Stress Concepts and Applications in Various Matrices with a Focus on Hair Cortisol and Analytical Methods
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Stress Definitions
1.2. Stress Classifications
2. Biomarkers of Stress in Different Animal Species
2.1. Biomarkers of Stress
2.2. Evaluation of Stress in Domestic Animals Including Ruminants, Birds, and Aquatic Organisms
3. Recent Advances in Stress Evaluation Indexes
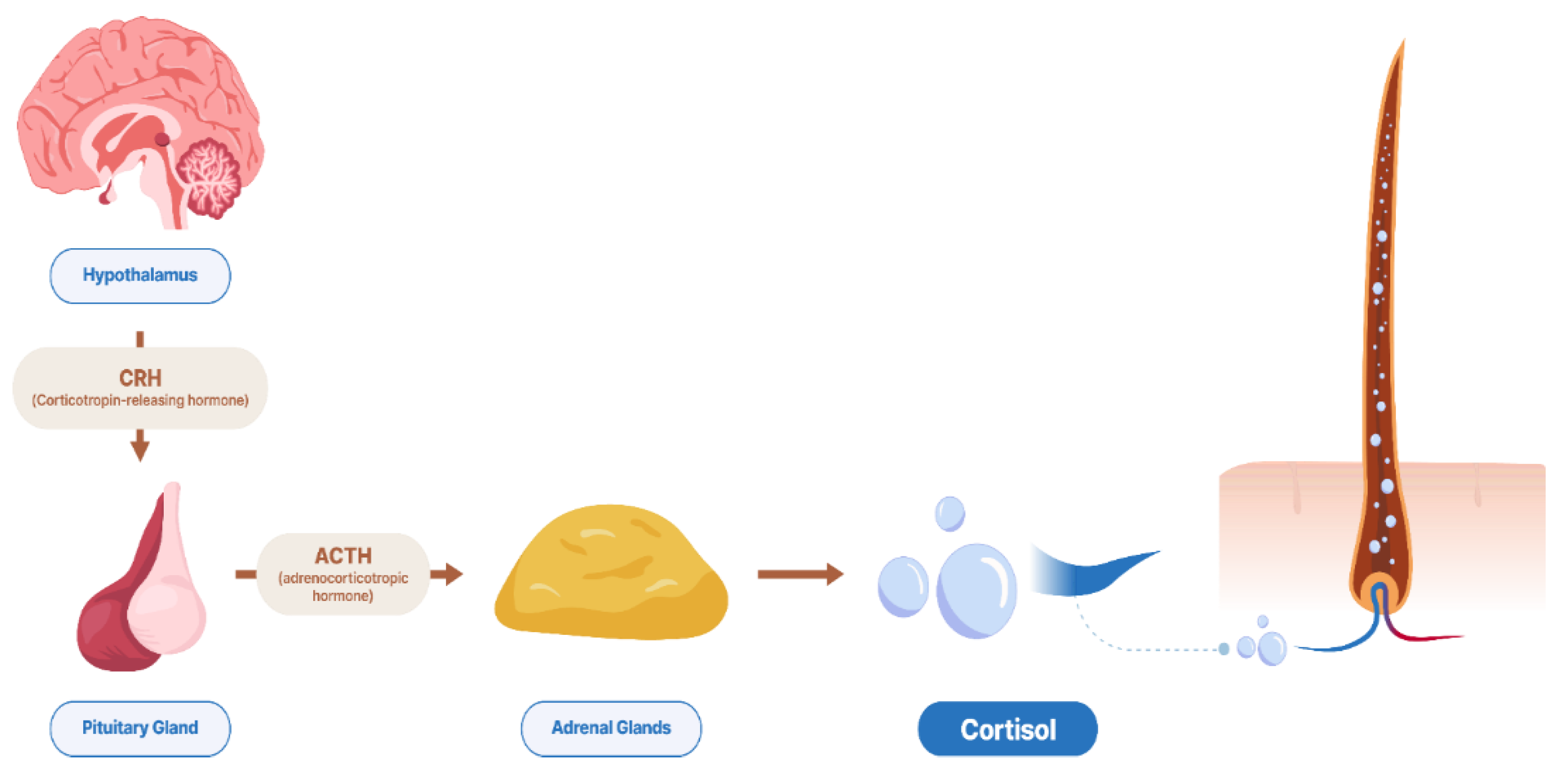
3.1. Measuring Hair Cortisol
3.2. Hair Biomatrix, Structure, and Mechanism for Incorporation of Hormone
3.3. Considerations for Hair Samples Collection
3.3.1. Areas of the Collection
3.3.2. Color of the Samples and Coats
3.3.3. Method of Sample Collection, Preservation, and Preparation for Hormone Extraction
3.3.4. Sex and Age
3.3.5. Extraction Method

4. Assay Methods for Detection of Stress Hormone
4.1. Mass Spectrometry
4.2. Radioimmunoassay
4.3. Enzyme-Linked Immunosorbent Assay or Enzyme Immunoassays
4.4. Multiplex Immunoassays
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K. Stress and Metabolic Disease. In Sociality, Hierarchy, Health. Comparative Biodemography: A Collection of Papers; Weinstein, M., Lane, M.A., Eds.; National Academies Press: Washington, DC, USA, 2014; Volume 11, pp. 247–267. [Google Scholar]
- Trevisi, E.; Bertoni, G. Some physiological and biochemical methods for acute and chronic stress evaluationin dairy cows. Ital. J. Anim. Sci. 2009, 8, 265–286. [Google Scholar] [CrossRef]
- Rudland, J.R.; Golding, C.; Wilkinson, T.J. The stress paradox: How stress can be good for learning. Med. Educ. 2020, 54, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hoirisch-Clapauch, S. Mechanisms affecting brain remodeling in depression: Do all roads lead to impaired fibrinolysis? Mol. Psychiatry 2022, 27, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Pollak, C.; Maier, H.B.; Moschny, N.; Jahn, K.; Bleich, S.; Frieling, H.; Neyazi, A. Epinephrine levels decrease in responders after electroconvulsive therapy. J. Neural Transm. Suppl. 2021, 128, 1917–1921. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Kim, B.W.; Lee, B.H.; Sung, K.I. Coat and hair color: Hair cortisol and serotonin levels in lactating Holstein cows under heat stress conditions. Anim. Sci. J. 2017, 88, 190–194. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Lohakare, J.; Son, J.; Kwon, E.; West, J.; Sung, K. Wool cortisol is a better indicator of stress than blood cortisol in ewes exposed to heat stress and water restriction. Animal 2014, 8, 128–132. [Google Scholar] [CrossRef]
- Ataallahi, M.; Ghassemi Nejad, J.; Park, K.H. Selection of appropriate biomatrices for studies of chronic stress in animals: A review. J. Anim. Sci. Technol. 2022, 64, 621. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Hughes, H.D.; Carroll, J.A.; Sanchez, N.C.B.; Richeson, J.T. Natural variations in the stress and acute phase responses of cattle. Innate Immun. 2014, 20, 888–896. [Google Scholar] [CrossRef]
- Mendoza, S.; Capitanio, J.; Mason, W.A. Chronic Social Stress: Studies 11 in Non-human Primates. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J., Eds.; CAB International: New York, NY, USA, 2000; pp. 227–247. [Google Scholar]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare, 1st ed.; CAB International: Wallingford, UK, 2000. [Google Scholar]
- Burnard, C.; Ralph, C.; Hynd, P.; Edwards, J.H.; Tilbrook, A. Hair cortisol and its potential value as a physiological measure of stress response in human and non-human animals. Anim. Prod. Sci. 2016, 57, 401–414. [Google Scholar] [CrossRef]
- Romero, L.M. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.A. Toxic stress: Effects, prevention and treatment. Children 2014, 1, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S. Heat stress: Impact on livestock well-being and productivity and mitigation strategies to alleviate the negative effects. Anim. Prod. Sci. 2018, 58, 1404–1413. [Google Scholar] [CrossRef]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Iqbal Yatoo, M.; Bhatt, P.; et al. Biomarkers in stress related diseases/disorders: Diagnostic, prognostic, and therapeutic values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef]
- Ewert, A.; Chang, Y. Levels of Nature and Stress Response. Behav. Sci. 2018, 8, 49. [Google Scholar] [CrossRef]
- Takahashi, A.; Flanigan, M.E.; McEwen, B.S.; Russo, S.J. Aggression, social stress, and the immune system in humans and animal models. Front. Behav. Neurosci. 2018, 12, 56. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Ataallahi, M.; Salmanzadeh, M.H.; Park, K.T.; Lee, H.G.; Shoae, A.; Rahimi, A.; Sung, K.I.; Park, K.H. Cortisol Extraction from Sturgeon Fin and Jawbone Matrices. J. Vis. Exp. 2019, 151, e59961. [Google Scholar] [CrossRef]
- Ataallahi, M.; Ghassemi Nejad, J.; Song, J.I.; Kim, J.S.; Park, K.H. Effects of feather processing methods on quantity of extracted corticosterone in broiler chickens. J. Anim. Sci. Technol. 2020, 62, 884. [Google Scholar] [CrossRef]
- Freeman, N.E.; Newman, A.E. Quantifying corticosterone in feathers: Validations for an emerging technique. Conserv. Physiol. 2018, 6, coy051. [Google Scholar] [CrossRef]
- Romero, L.M.; Fairhurst, G.D. Measuring corticosterone in feathers: Strengths, limitations, and suggestions for the future. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Baxter-Gilbert, J.H.; Riley, J.L.; Mastromonaco, G.F.; Litzgus, J.D.; Lesbarrères, D. A novel technique to measure chronic levels of corticosterone in turtles living around a major roadway. Conserv. Physiol. 2014, 2, cou036. [Google Scholar] [CrossRef] [PubMed]
- Mack, Z.; Fokidis, H. A novel method for assessing chronic cortisol concentrations in dogs using the nail as a source. Domest. Anim. Endocrionl. 2017, 59, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.T.; Vanderstichel, R.; Hovenga, C.; Lappin, M.R. Evaluation of hair and nail cortisol concentrations and associations with behavioral, physical, and environmental indicators of chronic stress in cats. J. Vet. Intern. Med. 2021, 35, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid extraction: Get the best out of faecal samples. Wien. Tierärztliche Mon. 2013, 100, 238–246. [Google Scholar]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Morrow, C.J.; Kolver, E.S.; Verkerk, G.A.; Matthews, L.R. Fecal glucocorticoid metabolites as a measure of adrenal activity in dairy cattle. Gen. Comp. Endocrinol. 2002, 126, 229–241. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Ghaseminezhad, M.; Sung, K.I.; Hoseinzadeh, F.; Cabibi, J.B.A.; Lee, J.H. A cortisol study; facial hair and nails. J. Steroids Horm. Sci. 2016, 7, 177. [Google Scholar]
- Ghassemi Nejad, J.; Jeong, C.; Shahsavarani, H.; Sung, K.I.; Lee, J. Embedded dental cortisol content: A pilot study. Endocrinol. Metab. Syndr. 2016, 5, 1000240. [Google Scholar]
- Hucklebridge, F.; Hussain, T.; Evans, P.; Clow, A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology 2005, 30, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.S.; Novak, M.A. Minireview: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 2012, 153, 4120–4127. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, O.; Jellestad, F.K.; Murison, R. A systematic review of studies utilizing hair glucocorticoids as a measure of stress suggests the marker is more appropriate for quantifying short-term stressors. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shokry, E.; Pereira, J.; Marques Junior, J.G.; da Cunha, P.H.J.; Noronha Filho, A.D.F.; da Silva, J.A.; Soares Fioravanti, M.C.; de Oliveira, A.E.; Antoniosi Filho, N.R. Earwax metabolomics: An innovative pilot metabolic profiling study for assessing metabolic changes in ewes during periparturition period. PLoS ONE 2017, 12, e0183538. [Google Scholar] [CrossRef]
- Herane-Vives, A.; Ortega, L.; Sandoval, R.; Young, A.H.; Cleare, A.; Espinoza, S.; Hayes, A.; Benöhr, J. Measuring Earwax Cortisol Concentration using a non-stressful sampling method. Heliyon 2020, 6, e05124. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic–pituitary–adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Almoosavi, S.S.; Ghoorchi, T.; Naserian, A.A.; Ramezanpor, S.; Ghaffari, M. Long-term impacts of late-gestation maternal heat stress on growth performance, blood hormones and metabolites of newborn calves independent of maternal reduced feed intake. Domest. Anim. Endocrinol. 2020, 72, 106433. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Lee, B.H.; Kim, J.Y.; Sung, K.I.; Lee, H.G. Daytime Grazing in Mountainous Areas Increases Unsaturated Fatty Acids and Decreases Cortisol in the Milk of Holstein Dairy Cows. Animals 2021, 11, 3122. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Lee, B.H.; Kim, J.Y.; Chemere, B.; Sung, K.I.; Lee, H.G. Effect of alpine grazing on plasma and hair cortisol, serotonin, and DHEA in dairy cows and its welfare impact. Doemst. Anim. Endocrinol. 2021, 75, 106581. [Google Scholar] [CrossRef]
- Ramón-Moragues, A.; Carulla, P.; Mínguez, C.; Villagrá, A.; Estellés, F. Dairy cows activity under heat stress: A case study in Spain. Animals 2021, 11, 2305. [Google Scholar] [CrossRef] [PubMed]
- Antoni, M.H.; Dhabhar, F.S. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer 2019, 125, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi Nejad, J.; Park, K.H.; Forghani, F.; Lee, H.G.; Lee, J.S.; Sung, K.I. Measuring hair and blood cortisol in sheep and dairy cattle using RIA and ELISA assay: A comparison. Biol. Rhythm Res. 2020, 51, 887–897. [Google Scholar] [CrossRef]
- Negrao, J.; Porcionato, M.; De Passille, A.; Rushen, J. Cortisol in saliva and plasma of cattle after ACTH administration and milking. J. Dairy Sci. 2004, 87, 1713–1718. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Lombardelli, R.; Bionaz, M. Plasma cortisol variations in dairy cows after some usual or unusual manipulations. Ital. J. Anim. Sci. 2005, 4, 200–202. [Google Scholar] [CrossRef]
- Bomfim, G.; Merighe, G.; de Oliveira, S.; Negrao, J. Acute and chronic effects of cortisol on milk yield, the expression of key receptors, and apoptosis of mammary epithelial cells in Saanen goats. J. Dairy Sci. 2022, 105, 818–830. [Google Scholar] [CrossRef]
- Rushen, J.; Pombourcq, E.; de Passillé, A.M. Validation of two measures of lameness in dairy cows. Appl. Anim. Behav. Sci. 2007, 106, 173–177. [Google Scholar] [CrossRef]
- Smith, R.; Dobson, H. Hormonal interactions within the hypothalamus and pituitary with respect to stress and reproduction in sheep. Domest. Anim. Endocrinol. 2002, 23, 75–85. [Google Scholar] [CrossRef]
- Von Borell, E. The biology of stress and its application to livestock housing and transportation assessment. Anim. Sci. J. 2001, 79, E260–E267. [Google Scholar] [CrossRef]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J.; Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Miró, S.; López-Martínez, M.J.; Ortín-Bustillo, A.; Franco-Martínez, L.; Rubio, C.P.; Muñoz-Prieto, A.; Tvarijonaviciute, A.; et al. Basics for the potential use of saliva to evaluate stress, inflammation, immune system, and redox homeostasis in pigs. BMC Vet. Res. 2022, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Moya, D.; Schwartzkopf-Genswein, K.; Veira, D. Standardization of a non-invasive methodology to measure cortisol in hair of beef cattle. Livest. Sci. 2013, 158, 138–144. [Google Scholar] [CrossRef]
- Davenport, M.D.; Tiefenbacher, S.; Lutz, C.K.; Novak, M.A.; Meyer, J.S. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol. 2006, 147, 255–261. [Google Scholar] [CrossRef]
- Koren, L.; Mokady, O.; Karaskov, T.; Klein, J.; Koren, G.; Geffen, E. A novel method using hair for determining hormonal levels in wildlife. Anim. Behav. 2002, 63, 403–406. [Google Scholar] [CrossRef]
- Shukla, R.; Basu, A.K.; Mandal, B.; Mukhopadhyay, P.; Maity, A.; Chakraborty, S.; Kumar Devrabhai, P. 11β Hydroxysteroid dehydrogenase—1 activity in type 2 diabetes mellitus: A comparative study. BMC Endocr. Disord. 2019, 19, 15. [Google Scholar] [CrossRef]
- Raul, J.S.; Cirimele, V.; Ludes, B.; Kintz, P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin. Biochem. 2004, 37, 1105–1111. [Google Scholar] [CrossRef]
- Stubsjøen, S.M.; Bohlin, J.; Dahl, E.; Knappe-Poindecker, M.; Fjeldaas, T.; Lepschy, M.; Palme, R.; Langbein, J.; Ropstad, E. Assessment of chronic stress in sheep (part I): The use of cortisol and cortisone in hair as non-invasive biological markers. Small Rumin. Res. 2015, 132, 25–31. [Google Scholar] [CrossRef]
- Stubsjøen, S.M.; Sørheim, K.; Chincarini, M.; Bohlin, J.; Brunberg, E.; Fuchs, B.; Palme, R.; Grøva, L. Exploring hair cortisone concentration as a novel tool to assess chronic stress in sheep with tick-borne fever. Small Rumin. Res. 2018, 164, 110–119. [Google Scholar] [CrossRef]
- Vanaelst, B.; Michels, N.; De Vriendt, T.; Huybrechts, I.; Vyncke, K.; Sioen, I.; Bammann, K.; Rivet, N.; Raul, J.S.; Molnar, D.; et al. Cortisone in hair of elementary school girls and its relationship with childhood stress. Eur. J. Pediatr. 2013, 172, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Xu, Y.; Yang, J.; Chen, Z.; Deng, H. Characteristics of novel hair-based biomarker for the activity assessment of 11β-hydroxysteroid dehydrogenase. Clin. Chim. Acta 2013, 426, 25–32. [Google Scholar] [CrossRef] [PubMed]
- López-Arjona, M.; Tecles, F.; Mateo, S.V.; Contreras-Aguilar, M.D.; Martínez-Miró, S.; Cerón, J.J.; Martínez-Subiela, S. Measurement of cortisol, cortisone and 11β-hydroxysteroid dehydrogenase type 2 activity in hair of sows during different phases of the reproductive cycle. Vet. J. 2020, 259–260, 105458. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, N.; Izawa, S.; Ogawa, N.; Shirotsuki, K.; Nomura, S. Association between hair cortisol and diurnal basal cortisol levels: A 30-day validation study. Psychoneuroendocrinology 2020, 116, 104650. [Google Scholar] [CrossRef] [PubMed]
- Short, S.J.; Stalder, T.; Marceau, K.; Entringer, S.; Moog, N.K.; Shirtcliff, E.A.; Wadhwa, P.D.; Buss, C. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology 2016, 71, 12–18. [Google Scholar] [CrossRef]
- Ataallahi, M.; Ghassemi Nejad, J.; Takahashi, J.; Song, Y.H.; Sung, K.I.; Yun, J.I.; Park, K.H. Effects of environmental changes during different seasons on hair cortisol concentration as a biomarker of chronic stress in Korean native cattle. Int. J. Agric. Biol. 2019, 21, 1166–1172. [Google Scholar]
- Jang, W.J.; Choi, J.Y.; Park, B.; Seo, J.H.; Seo, Y.H.; Lee, S.K.; Jeong, C.H.; Lee, S.Y. Hair metabolomics in animal studies and clinical settings. Molecules 2019, 24, 2195. [Google Scholar] [CrossRef]
- Arck, P.C.; Slominski, A.; Theoharides, T.C.; Peters, E.M.; Paus, R. Neuroimmunology of stress: Skin takes center stage. J. Investig. Dermatol. 2006, 126, 1697–1704. [Google Scholar] [CrossRef]
- Boumba, V.A.; Ziavrou, K.S.; Vougiouklakis, T. Hair as a biological indicator of drug use, drug abuse or chronic exposure to environmental toxicants. Int. J. Toxicol. 2006, 25, 143–163. [Google Scholar] [CrossRef]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal effects on hair follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef]
- Wennig, R. Potential problems with the interpretation of hair analysis results. Forensic Sci. Int. 2000, 107, 5–12. [Google Scholar] [CrossRef]
- Heimbürge, S.; Kanitz, E.; Tuchscherer, A.; Otten, W. Within a hair’s breadth–Factors influencing hair cortisol levels in pigs and cattle. Gen. Comp. Endocrinol. 2020, 288, 113359. [Google Scholar] [CrossRef] [PubMed]
- Heimbürge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Burnett, T.A.; Madureira, A.M.; Silper, B.F.; Nadalin, A.; Tahmasbi, A.; Veira, D.M.; Cerri, R.L. Factors affecting hair cortisol concentrations in lactating dairy cows. J. Dairy Sci. 2014, 97, 7685–7690. [Google Scholar] [CrossRef] [PubMed]
- Tennenhouse, E.M.; Putman, S.; Boisseau, N.P.; Brown, J.L. Relationships between steroid hormones in hair and social behaviour in ring-tailed lemurs (Lemur catta). Primates 2017, 58, 199–209. [Google Scholar] [CrossRef]
- Fourie, N.H.; Brown, J.L.; Jolly, C.J.; Phillips-Conroy, J.E.; Rogers, J.; Bernstein, R.M. Sources of variation in hair cortisol in wild and captive non-human primates. Zoology 2016, 119, 119–125. [Google Scholar] [CrossRef]
- Pragst, F.; Balikova, M.A. State of the art in hair analysis for detection of drug and alcohol abuse. Clin. Chim. Acta 2006, 370, 17–49. [Google Scholar] [CrossRef]
- Baier, F.; Grandin, T.; Engle, T.; Edwards-Callaway, L. Evaluation of hair characteristics and animal age on the impact of hair cortisol concentration in feedlot steers. Front. Vet. Sci. 2019, 6, 323. [Google Scholar] [CrossRef]
- Tallo-Parra, O.; Manteca, X.; Sabes-Alsina, M.; Carbajal, A.; Lopez-Bejar, M. Hair cortisol detection in dairy cattle by using EIA: Protocol validation and correlation with faecal cortisol metabolites. Animal 2015, 9, 1059–1064. [Google Scholar] [CrossRef]
- del Rosario, M.; Valdez, R.A.; Lemus-Ramirez, V.; Vázquez-Chagoyán, J.C.; Villa-Godoy, A.; Romano, M.C. Effects of adrenocorticotropic hormone challenge and age on hair cortisol concentrations in dairy cattle. Can. J. Vet. Res. 2011, 75, 216–221. [Google Scholar]
- Braun, U.; Michel, N.; Baumgartner, M.R.; Hässig, M.; Binz, T.M. Cortisol concentration of regrown hair and hair from a previously unshorn area in dairy cows. Res. Vet. Sci. 2017, 114, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Uetake, K.; Morita, S.; Sakagami, N.; Yamamoto, K.; Hashimura, S.; Tanaka, T. Hair cortisol levels of lactating dairy cows in cold-and warm-temperate regions in Japan. Anim. Sci. J. 2018, 89, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Marti, S.; Wilde, R.; Moya, D.; Heuston, C.; Brown, F.; Schwartzkopf-Genswein, K. Effect of rest stop duration during long-distance transport on welfare indicators in recently weaned beef calves. J. Anim. Sci. 2017, 95, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Creutzinger, K.C.; Stookey, J.M.; Marfleet, T.W.; Campbell, J.R.; Janz, D.M.; Marqués, F.J.; Seddon, Y.M. An investigation of hair cortisol as a measure of long-term stress in beef cattle: Results from a castration study. Can. J. Anim. Sci. 2017, 97, 499–509. [Google Scholar] [CrossRef]
- Lockwood, S.A.; Kattesh, H.G.; Rhinehart, J.D.; Strickland, L.G.; Krawczel, P.D.; Wilkerson, J.B.; Kirkpatrick, F.D.; Saxton, A.M. Relationships among temperament, acute and chronic cortisol and testosterone concentrations, and breeding soundness during performance testing of Angus bulls. Theriogenology 2017, 89, 140–145. [Google Scholar] [CrossRef]
- Levine, B.S.; Kerrigan, S. Principles of Forensic Toxicology, 5th ed.; Springer Nature: Cham, Switzerland, 1999. [Google Scholar]
- Smyth, N. Cortisol Secretion in Saliva and Hair: Methodological Considerations and Relationships with State and Trait Well-being. Ph.D. Thesis, University of Westminster, London, UK, 2013. [Google Scholar]
- Ghassemi Nejad, J.; Ataallahi, M.; Park, K.H. Methodological validation of measuring Hanwoo hair cortisol concentration using bead beater and surgical scissors. J. Anim. Sci. Technol. 2019, 61, 41. [Google Scholar] [CrossRef]
- Duran, M.C.; Janz, D.M.; Waldner, C.L.; Campbell, J.R.; Marques, F.J. Hair cortisol concentration as a stress biomarker in horses: Associations with body location and surgical castration. J. Equine Vet. Sci. 2017, 55, 27–33. [Google Scholar] [CrossRef]
- Casal, N.; Manteca, X.; Peña, R.; Bassols, A.; Fàbrega, E. Analysis of cortisol in hair samples as an indicator of stress in pigs. J. Vet. Behav. 2017, 19, 1–6. [Google Scholar] [CrossRef]
- Acker, M.; Mastromonaco, G.; Schulte-Hostedde, A.I. The effects of body region, season and external arsenic application on hair cortisol concentration. Conserv. Physiol. 2018, 6, coy037. [Google Scholar] [CrossRef]
- Carlitz, E.H.D.; Kirschbaum, C.; Miller, R.; Rukundo, J.; van Schaik, C.P. Effects of body region and time on hair cortisol concentrations in chimpanzees (Pan troglodytes). Gen. Comp. Endocrinol. 2015, 223, 9–15. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.B.; Rogers, A.R.; Schütz, K.E. Effect of solar radiation on dairy cattle behaviour, use of shade and body temperature in a pasture-based system. Appl. Anim. Behav. Sci. 2008, 109, 141–154. [Google Scholar] [CrossRef]
- Accorsi, P.A.; Carloni, E.; Valsecchi, P.; Viggiani, R.; Gamberoni, M.; Tamanini, C.; Seren, E. Cortisol determination in hair and faeces from domestic cats and dogs. Gen. Comp. Endocrinol. 2008, 155, 398–402. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Lee, H.G. Coat colour affects cortisol and serotonin levels in the serum and hairs of Holstein dairy cows exposed to cold winter. Domest. Anim. Endocrinol. 2022, 82, 106768. [Google Scholar] [CrossRef] [PubMed]
- Kittilsen, S.; Schjolden, J.; Beitnes-Johansen, I.; Shaw, J.; Pottinger, T.G.; Sørensen, C.; Braastad, B.O.; Bakken, M.; Øverli, Ø. Melanin-based skin spots reflect stress responsiveness in salmonid fish. Hormones Behav. 2009, 56, 292–298. [Google Scholar] [CrossRef]
- Kersey, D.C.; Dehnhard, M. The use of noninvasive and minimally invasive methods in endocrinology for threatened mammalian species conservation. Gen. Comp. Endocrinol. 2014, 203, 296–306. [Google Scholar] [CrossRef]
- Bennett, A.; Hayssen, V. Measuring cortisol in hair and saliva from dogs: Coat color and pigment differences. Domest. Anim. Endocrinol. 2010, 39, 171–180. [Google Scholar] [CrossRef]
- Comin, A.; Veronesi, M.C.; Montillo, M.; Faustini, M.; Valentini, S.; Cairoli, F.; Prandi, A. Hair cortisol level as a retrospective marker of hypothalamic–pituitary–adrenal axis activity in horse foals. Vet. J. 2012, 194, 131–132. [Google Scholar] [CrossRef]
- Trut, L.N. Early Canid Domestication: The Farm-Fox Experiment: Foxes bred for tamability in a 40-year experiment exhibit remarkable transformations that suggest an interplay between behavioral genetics and development. Am. Sci. 1999, 87, 160–169. [Google Scholar] [CrossRef]
- Newman, A.E.; Pradhan, D.S.; Soma, K.K. Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: Jugular versus brachial plasma. Endocrinology 2008, 149, 2537–2545. [Google Scholar] [CrossRef]
- Gratacos-Cubarsi, M.; Castellari, M.; Valero, A.; García-Regueiro, J. Hair analysis for veterinary drug monitoring in livestock production. J. Chromatogr. B. 2006, 834, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi Nejad, J.; Lee, B.H.; Kim, J.Y.; Kim, B.W.; Chemere, B.; Park, K.H.; Sung, K.I. Comparing hair cortisol concentrations from various body sites and serum cortisol in Holstein lactating cows and heifers during thermal comfort zone. J. Vet. Behav. 2019, 30, 92–95. [Google Scholar] [CrossRef]
- Ling, J.; Robbins, L.B.; Xu, D. Food security status and hair cortisol among low-income mother-child dyads. West. J. Nurs. Res. 2019, 41, 1813–1828. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Novak, M.; Hamel, A.; Rosenberg, K. Extraction and analysis of cortisol from human and monkey hair. J. Vis. Exp. 2014, 83, e50882. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, L.E.; Brangs, H.C.; Burden, D.W. Bead Beating: A Primer; OPS Diagnostics LLC: Lebanon, NJ, USA, 2014; Volume 12, pp. 1–20. [Google Scholar]
- Comin, A.; Peric, T.; Corazzin, M.; Veronesi, M.C.; Meloni, T.; Zufferli, V.; Cornacchia, G.; Prandi, A. Hair cortisol as a marker of hypothalamic-pituitary-adrenal axis activation in Friesian dairy cows clinically or physiologically compromised. Livest. Sci. 2013, 152, 36–41. [Google Scholar] [CrossRef]
- Slominski, R.; Rovnaghi, C.R.; Anand, K.J. Methodological considerations for hair cortisol measurements in children. Ther. Drug Monit. 2015, 37, 812. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, F.; Carrasco, J.; Pastor-Ciurana, J.; Belda, X.; Rami-Bastante, A.; Bacci, M.L.; Armario, A. Validation of the long-term assessment of hypothalamic-pituitary-adrenal activity in rats using hair corticosterone as a biomarker. FASEB J. 2015, 29, 859–867. [Google Scholar] [CrossRef]
- Gow, R.; Thomson, S.; Rieder, M.; Van Uum, S.; Koren, G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci. Int. 2010, 196, 32–37. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Tietze, A.; Skoluda, N.; Dettenborn, L. Hair as a retrospective calendar of cortisol production—Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 2009, 34, 32–37. [Google Scholar] [CrossRef]
- Konstantinou, G.N. Enzyme-linked immunosorbent assay (ELISA). In Food Allergens; Lin, J., Alcocer, M., Eds.; Human Press: Bergen County, NJ, USA, 2017; Volume 1592, pp. 79–94. [Google Scholar]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef]
- Engvall, E.; Jonsson, K.; Perlmann, P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim. Biophys. Acta 1971, 251, 427–434. [Google Scholar] [CrossRef]
- Elshal, M.F.; McCoy, J.P. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods 2006, 38, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.X.; McElhaney, J.E.; Walston, J.D.; Xie, D.; Fedarko, N.S.; Kuchel, G.A. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 879–884. [Google Scholar] [CrossRef]
- Mountjoy, K.G. ELISA versus LUMINEX assay for measuring mouse metabolic hormones and cytokines: Sharing the lessons I have learned. J. Immunoass. Immunochem. 2021, 42, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Ghassemi Nejad, J.; Roh, S.G.; Lee, H.G. Heat-shock proteins gene expression in peripheral blood mononuclear cells as an indicator of heat stress in beef calves. Animals 2020, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Ghassemi Nejad, J.; Peng, D.Q.; Jung, U.S.; Kim, M.J.; Jo, Y.H.; Jo, J.H.; Lee, J.S.; Lee, H.G. Identification of heat shock protein gene expression in hair follicles as a novel indicator of heat stress in beef calves. Animal 2020, 14, 1502–1509. [Google Scholar] [CrossRef]
| Properties | Biomatrices | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hair/wool | Feather | Nail/ Teeth/Scale 1 | Earwax | Feces | Urine | Sweat | Saliva | Milk | Blood | |
| Stressful-sampling procedure | Low 1 | Low | No | Low | Low | Low | Low | Low 2 | Low | High |
| Sampling effects on results | No | Low | No | No | No | No | Yes | Yes | No | Yes |
| Painful-sampling procedure | Low | Low | No | Low | Low | Low | Low | Low | Low | High |
| Liability to external contamination | High | High | Low | Low | High | High | High | High | Low | Low |
| Application acute stress | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Application chronic stress | Yes | Yes | Yes | Yes | Yes | Yes | No 3 | No 4 | No 5 | No 6 |
| Possibility of repeated sampling | No | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Liability to blood contamination of samples | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No |
| Effect of pH on composition | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Affected by location of sample collection | Yes | No | No | No | Yes | Yes | Yes | No | No | No |
| Need for vet personnel for sample collection | No | No | No | Yes | No | Yes | No | No | No | Yes |
| Time periods of cortisol represented | Weeks, Months, Years | Weeks, Months, Years | Weeks, Months, Years | Weeks, Months | Two to four days | One to two days | Single point, hour | Single point, hour | 4–10 h | Single point, hour |
| Measuring cortisol forms | Unbound | Unbound | Unbound | Unbound | Unbound | Unbound | Unbound | Unbound | Bound and unbound | Bound and unbound |
| Storage condition | Room temperature | Room temperature | Refrigeration, freezing | Refrigeration, freezing | Refrigeration, freezing | Refrigeration, freezing | Refrigeration, freezing | Refrigeration, freezing | Refrigeration, freezing | Refrigeration, freezing |
| Analytical costs | High | High | High | Medium | Medium | Medium | High | Medium | Medium | Medium |
| Strengths | Considerations |
|---|---|
| The procedure of hair sampling is a non-invasive method and simple. | Difficulty in the collection of short hair species or offspring. |
| At most 1g of hair sample is required. | Cannot access hair loss people and animals. |
| Ease of storage and stability at room temperature for years and hair is resistant to chemical decomposition rather than other biological matrices. | Requiring to wrap hair samples in aluminum foil to maintain the integrity and to avoid extra contamination while storing at room temperature. |
| Retrospective calendar of cortisol secretion - provides a window to the past. | Hair samples do not reflect the immediate or recent exposure to cortisol secretion. |
| Hair characteristics including hair color [7] do not influence the amount of cortisol. Sampling is possible by researcher. | Possible confounding factors: age, sex, hair growth rate, and body weight. |
| Easily can be transferred to the laboratory for analysis. | Still need to determine the most effective cortisol extraction method (methods of cortisol extraction differ between studies). |
| Characters | RIA Assay | EIA Assay | Luminex Assay |
|---|---|---|---|
| Easy to perform with a simple procedure | No | Yes | Yes |
| Immunoassay type | Yes | Yes | Yes |
| Antigen and antibody reaction | Yes, by immune methods | Yes | Yes |
| High specificity and sensitivity | Yes | Yes | Yes |
| Useful in diagnosis and research | Yes | Yes | Yes |
| Generally safe and eco-friendly | No | Yes | Yes |
| Radioactive substances requirement | Yes | No | No |
| Cost-effective assay | No | Yes | Yes |
| Reagents cost | High cost | Low cost | Low cost |
| Time duration of the experimental procedure | Time consuming | Short and fast | Save time and money compared to multiple single-analyte ELISAs |
| An efficient and highly skilled handler is needed | Yes | No | No |
| Disposal requires special care | Yes, to avoid radiation exposure | No | No |
| Disposal of waste is simple Measures multiple analytes in one sample Track biological interactions in real time | No No No | Yes No No | Yes Yes Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghassemi Nejad, J.; Ghaffari, M.H.; Ataallahi, M.; Jo, J.-H.; Lee, H.-G. Stress Concepts and Applications in Various Matrices with a Focus on Hair Cortisol and Analytical Methods. Animals 2022, 12, 3096. https://doi.org/10.3390/ani12223096
Ghassemi Nejad J, Ghaffari MH, Ataallahi M, Jo J-H, Lee H-G. Stress Concepts and Applications in Various Matrices with a Focus on Hair Cortisol and Analytical Methods. Animals. 2022; 12(22):3096. https://doi.org/10.3390/ani12223096
Chicago/Turabian StyleGhassemi Nejad, Jalil, Morteza Hosseini Ghaffari, Mohammad Ataallahi, Jang-Hoon Jo, and Hong-Gu Lee. 2022. "Stress Concepts and Applications in Various Matrices with a Focus on Hair Cortisol and Analytical Methods" Animals 12, no. 22: 3096. https://doi.org/10.3390/ani12223096
APA StyleGhassemi Nejad, J., Ghaffari, M. H., Ataallahi, M., Jo, J.-H., & Lee, H.-G. (2022). Stress Concepts and Applications in Various Matrices with a Focus on Hair Cortisol and Analytical Methods. Animals, 12(22), 3096. https://doi.org/10.3390/ani12223096








