Simple Summary
Growth qualities are critical economic traits that substantially impact pork yield. Here, we evaluated the effect of somatostatin (SS) DNA vaccines on growth promotion in fattening pigs. Our results demonstrate that immunisation with SS DNA vaccine induces cellular and humoral immunity in fattening pigs, producing anti-SS antibodies that neutralise endogenous SS, thereby promoting daily gain and slaughter weight. In addition, the vaccine does not pose a safety challenge. Consequently, it is possible to accelerate the growth of fattening pigs through the immune SS DNA vaccine, which will bring more economic benefits to the company and the farmer.
Abstract
This study aimed to evaluate the efficacy and safety of the SS DNA vaccine on growing pigs. Randomly, 147 pigs were divided into four groups, treatment 1 (T1, 3 × 109 CFU/mL, n = 39), T2 (3 × 108 CFU/mL, n = 35), T3 (3 × 107 CFU/mL, n = 35) and control group (phosphate-buffered saline, n = 38). All animals received two vaccinations separated by 45 days and the same diet and management. The results showed that all treatment groups (T1, T2 and T3) had significantly higher slaughter weight (d 185) than the Ctrl group (p < 0.05), and daily gain between 50 and 110 days of age was significantly higher in all treatment groups than in the Ctrl group (p < 0.05). Antibody-positive pigs have significantly higher daily weight gain than that in antibody-negative pigs (p < 0.05). The results of the meat quality analysis showed no significant changes between the P (antibody-positive pigs) and N (antibody-negative pigs) groups. Furthermore, the results showed that antibody titres at 110 and 185 days had a significant positive correlation with the daily weight gain (p < 0.05) and a significant negative correlation with the backfat thickness (p < 0.05). Evaluating the safety of vaccines by PCR amplification of target genes (GS/2SS), faecal, soil and water samples had no target genes detected by PCR amplification in these samples after 5 days, and no GS/2SS were detected in the blood and tissues for the experimental period. Moreover, no abnormalities were found in pathological sections of the P group compared with the N group. In conclusion, SS DNA vaccines can promote the growth of fattening pigs to a certain extent without altering the meat quality, and it has no effects on the safety of the surrounding environment.
1. Introduction
Growth hormone (GH) is a critical hormone in growth regulation, by modulating the growth hormone insulin-like growth factor (GH-IGF) axis and promoting cellular protein synthesis, cell division, and proliferation. Hence, it can accelerate the growth of muscle, bone, and other animal tissues [1]. Studies have shown that somatostatin (SS) has a wide range of inhibitory effects on GH, prolactin (PRL), thyroid-stimulating hormone (TSH), glucagon and insulin [2]. In addition, SS also inhibits digestive system functions, including digestive juice secretion, intestinal motility and blood flow in the small intestine [3,4,5]. SS exerts its biological effects mainly by binding to somatostatin receptors (SSTRs), which are widely expressed in the body’s tissues [6]. Passive [7] or active [8,9] immunisation against somatostatin through immunological methods using specific antibodies to neutralise endogenous somatostatin to block somatostatin-somatostatin receptor binding and promote the release of growth hormones and other hormones to improve the efficiency of nutrient uptake in animals to promote growth. The current study evaluated the effect of the SS DNA vaccine (pVGS/2SS-asd) on growth traits in fattening pigs through nasal immunisation.
It is well known that somatostatin has an inhibitory effect on animal growth. A previous study demonstrated that the production of antibodies to SS by the body exposed to active immunisation releases their growth inhibitory effect [10] and promote the growth of animals such as sheep [11,12] and heifer [8]. All of the above evidence suggests that SS-active immunisation is an effective method to promote animal growth. However, due to their high preparation costs, the promotion of synthetic peptides and recombinant protein and their application in livestock production is limited.
The advent of genetically engineered vaccines has brought new ideas to active immunisation with somatostatin. DNA vaccines have many advantages compared to conventional vaccines, including straightforward design, low production costs, ease of transport, no unsafe infectious agents involved, and encoding of multiple immunogenic epitopes [13,14]. However, DNA vaccines also have some disadvantages, such as having relatively poor immunogenicity [15], and the insertion of foreign DNA into the host genome may cause the cell to become cancerous [16]. In addition, the live attenuated Salmonella strain has the potential to spread across the natural environment through faeces; this could lead to contamination of the environment and a virulent reversion through the process of survival ex vivo [17,18]. Therefore, the objective of this study was to (1) evaluate the effects of the bacteria-delivered SS DNA vaccine on the growth of fattening pigs and (2) to evaluate the safety of SS DNA vaccine, including the surrounding environment and insertion of foreign DNA into the host genome.
2. Materials and Methods
2.1. Preparation of Bacterial Vaccine
The SS DNA vaccine (pGS/2SS-asd) was constructed previously in our laboratory using the pVAX-asd eukaryotic expression plasmid [19]. Before immunisation, bacteria were plated on LB agar in triplicate to determine the number of colony-forming units (CFU) and were adjusted approximately 3 × 109 CFU/mL by PBS.
2.2. Animals and Immunisation Protocol
A total of 147 cross-bred starter pigs (Duroc-Landrace-Yorkshire), around 50 days old, were selected from a pig farm in Hubei, China (Hubei Jinlin Original Breed Livestock Co., Ltd.). All the animals were in good general health, and their physical condition was nearly the same size in each group. During the test period, the animals were fed and watered ad libitum. The barns were equipped with ventilation, pens were cleaned twice daily and animal behavioral observations were made daily. The animal handling procedures and all experimental protocols were approved by the Huazhong Agricultural University Animal Care and Use Committee (HZAUSW-2018-029).
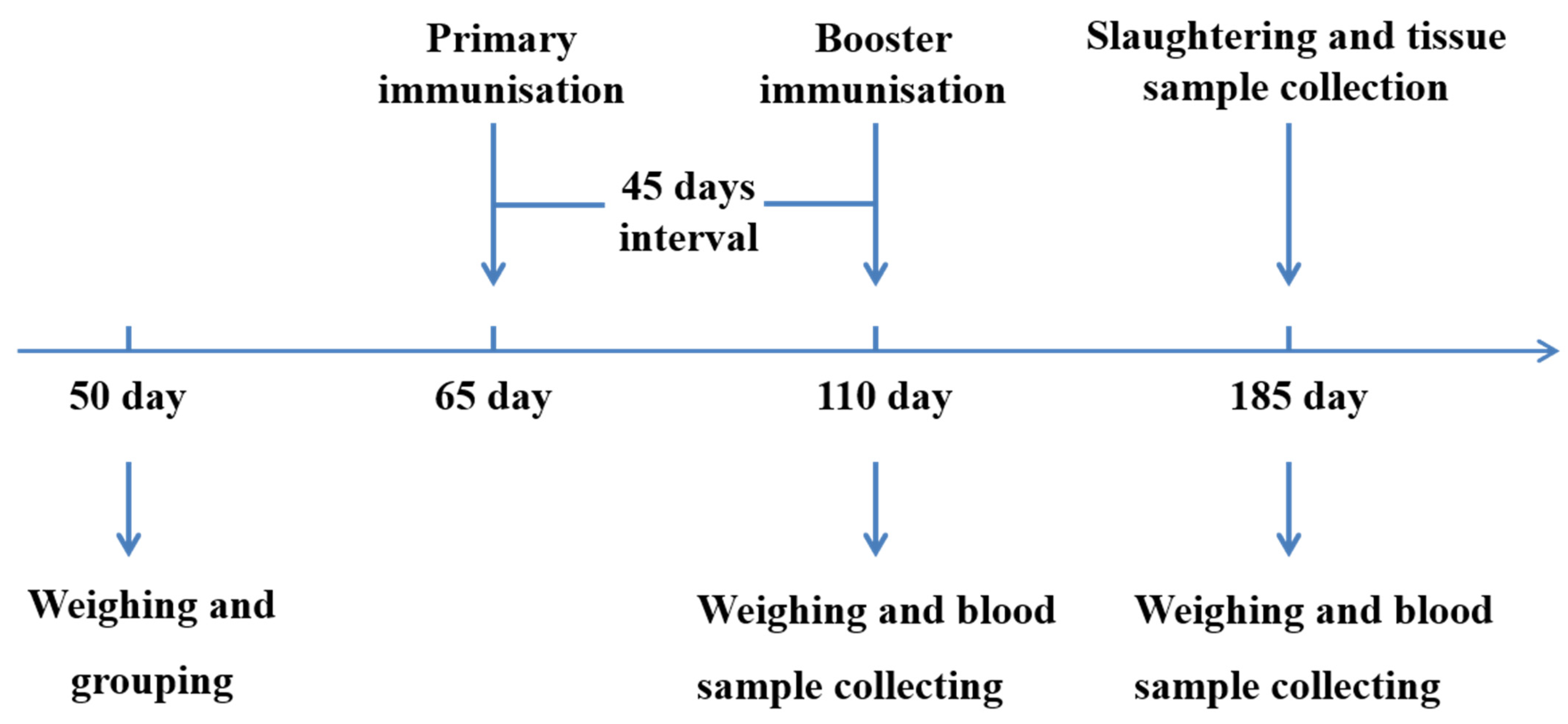
All animals were divided into four groups: groups T1 (3 × 109 CFU/mL, n = 39), T2 (3 × 108 CFU/mL, n = 35) and T3 (3 × 107 CFU/mL, n = 35) were nasally immunised twice (09:00 and 15:00) a day with 15 mL of the SS DNA vaccine for 3 days, respectively. The control group (n = 38) was nasally immunised twice daily with 15 mL of PBS for 3 days. All animals were immunised twice with an interval of 45 days (Figure 1).
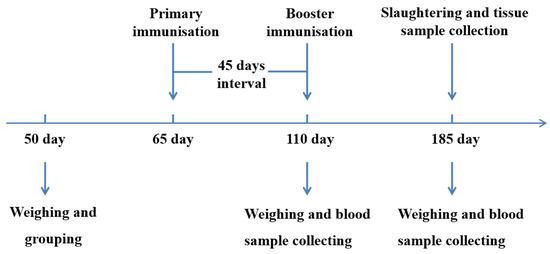
Figure 1.
An outline explains the experimental design. Experimental pigs were immunised with SS DNA vaccine on days 65 and 110, and the control group was administered PBS. All pigs were slaughtered on days 185.
2.3. Blood Collection and Antibody Titre Detection
The blood samples of pigs were collected from the jugular vein on days 110 and 185 of the experiment. The serum was separated by centrifugation and stored at −80 °C until further analysis.
Indirect ELISA was used to detect SS antibody titres in pig plasma. For detailed steps refer to the previous study [20]; those that differ from them include (1) each well of the 96-well micro titre plate was coated 100 ng/100 μL of SS antigen (Sangon Biotech (Shanghai) Co., Ltd.; Shanghai, China); (2) serum samples (100 μL) diluted with PBS (1:25, 1:50, 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:6400). Finally, under the same dilution factor, if the OD 450 value of the experimental group was greater than the mean of OD 450 value in the control group + 2SD (standard deviation), then it was judged as positive [21,22].
2.4. Detections of Hormones
ELISA Kit (MLBIO Biotechnology Co., Ltd.; Shanghai, China) specific to porcine was used to measure IL-4 (mL002314), IFN-γ (mL002333), GH (mL002349) and IGF-1 (mL002344) concentrations. For specific operation methods, refer to the product manual. The intra- and inter-assay coefficients of variation were less than 10.0% and 10.0% for IL-4, IFN-γ, GH, and IGF-1. The assay sensitivity were 0.75 ng/mL for GH, 5 ng/mL for IGF-1 and 4 ng/mL for IL-4, 2.5 ng/mL for IFN-γ.
2.5. Meat Quality and Backfat Thickness Analysis
The experimental pigs were slaughtered on day 185, and the longissimus dorsi was collected and stored on ice for meat quality analysis. Backfat thickness was tested with ultrasonography before slaughter. Subsequently, ten pigs were randomly selected from each antibody-positive and -negative groups for meat quality determination. Each indicator was measured as follows: The pH value was measured at the center of the longissimus dorsi after 1 h of slaughter (pH1 value) and again after 24 h of refrigeration at 4 °C (pH24 value), and the meat was taken for pH measurement on three different sides using a pH meter; meat samples from the longissimus dorsi cross-section within 2 h of slaughter were visually inspected and scored on a 5-point scale for colour and marbling using the National Pork Producers Council’s (NPPC) Meat Colour Scale and Marbling Scale; The sample is cut into 1 cm3 squares and the cut resistance is measured along the vertical direction of the muscle fibres using a mass spectrometer; moisture, ash, crude protein and intramuscular fat were determined according to the method of the national standard GB5009.3-2016 “National Standard for Food Safety Determination of Moisture in Food”.
2.6. Environmental Safety Testing
Fusion gene GS/2SS in bacteria from faecal, water, and soil samples was detected by a PCR method to evaluate the safety of the SS DNA vaccine for the surrounding environment. The faecal, water and soil samples were collected from immunised pigs on days 3, 5, 7, 10, 15 and 30 after immunisation. Faecal (1 g) and soil aliquots (1 g) were placed into 9 mL of sterile PBS, respectively. These samples were serially diluted in a 10-fold series with sterile PBS, up to a dilution of 103-fold. The 10-fold dilution series (200 µL of each dilution) were plated onto MacConkey agar (Tianhe, Hangzhou, China) and incubated at 37 °C for 24 h. Water samples (200 µL) were also plated onto MacConkey agar for incubation. All suspect colonies were picked and the target fragment GS/2SS of the vaccine plasmid DNA was detected by PCR (Primer: F: GCTGTGATGAATGAAACCGTAGA; R: GCAACAGGAGGGATACATAGAGG). Amplification was carried out under the following conditions: Pre-denaturing at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 40 s, annealing at 58 °C for 60 s, and extension at 72 °C for 40 s in a total volume of 20 µL of mixed liquids, including DNA (1 µL), RNase-free water (8 µL), sense and antisense (1 µL) and TaqMix (10 µL).
2.7. Data Statistics
The data were analysed using the MIXED procedure of SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA). The mean values were compared using Tukey’ multiple range test with a significance level of p < 0.05. Nonparametric data were analysed using the chi-squared test. A bivariate analysis of Pearson correlation was performed. Variability in the data was expressed as the standard error of means (SEM), and p < 0.05 was considered statistically significant.
3. Results
3.1. Effect on Serum Anti-SS Antibody Titre Levels and Positivity Rate in Pigs
As shown in Table 1, anti-SS antibody titre levels in serum were analysed. On day 45 after the primary immunisation (d 110), there were no significant differences between the treatment groups (p > 0.05), where the highest antibody titre was in the T1 group at 268.75. In addition, there was no significant difference in antibody positivity between the groups (p > 0.05). On day 75 after the booster immunisation (d 185), there was no significant difference in antibody titre levels between the treatment groups (p > 0.05). Both antibody titres and antibody positivity rates were at low levels compared to d 110.

Table 1.
The antibody titre and antibody-positive rate in pigs at different doses of the SS DNA vaccine.
3.2. Effect of Hormone Levels in the Serum
IL-4 and IFN-γ were detected by the ELISA kit (Table 2). There was no significant difference in IL-4 levels between the antibody-positive (P) and –negative (N) groups on either day 110 or day 185 (p > 0.05). In addition, there was no significant difference in IFN-γ levels between the P and N groups at day 110 and day 185 (p > 0.05).

Table 2.
The serum of the IL-4 and IFN-γ levels in antibody-positive and antibody-negative pigs at different periods.
Furthermore, we measured and analysed serum concentrations of GH and IGF-1 (Table 3). The results showed no significant difference in GH and IGF-1 levels between the P and N groups at 110 days (p > 0.05). Similarly, there was no significant difference in GH and IGF-1 concentrations between the P and N groups on days 185 (p > 0.05).

Table 3.
The serum of the GH and IGF-1 levels in antibody-positive and antibody-negative pigs at different period.
3.3. Effect on the Growth of the Fattening Pigs
As shown in Table 4, this study evaluated the effect of different concentrations of SS DNA vaccines on body weight and daily gain. The results revealed that the weight in pigs from the T3 group (59.44 ± 0.688) was significantly higher than the Ctrl group (56.42 ± 0.681; p < 0.05) at 110 days. At slaughter (d 185) in fattening pigs, weight was significantly higher in all treatment groups (T1, T2 and T3) than in the Ctrl group (p < 0.05). Furthermore, we analysed the daily gain of pigs at different periods. At 50 to 110 days, daily gain was significantly higher in treatment groups (T1, T2 and T3) than that in the Ctrl group (p < 0.05). Between 110 and 185 days of age, a significant increase in daily weight gain in the T1 and T3 groups compared to the Ctrl group (p < 0.05). Throughout the experimental period (days 50 to 185), the daily weight gain was significantly higher in the T1 group than that in the Ctrl group (0.74 ± 0.012 vs. 0.69 ± 0.012, p < 0.05).

Table 4.
Mean weight and daily gain of each period after immunisation of fattening pigs with different doses of SS DNA vaccine.
In addition, our study analysed the daily gain of antibody-positive (P) and antibody-negative (N) pigs (Table 5). The results showed that between 50 and 110 days of age, the daily gain of the P group was significantly higher than that of the N group by 0.09 kg/d (p < 0.05). Compared to the N group, the daily weight gain was significantly higher in the P group between 110 and 185 days of age (p < 0.05). Meanwhile, the daily gain of the P group (0.75 ± 0.012) was significantly higher than that of the N group (0.71 ± 0.007) throughout the experimental period (days 50 to 185) (p < 0.05).

Table 5.
The daily gain of antibody-positive and antibody-negative pigs at different period.
3.4. Effect on Meat Quality and Backfat Thickness of Fattening Pigs
Firstly, we tested the PH at 1 h and 24 h and there was no significant difference between the antibody-positive (P) and -negative (N) pigs (p > 0.05). No significant difference in meat colour and marbling was found between groups P and N (p > 0.05). Similarly, the differences in cut resistance, moisture, ash and crude protein between the P and N groups were insignificant (p > 0.05). Furthermore, there was no significance in intramuscular fat in the P group compared to the N group (p > 0.05). In addition, ultrasound was used to test the backfat thickness of fattening pigs, and the results showed that the P group was lower than that of the N group, but the difference was not significant (p > 0.05) (Table 6).

Table 6.
Comparison of meat quality and backfat thickness between antibody-positive pigs and antibody-negative pigs.
3.5. Correlation Coefficients of Antibody Titres with Daily Weight Gain and Backfat Thickness
As shown in Table 7, the correlation coefficients between antibody titres with daily weight gain and backfat thickness were analysed. Antibody titres at 110 days were significantly and positively correlated (r = 0.664) with daily weight gain between 50 and 110 days in fattening pigs (p < 0.01). Antibody titres at 185 days were significantly and positively correlated (r = 0.447) with daily weight gain between 110 and 185 days in fattening pigs (p < 0.05). Throughout the trial (d 50 to d 185), both days 110 (r = 0.396) and 185 (r = 0.446) antibody titres were significantly and positively correlated with daily weight gain (p < 0.05). In addition, our study results showed a significant negative correlation (r = −0.406) between backfat thickness and antibody titres at 110 days (p < 0.05).

Table 7.
Correlation coefficients of antibody titre with daily weight gain and backfat thickness.
3.6. Safety of SS DNA Vaccines
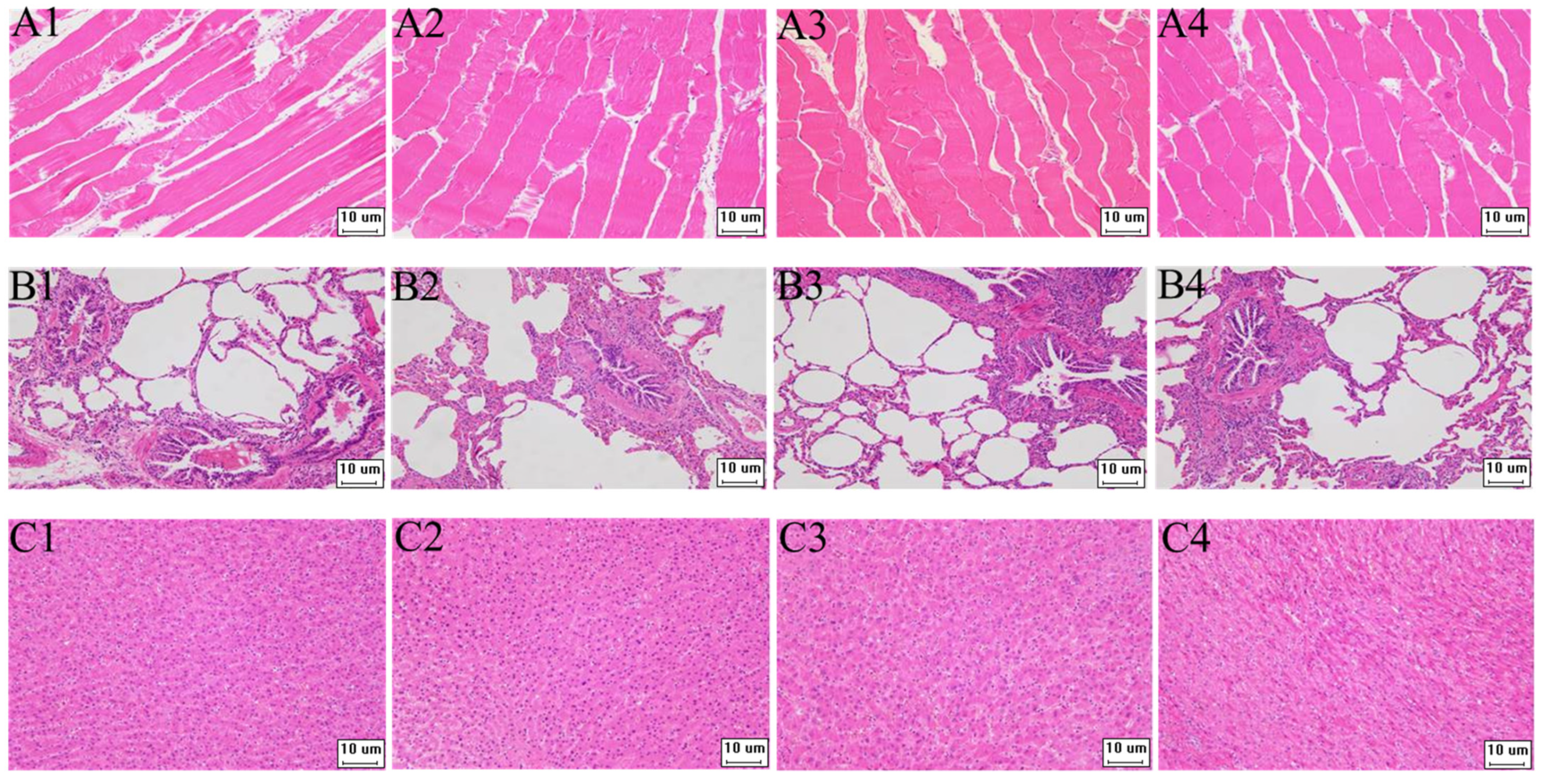
The target fragment GS/2SS of the vaccine plasmid DNA was amplified by PCR and no gene integration was detected within the sensitivity range of the PCR compared to the positive control (Supplementary Figure S1). Furthermore, our study investigated the safety of the environment around the barn. Soil samples were collected at 3, 5, 7, 10, 15 and 30 days after immunisation at 1, 3 and 5 m east, west, south and north of the barn and at 7 m from the air vent, respectively. The target fragment GS/2SS was not detected within the sensitivity range of PCR (Supplementary Figure S2). On the 3rd, 5th, 7th, 10th, 15th and 30th days after immunisation, faecal and water samples were collected by geometric sampling in the test pens, and the target fragment GS/2SS of the vaccine plasmid was detected by PCR. Within the sensitivity range of the PCR, the recombinant fragments were amplified on days 3 and 5 in the water samples and on day 5 in the faecal samples. However, no amplified bands were found in either the water or faecal samples after day 5 (Supplementary Figure S3). Compared with the antibody-negative pigs, the tissue morphology of all samples in the antibody-positive pigs was normal, and no pathological changes were observed (Figure 2).
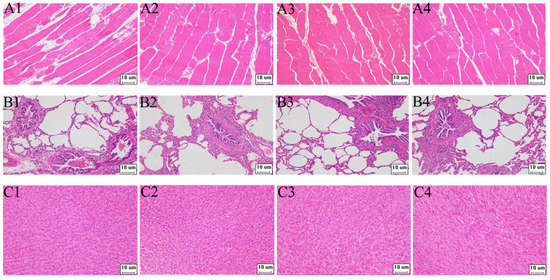
Figure 2.
The pathological paraffin tissues section of pigs (200×). (A–C) are paraffin tissue sections of muscle, lung, and liver tissue, respectively; 1–3 are antibody-positive pigs, and 4 is antibody-negative pigs.
4. Discussion
Compared to standard protein vaccinations, the clinical benefits of DNA vaccines include low cost, vaccine durability, high productivity and facile antigen customisation. On the other hand, clinical investigations revealed that the immunogenicity of DNA vaccines was relatively low [23]. In the present study, when we constructed a vaccine, the hepatitis B surface antigen gene (HBsAg-S) was inserted into the vector to address this problem. HBsAg-S can further improve immune response to DNA vaccines [24]. HBsAg-S was inserted into a gene vaccine previously constructed in the laboratory, and antibody positivity rates in serum after 14 days of immunisation ranged between 40% and 100% [25]. However, our results show antibody positivity rates between 30.77% and 40.00%. The relatively low antibody positivity rate after this study might be due to too long between immunisation and blood collection, which means our experimental design is imperfect. Wang et al. reported that antibody positivity rates were between 44.44% and 83.33% two weeks after immunisation, but between 33.33% and 61.11% four weeks after immunisation [22], suggesting a gradual decrease in antibody positivity over time. On the other hand, the dose of vaccine may also be a significant contributor to this phenomenon. Han et al. reported that oral administration of a 5 × 1010 CFU/mL concentration of SS DNA vaccine induced 100% antibody positivity in piglets, while 5 × 109 CFU/mL and 5 × 108 CFU/mL concentrations induced 30% and 20% antibody positivity, respectively [20]. Furthermore, we examined the levels of cytokines in the serum, and the results showed that the concentrations of IL-4 and IFN-γ in the P group were not significantly different from those in the N group. On the one hand, this may be because of the long interval between immunisation and blood collection. On the other hand, it may be related to the physiological hormonal compensation of the animal organism. T helper (Th) cells play a key role in regulating immune response, including th1 and th2 cells. IFN-γ production by Th1 cells is associated with cell-mediated immune function, and IL-4 produced by Th2 cells assists in the humoral immune response [26,27]. Moreover, research reported that IFN-γ and IL-4 significantly enhanced specific Th1 and Th2 cell immune responses, respectively [28]. In our study, although the insertion of HBsAg-S improved the immunogenicity of the SS DNA vaccine, a high antibody positivity rate was still not obtained. In addition to increasing the dose of the vaccine, the addition of immuno-adjuvant [29,30] is one of the important approaches to improve the antibody-positivity rate of the SS DNA vaccine.
Immunisation against SS has been reported to enhance growth in various species, including mice [31], lambs [9], rats [22], and cattle [8]. Previous studies in the laboratory have reported that the SS DNA vaccine is attributable to improving the growth performance of piglets through an influence on GH secretion [20]. In this study, we evaluated the effect of the SS DNA vaccine on growth promotion in fattening pigs. We showed that the weight at slaughter and daily weight gain throughout the feeding period was significantly higher in the SS DNA vaccine immunised group than in the Ctrl group. Several studies have shown significant positive correlations between anti-SS antibodies and average daily gain [20,32], which is consistent with our results, suggesting that the specific antibodies produced by vaccination neutralised endogenous SS in pigs and promoted growth in fattening pigs. Furthermore, the results did not reveal a significant dose-dependence of daily weight gain in fattening pigs, which is the same as the previous results of Liang et al. [33]. Because of the decrease in antibody positivity at higher doses, the loss of dose-dependence may be related to the immune stress induced by higher doses of vaccine [34]. This study is the first to analyse the meat quality after SS immunisation. The results showed no significant changes in the P group compared to the N group, including pH1, pH24, meat colour, marbling, cut resistance, moisture, ash, intramuscular fat and crude protein. Immunisation promotes the secretion of GH, which causes a decrease in intramuscular fat by stimulating lipolysis and regulating lipid deposition in adipose tissue [35], which may be an indirect cause of the significant negative correlation between backfat thickness and antibody titres.
DNA vaccine safety concerns are genetic, immunologic, toxic, and environmental [13]. Regulatory agencies, such as the European Medicines Agency (EMA), the World Health Organisation (WHO) and the Food and Drug Administration (FDA), have published guidelines for DNA vaccines [36]. Before any DNA vaccine can be submitted for regulatory approval, it needs to be tested for safety and performance [37]. In this study, genomic DNA was collected from pig blood, lung liver, and muscle tissues. No fusion gene fragment GS/2SS was detected, which is consistent with the experimental results of Liang et al. [33] and Han et al. [20]. Gene integration in DNA vaccines has not been reported to date and is theoretically less likely than the natural mutation rate of the genome [33]. However, the GS/2SS gene was observed in day 3 and 5 water samples and day 5 faecal samples. Since the study was conducted by nasal immunisation through aerosolising the bacteriological solution, it was difficult to avoid spreading in pen during the vaccination. Some studies have reported that the live attenuated Salmonella strain can spread in the natural environment by faecal [17]. Importantly, No GS/2SS gene was detected between 5 and 30 days after immunisation, suggesting that the vaccine bacterium has a limited ability to survive in the natural environment and is not able to spread over a large, prolonged period of time. Finally, compared to antibody-negative pigs, no significant pathological toxicity changes were observed in pathological sections of lung, liver, and muscle tissues in antibody-positive pigs, indicating that the SS DNA vaccine does not produce toxicity while promoting growth in fattening pigs.
5. Conclusions
In conclusion, nasal immunisation with SS DNA vaccine in our study resulted in the production of anti-SS antibodies in a proportion of fattening pigs, which can increase their daily gain and slaughter weight to a limited extent. Moreover, while promoting pig growth, there was no significant effect on meat quality. Finally, the vaccine safety was assessed, and no genomic integration, histopathology or contamination of the surrounding environment of animals was found. However, it needs to confirm whether optimising the sampling time between immunisations could improve the antibody positivity rate or whether further research is warranted to make this vaccine more efficient by trying vaccines with different immuno-adjuvants and routes of administration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12223072/s1, Figure S1: Electrophoresis analysis of GS/2SS PCR products extracted from blood and tissue samples of fattening pigs; Figure S2: Electrophoretogram of PCR amplification products of DNA recombinant fragment GS/2SS in soil samples of somatostatin DNA vaccine release environment; Figure S3: Electrophoretogram of PCR amplification products of DNA recombinant fragment GS/2SS in water and fecal samples of somatostatin DNA vaccine release environment.
Author Contributions
C.C. wrote the manuscript. C.C. and Z.Z. conceived and designed the experiment data curation. C.C, Z.Z., C.D. and K.N. contributed animals’ arrangement and sample collection. A.L. and L.Y. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the earmarked fund for CARS36 and the National Natural Science Fund (No. 31772602).
Institutional Review Board Statement
Use of animals and all experimental procedures were performed following the guidelines of the Committee of Animal Research Institute, Huazhong Agricultural University, China (HZAUSW-2018-029).
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author.
Acknowledgments
The authors wish to acknowledge Hubei Jinlin Original Breed Livestock Co., Ltd. for their care of the animals and their assistance in sampling during the study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Gan, Y.; Buckels, A.; Ying, L.; Yue, Z.; Frank, S.J. Human GH Receptor-IGF-1 Receptor Interaction: Implications for GH Signaling. Mol. Endocrinol. 2014, 28, me20141174. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Stalla, G.K. Somatostatin receptors: From signaling to clinical practice. Front. Neuroendocrinol. 2013, 34, 228–252. [Google Scholar] [CrossRef] [PubMed]
- Gyr, K.E.; Meier, R. Pharmacodynamic effects of Sandostatin® in the gastrointestinal tract. Metabolism 1992, 54 (Suppl. S1), 14–19. [Google Scholar] [CrossRef]
- Vonderohe, M.R.; Camilleri, M.; Thomforde, G.M.; Klee, G.G. Differential regional effects of octreotide on human gastrointestinal motor function. Gut 1995, 36, 743–748. [Google Scholar] [CrossRef]
- Paran, H.; Neufeld, D.; Kaplan, O.; Klausner, J.; Freund, U. Octreotide for treatment of postoperative alimentary-tract fistulas. World J. Surg. 1995, 19, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.C. Somatostatin and its receptor family. Front Neuroendocr. 1999, 20, 157–198. [Google Scholar] [CrossRef] [PubMed]
- Lakeh, A.A.B.; Farahmand, H.; Kloas, W.; Mirvaghefi, A.; Trubiroha, A.; Peterson, B.C.; Wuertz, S. Growth enhancement of rainbow trout (Oncorhynchus mykiss) by passive immunization against somatostatin-14. Aquac. Int. 2016, 24, 11–21. [Google Scholar] [CrossRef]
- Dawson, J.M.; Soar, J.B.; Buttery, P.J.; Craigon, J.; Gill, M.; Beever, D.E. The effect of immunization against somatostatin and β-agonist administration alone and in combination on growth and carcass composition in young steers. Anim. Sci. 1997, 64, 37–51. [Google Scholar] [CrossRef]
- Spencer, G.S.G. Immuno-neutralization of somatostatin and its effects on animal production. Domest. Anim. Endocrinol. 1986, 3, 55–68. [Google Scholar] [CrossRef]
- Liu, X.B.; Liang, A.X.; Feng, X.G.; Guo, A.Z.; Ke, C.Y.; Zhang, S.J.; Yang, L.G. Oral and intranasal administration of somatostatin DNA vaccine mediated by attenuated Salmonella Enterica Serovar Typhimurium to promote growth of piglets. Animal 2011, 5, 1231–1236. [Google Scholar] [CrossRef]
- Spencer, G.; Garssen, G.J.; Bergström, P. A novel approach to growth promotion using auto-immunisation against somatostatin. I. Effects on growth and hormone levels in lambs. Livest. Prod. Sci. 1983, 10, 469–477. [Google Scholar] [CrossRef]
- Deligeorgis, S.; Rogdakis, E.; Mantzios, A.; Nikolaou, E.; Evangelatos, G.; Sotiriadis-Vlahos, C. A note on the effect of active immunization against somatostatin on milk production and growth in sheep. Anim. Sci. 2010, 46, 304–308. [Google Scholar] [CrossRef]
- Schalk, J.A.C.; Mooi, F.R.; Berbers, G.A.M.; van Aerts, L.; Ovelgonne, H.; Kimman, T.G. Preclinical and clinical safety studies on DNA vaccines. Hum. Vaccines 2006, 2, 45–53. [Google Scholar] [CrossRef]
- Li, L.; Petrovsky, N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef]
- Kim, D.; Hung, C.-F.; Wu, T.C.; Park, Y.-M. DNA vaccine with alpha-galactosylceramide at prime phase enhances anti-tumor immunity after boosting with antigen-expressing dendritic cells. Vaccine 2010, 28, 7297–7305. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.A.; Haigwood, N.L. DNA vaccine strategies: Candidates for immune modulation and immunization regimens. Methods 2003, 31, 207–216. [Google Scholar] [CrossRef]
- Pusterla, N.; Hilton, H.; Wattanaphansak, S.; Collier, J.R.; Mapes, S.M.; Stenbom, R.M.; Gebhart, C. Evaluation of the humoral immune response and fecal shedding in weanling foals following oral and intra-rectal administration of an avirulent live vaccine of Lawsonia intracellularis. Vet. J. 2009, 182, 458–462. [Google Scholar] [CrossRef]
- Kim, S.W.; Kang, H.Y.; Jin, H.; Sang, W.G.; Lee, J.H. Construction of a conditional lethal Salmonella mutant via genetic recombination using the ara system and asd gene. J. Microbiol. Methods 2011, 87, 202–207. [Google Scholar] [CrossRef]
- Liang, A.X.; Han, L.; Hua, G.H.; Geng, L.Y.; Sang, L.; Liu, X.B.; Guo, A.Z.; Yang, L.G. Construction of a fusion protein expression vector pGS/2SS-M4GFP without antibiotic resistance gene and its subcellular localization in different cell lines. Biologicals 2009, 37, 37–43. [Google Scholar] [CrossRef]
- Han, Y.G.; Liang, A.X.; Han, L.; Guo, A.Z.; Jiang, X.P.; Yang, L.G. Efficacy and Safety of an Oral Somatostatin DNA Vaccine without Antibiotic Resistance Gene in Promoting Growth of Piglets. Scand. J. Immunol. 2014, 79, 244–250. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, F.; Chang, H.; Peng, B.; Wu, J. Comparison of protection against H5N1 influenza virus in mouse offspring provided by maternal vaccination with HA DNA and inactivated vaccine. Arch. Virol. 2013, 158, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Li, H.; Ahmad, S.; Cao, S.X.; Xue, L.Q.; Xing, Z.F.; Feng, J.Z.; Liang, A.X.; Yang, L.G. Effect of a DNA vaccine harboring two copies of inhibin α (1-32) fragments on immune response, hormone concentrations and reproductive performance in rats. Theriogenology 2012, 78, 393–401. [Google Scholar] [CrossRef]
- Kobiyama, K.; Jounai, N.; Aoshi, T.; Tozuka, M.; Takeshita, F.; Coban, C.; Ishii, K.J. Innate Immune Signaling by, and Genetic Adjuvants for DNA Vaccination. Vaccines 2013, 1, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.; Coit, D.; Medina-Selby, M.A.; Kuo, C.H.; Nest, G.V.; Burke, R.L.; Bull, P.; Urdea, M.S.; Graves, P.V. Antigen Engineering in Yeast: Synthesis and Assembly of Hybrid Hepatitis B Surface Antigen-Herpes Simplex 1 gD Particles. Nat. Biotechnol. 1985, 3, 323–326. [Google Scholar] [CrossRef]
- Dan, X.; Han, L.; Riaz, H.; Luo, X.; Liu, X.; Chong, Z.; Yang, L. Construction and evaluation of the novel DNA vaccine harboring the inhibin α (1-32) and the RFamide related peptide-3 genes for improving fertility in mice. Exp. Anim. 2016, 65, 17–25. [Google Scholar] [CrossRef]
- Li-Weber, M.; Laur, O.; Davydov, I.V.; Hu, C.G.; Salgame, P.; Krammer, P.H. What controls tissue-specific expression of the IL-4 gene? Immunobiology 1997, 198, 170–178. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Pillars article: Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 2005, 175, 5–14. [Google Scholar]
- Inagawa, H.; Nishizawa, T.; Honda, T.; Nakamoto, T.; Takagi, K.; Soma, G. Mechanisms by which chemotherapeutic agents augment the antitumor effects of tumor necrosis factor: Involvement of the pattern shift of cytokines from Th2 to Th1 in tumor lesions. Anticancer Res. 1998, 18, 3957–3964. [Google Scholar]
- Hartoonian, C.; Sepehrizadeh, Z.; Mahdavi, M.; Arashkia, A.; Jang, Y.S.; Ebtekar, M.; Yazdi, M.T.; Negahdari, B.; Nikoo, A.; Azadmanesh, K. Modulation of hepatitis C virus core DNA vaccine immune responses by co-immunization with CC-chemokine ligand 20 (CCL20) gene as immunoadjuvant. Mol. Biol. Rep. 2014, 41, 5943–5952. [Google Scholar] [CrossRef]
- Marin, A.; Chowdhury, A.; Valencia, S.M.; Zacharia, A.; Kirnbauer, R.; Roden, R.B.S.; Pinto, L.A.; Shoemaker, R.H.; Marshall, J.D.; Andrianov, A.K. Next generation polyphosphazene immunoadjuvant: Synthesis, self-assembly and in vivo potency with human papillomavirus VLPs-based vaccine. Nanomed. Nanotechnol. Biol. Med. 2021, 33, 102359. [Google Scholar] [CrossRef]
- Han, L.; Liang, A.X.; Zhang, J.; Fang, M.; Hua, G.H.; Sang, L.; Geng, L.Y.; Wang, H.R.; Yang, L.G. Evaluation of the VP22 gene adjuvant for enhancement of DNA vaccine against somatostatin in mice. Anim. Int. J. Anim. Biosci. 2008, 2, 1569–1574. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xue, C.L.; Liang, A.X.; Mao, D.G.; Han, L.; Liu, X.B.; Zhang, J.; Yang, L.G. Effect of genetic adjuvants on immune respondance, growth and hormone levels in somatostatin DNA vaccination-induced Hu lambs. Vaccine 2010, 28, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Cao, S.; Li, H.; Yao, Y.; Moaeen-Ud-Din, M.; Yang, L. Construction and evaluation of the eukaryotic expression plasmid encoding two copies of somatostatin genes fused with hepatitis B surface antigen gene S. Vaccine 2008, 26, 2935–2941. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Keller, S.E.; Schleifer, S.J. Stress and immunomodulation: The role of depression and neuroendocrine function. J. Immunol. 1985, 135, 827s. [Google Scholar] [PubMed]
- Chaves, V.E.; Mesquita Junior, F.; Bertolini, G.L. The metabolic effects of growth hormone in adipose tissue. Endocrine 2013, 44, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Myhr, A.I. DNA Vaccines: Regulatory Considerations and Safety Aspects. Curr. Issues Mol. Biol. 2017, 22, 79–88. [Google Scholar] [CrossRef]
- Klug, B.; Reinhardt, J.; Robertson, J. Current status of regulations for DNA vaccines. In Gene Vaccines; Springer: Berlin/Heidelberg, Germany, 2012; pp. 285–295. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).