas, J.; Newton, A.; Diaz, A.; Bullock, J. Enhancement of Biodiversity and Ecosystem Services by Ecological Restoration: A Meta-Analysis. Science 2009, 325, 1121–1124. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, J.; Gao, H.; Cai, Z.; Zhou, X.; Li, S.; Zhang, T. Musk deer (Moschus spp.) face redistribution to higher elevations and latitudes under climate change in China. Sci. Total Environ. 2019, 704, 135335. [Google Scholar] [CrossRef]
- You, Z.Y.; Lu, B.G.; Luo, N.L.; Xie, F.; Yang, K.; Yang, N. Habitat suitability assessment of Moschus sifanicus based on MaxEnt modeling in Chaqingsongduo National Nature Reserve Sichuan Province. J. Nat. Conserv. 2021, 47, 555–561. [Google Scholar] [CrossRef]
- Yang, Q.; Meng, X.; Xia, L.; Feng, Z. Conservation status and causes of decline of musk deer (Moschus spp.) in China. Biol. Conserv. 2003, 109, 333–342. [Google Scholar] [CrossRef]
- Tong, M.; Pan, S.X.; Wang, X.W.; An, T.H.; Feng, J.C.; Meng, X.X. Summer habitat selection of Alpine musk deer in Xinglongshan National Nature Reserve, Northwestern China. Zool. Res. 2010, 31, 610–616. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.P.; Xu, T.; Jun, Q.; Zhang, Y.; Meng, X. Population distribution, quantitative characteristics and influence factors of the wild Alpine musk deer in Xinglongshan National Natural Reserve, Gansu Province. Acta Ecol. Sin. 2020, 40. [Google Scholar] [CrossRef]
- Buchholz, R.; Hanlon, E.M. Ecotourism, wildlife management, and behavioural biologists. In Behavioural Responses to a Changing World; 2012; Available online: https://academic.oup.com/book/9434/chapter-abstract/156309250?redirectedFrom=fulltext&login=false (accessed on 3 June 2022).
- Bhattacharya, T.; Kittur, S.; Sambandam, S.; Rawat, G. Diet Overlap between Wild Ungulates and Domestic Livestock in the Greater Himalaya: Implications for Management of Grazing Practices. Proc. Zool. Soc. 2012, 65, 11–21. [Google Scholar] [CrossRef]
- Buckland, S.T.; Rexstad, E.; Marques, T.A.; Oedekoven, C.S. Distance Sampling: Methods and Applications; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Pal, R.; Bhattacharya, T.; Qureshi, Q.; Buckland, S.T.; Sathyakumar, S. Using distance sampling with camera traps to estimate the density of group-living and solitary mountain ungulates. Oryx 2021, 55, 668–676. [Google Scholar] [CrossRef]
- Pal, R.; Panwar, A.; Goyal, S.P.; Sathyakumar, S. Space Use by Woolly Wolf Canis lupus chanco in Gangotri National Park, Western Himalaya, India. Front. Ecol. Evol. 2022, 9. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species–Elith–2010–Methods in Ecology and Evolution–Wiley Online Library. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Hill, N.J.; Tobin, A.J.; Reside, A.E.; Pepperell, J.G.; Bridge, T.C. Dynamic habitat suitability modelling reveals rapid poleward distribution shift in a mobile apex predator. Glob. Change Biol. 2016, 22, 1086–1096. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Lamsal, P.; Kumar, L.; Aryal, A.; Atreya, K. Future climate and habitat distribution of Himalayan Musk Deer (Moschus chrysogaster). Ecol. Inform. 2018, 44, 101–108. [Google Scholar] [CrossRef]
- Khadka, K.K.; James, D.A. Modeling and mapping the current and future climatic-niche of endangered Himalayan musk deer. Ecol. Inform. 2017, 40, 1–7. [Google Scholar] [CrossRef]
- Han, C.; Liu, Y.; Zhang, C.; Li, Y.; Zhou, T.; Khan, S.; Chen, N.; Zhao, C.M. Effects of Three Plantation Coniferous Species on Plant-Soil Feedbacks and Soil Physical and Chemical Properties in Semi-Arid Mountain Ecosystems. For. Ecosyst. 2020, 8. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, Z.; Zhang, L.; Wang, X.; Li, M.; Xiang, Z. Activity Rhythms of Coexisting Red Serow and Chinese Serow at Mt. Gaoligong as Identified by Camera Traps. Animals 2019, 9, 1071. [Google Scholar] [CrossRef]
- Wu, T.W.; Lu, Y.X.; Fang, Y.J.; Xin, X.G.; Liu, X.H. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Kass, J.M.; Vilela, B.; Aiellolammens, M.E.; Muscarella, R.; Anderson, R.P. Wallace: A Modular Platform for Reproducible Modeling of Species Niches and Distributions. 2018. Version 1.0.6.1. Available online: https://besjournals.onlinelibrary.wiley.com/doi/full/10.1111/2041-210X.12945 (accessed on 3 June 2022).
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Fouquet, A.; Ficetola, G.F.; Haigh, A.; Gemmell, N. Using ecological niche modelling to infer past, present and future environmental suitability for Leiopelma hochstetteri, an endangered New Zealand native frog. Biol. Conserv. 2010, 143, 1375–1384. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, R. Study on the Phonological Period about Rosaceae in Xinglongshan National Nature Reserve, Gansu Province. For. Sci. Technol. 2017, 69–71. [Google Scholar] [CrossRef]
- LI, S.; Mcshea, W.J.; Wang, D.J.; Shao, L.K.; Shi, X.G. The use of infrared-triggered cameras for surveying phasianids in Sichuan Province, China. Ibis 2010, 152, 299–309. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overlap: Estimates of Coefficient of Overlapping for Animal Activity Patterns. 2016. Available online: https://CRAN.Rproject.org/package=overlap (accessed on 7 January 2022).
- Rowcliffe, J.M.; Kays, R.; Kranstauber, B.; Carbone, C.; Jansen, P.A. Quantifying levels of animal activity using camera trap data. Methods Ecol. Evol. 2014, 5, 1170–1179. [Google Scholar] [CrossRef]
- Rowcliffe, M. Activity: Animal Activity StatistIEs. 2016. Available online: https://CRAN.R-project.org/package=activity (accessed on 7 January 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 7 January 2022).
- Bista, D.; Baxte, G.S.; Hudson, N.J.; Murray, P.J. Seasonal resource selection of an arboreal habitat specialist in a human-dominated landscape: A case study using red panda. Curr. Zool. 2022. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Guo, Z.; Dai, L.J.; Wu, Y.Q.; Liu, H.Y.; Li, Y.F.; Chen, H.; Zhang, Y.N.; Zhao, Y.X.; et al. Integrating Maxent model and landscape ecology theory for studying spatiotemporal dynamics of habitat: Suggestions for conservation of endangered Red-crowned crane. Ecol. Indic. 2020, 116, 106472. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Sun, Z.; An, B.; Zhang, D.; Chen, L. Alpine Musk Deer (Moschus chrysogaster) Adjusts to a Human-Dominated Semi-Arid Mountain Ecosystem. Animals 2022, 12, 3061. https://doi.org/10.3390/ani12213061
Zhang L, Sun Z, An B, Zhang D, Chen L. Alpine Musk Deer (Moschus chrysogaster) Adjusts to a Human-Dominated Semi-Arid Mountain Ecosystem. Animals. 2022; 12(21):3061. https://doi.org/10.3390/ani12213061
Chicago/Turabian StyleZhang, Lixun, Zhangyun Sun, Bei An, Dexi Zhang, and Liuyang Chen. 2022. "Alpine Musk Deer (Moschus chrysogaster) Adjusts to a Human-Dominated Semi-Arid Mountain Ecosystem" Animals 12, no. 21: 3061. https://doi.org/10.3390/ani12213061
APA StyleZhang, L., Sun, Z., An, B., Zhang, D., & Chen, L. (2022). Alpine Musk Deer (Moschus chrysogaster) Adjusts to a Human-Dominated Semi-Arid Mountain Ecosystem. Animals, 12(21), 3061. https://doi.org/10.3390/ani12213061





