Feeding Strategies for Adapting Lake Sturgeon (Acipenser fulvescens) Larvae to Formulated Diets at Early Life Stages
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Feeding Trial I
2.2. Feeding Trial II
2.3. Feeding Trial III
2.4. Data Analysis
3. Results
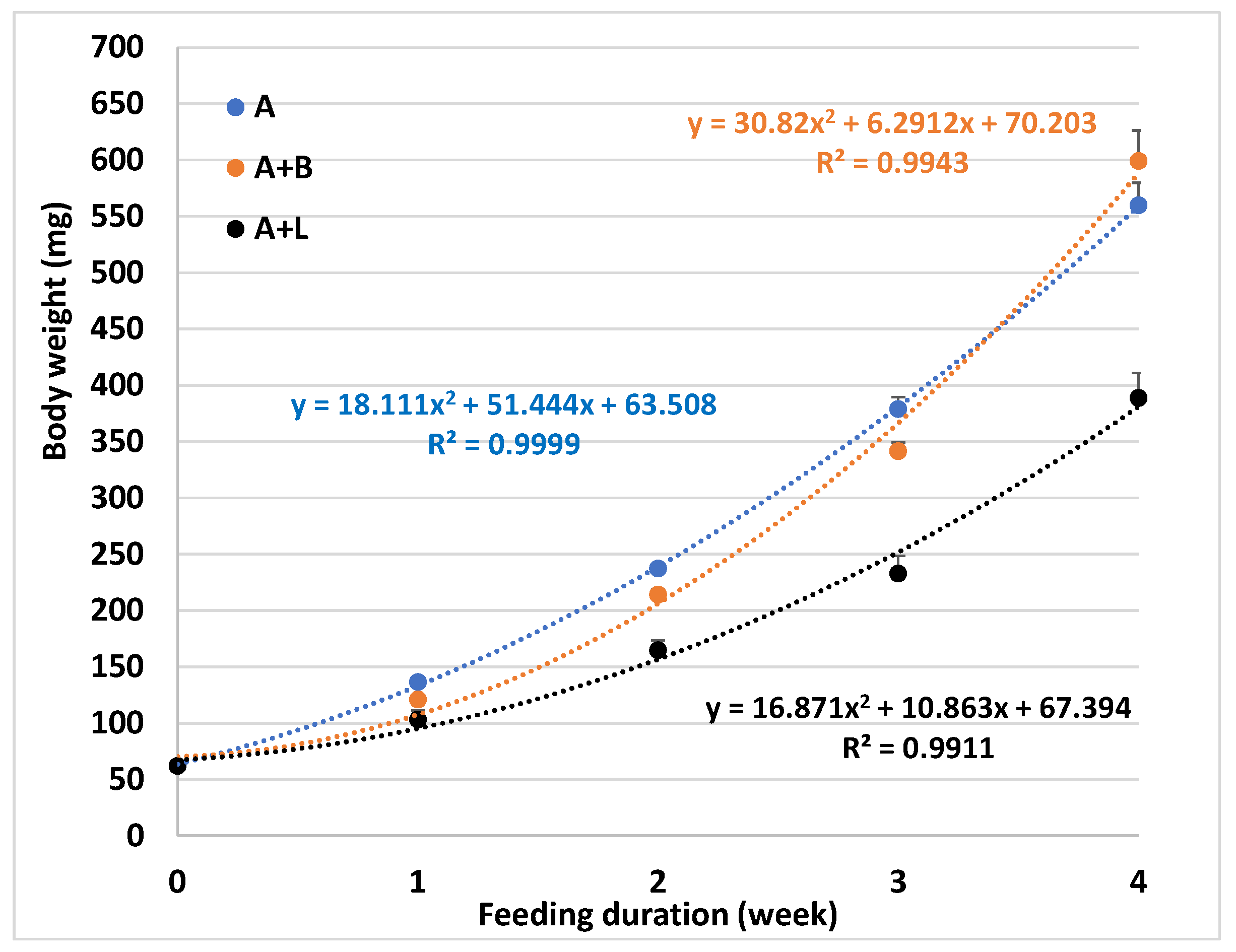
3.1. The Potential of Substituting Artemia with Formulated Feed
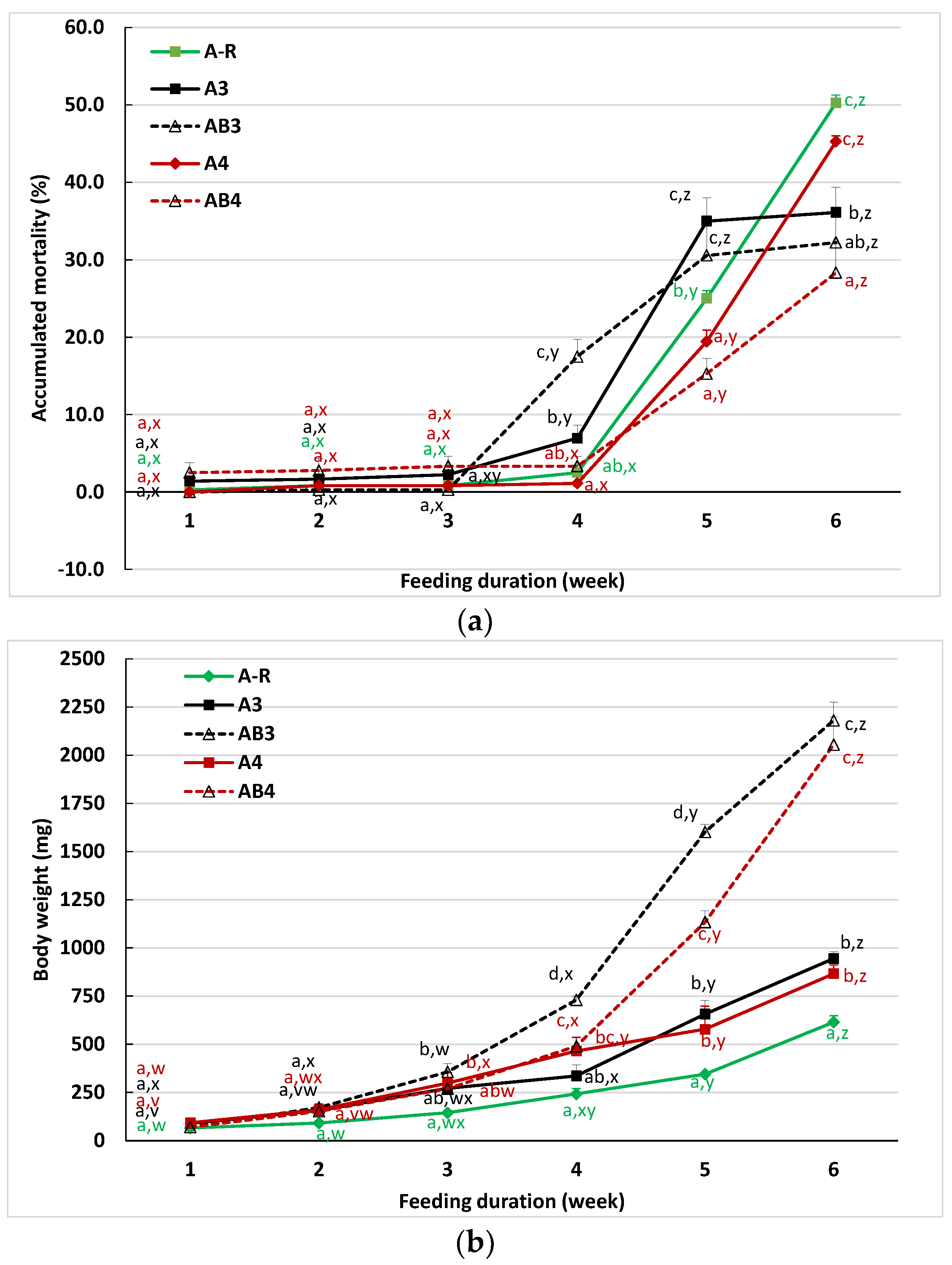
3.2. Effect of Different Feedings on the Adaptation of Lake Sturgeon to Formulated Feed
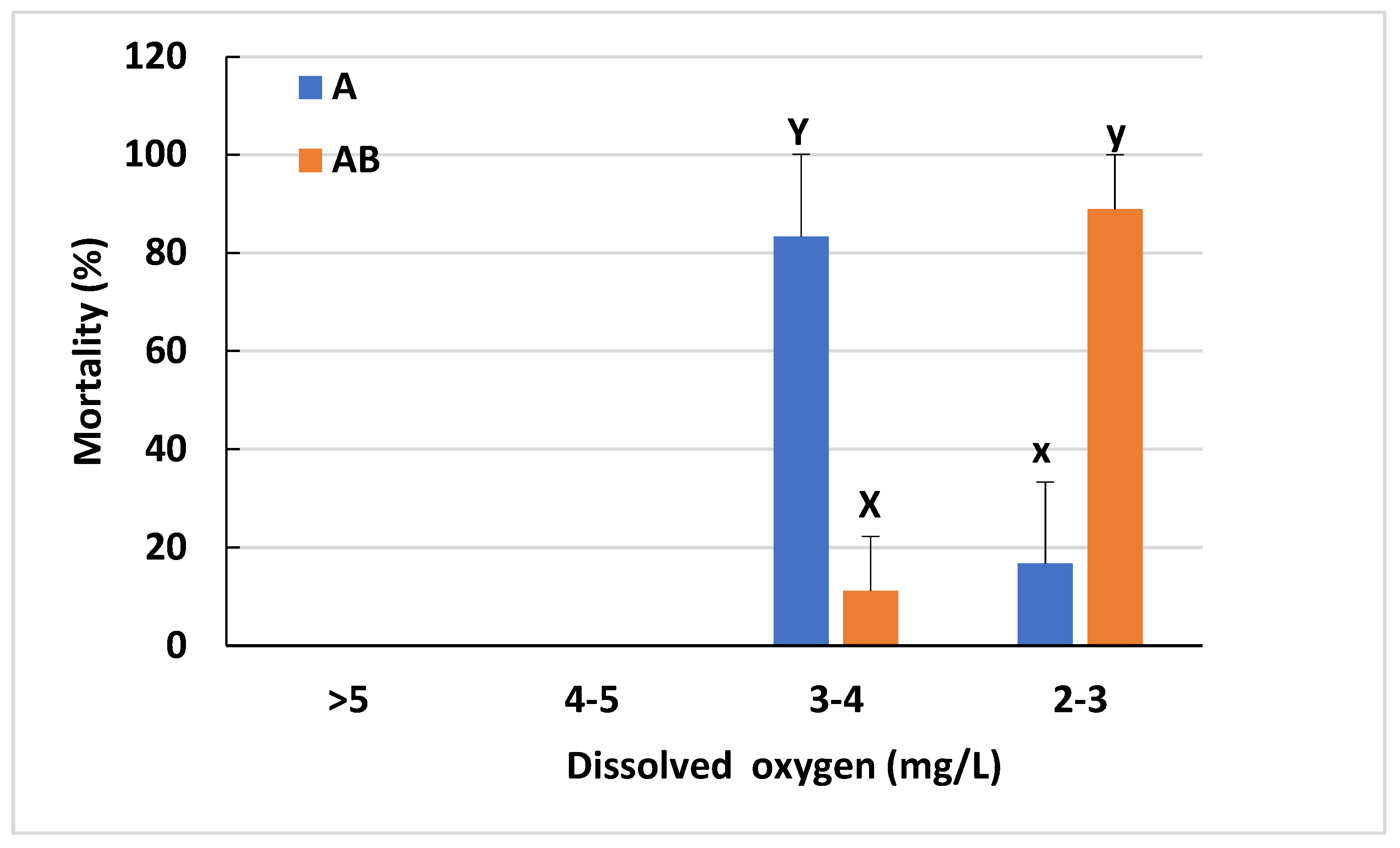
3.3. Effect of Different Feedings on Hypoxia Tolerance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneeberger, P.J.; Elliott, R.F.; Jonas, J.L.; Hart, S. Benthivores. In The State of Lake Michigan in 2000; Holey, M.E., Trudeau, T.N., Eds.; Great Lakes Fisheries Commission: Ann Arbor, MI, USA, 2005; ISSN 1090-1051. [Google Scholar]
- Michigan Natural Features Inventory. Acipenser fulvescens. Available online: https://mnfi.anr.msu.edu/abstracts/zoology/Acipenser_fulvescens.pdf (accessed on 1 November 2020).
- Schloesser, J.T.; Quinlan, H.R. Population Status and Demographics of Lake Sturgeon in the Bad and White rivers, Wisconsin. J. Fish Wildl. Manag. 2019, 10, 442–457. [Google Scholar] [CrossRef] [Green Version]
- Schram, S.T.; Lindgren, J.; Evrard, L. Reintroduction of Lake Sturgeon in the St. Louis River, Western Lake Superior. N. Am. J. Fish Manag. 1999, 19, 815–823. [Google Scholar] [CrossRef]
- Haxton, T.; Whelan, G.; Bruch, R. Historical Biomass and Sustainable Harvest of Great Lakes Lake Sturgeon (Acipenser fulvescens Rafinesque, 1817). J. Appl. Ichthyol. 2014, 30, 1371–1378. [Google Scholar] [CrossRef]
- Peterson, D.L.; Vecsei, P.; Jennings, C.A. Ecology and Biology of the Lake Sturgeon: A Synthesis of Current Knowledge of a Threatened North American Acipenseridae. Rev. Fish Biol. Fish. 2007, 17, 59–76. [Google Scholar] [CrossRef] [Green Version]
- Noakes, D.L.G.; Beamish, F.W.H.; Rossiter, A. Conservation Implications of Behaviour and Growth of the Lake Sturgeon, Acipenser fulvescens, in Northern Ontario. Environ. Biol. Fish. 1999, 55, 135–144. [Google Scholar] [CrossRef]
- Crossman, J.A.; Scribner, K.T.; Duong, Y.T.; Davis, C.A.; Forsythe, P.S.; Baker, E.A. Gamete and Larval Collection Methods and Hatchery Rearing Environment Affect Levels of Genetic Diversity in Early Life Stages of Lake Sturgeon (Acipenser fulvescens). Aquaculture 2011, 310, 312–324. [Google Scholar] [CrossRef]
- DiLauro, M.N.; Krise, W.F.; Fynn-Aikins, K. Growth and Survival of Lake Sturgeon Larvae Fed Formulated Diets. Prog. Fish Cult. 1998, 60, 293–296. [Google Scholar] [CrossRef]
- Bauman, J.M.; Woodward, B.M.; Baker, E.A.; Marsh, T.L.; Scribner, K.T. Effects of Family, Feeding Frequency, and Alternate Food Type on Body Size and Survival of Hatchery-Produced and Wild-Caught Lake sturgeon Larvae. N. Am. J. Aquac. 2016, 78, 136–144. [Google Scholar] [CrossRef]
- Valentine, S.A.; Bauman, J.M.; Scriber, K.T. Effects of Alternative Food Types on Body Size and Survival of Hatchery-Reared Lake Sturgeon. N. Am. J. Aquac. 2017, 79, 275–282. [Google Scholar] [CrossRef]
- Lee, S.; Zhao, H.; Li, Y.; Binkowski, F.P.; Deng, D.F.; Shepherd, B.S.; Hung, S.S.O.; Bai, S.C. Evaluation of Formulated Feed for Juvenile Lake Sturgeon Based on Growth Performance and Nutrient Retention. N. Am. J. Aquac. 2018, 80, 223–236. [Google Scholar] [CrossRef]
- Yang, S.; Zhai, S.W.; Shepherd, B.S.; Binkowski, F.P.; Hung, S.S.O.; Sealey, W.M.; Deng, D.F. Determination of Optimal Feeding Rates for Juvenile Lake Sturgeon (Acipenser fulvescens) Fed a Formulated Dry Diet. Aquac. Nutr. 2019, 25, 1171–1182. [Google Scholar] [CrossRef]
- Sorgeloos, P.; Bossuyt, E.; Laviña, E.; Baeza-Mesa, M.; Persoone, G. Decapsulation of Artemia Cysts: A Simple Technique of the Improvement of the Use of Brine Shrimp in Aquaculture. Aquaculture 2019, 12, 311–315. [Google Scholar] [CrossRef]
- Deng, D.F.; Koshio, S.; Yokoyama, S.; Bai, S.C.; Shao, Q.; Cui, Y.; Hung, S.S.O. Effects of Feeding Rate on Growth Performance of White Sturgeon (Acipenser transmontanus) Larvae. Aquaculture 2003, 217, 589–598. [Google Scholar] [CrossRef]
- Sealey, W.M.; O’Neill, T.J.; Peach, J.T.; Gaylord, T.G.; Barrows, F.T.; Block, S.S. Refining Inclusion Levels of Grain Distiller’s Dried Yeast in Commercial Type and Plant Based Diets for Juvenile Rainbow Trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2015, 46, 434–444. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, H.; Zai, S.; Shepherd, B.; Wen, H.; Deng, D.F. A Defatted Microalgae Meal (Haematococcus pluvialis) as a Partial Protein Source to Replace Fishmeal for Feeding Juvenile Yellow Perch Perca flavescens. J. Appl. Phycol. 2019, 31, 1197–1205. [Google Scholar] [CrossRef]
- Buckley, L.; Caldarone, E.M.; Ong, T.L. RNA–DNA Ratio and Other Nucleic Acid-based Indicators for Growth and Condition of Marine Fishes. Hydrobiologia 1999, 401, 265–277. [Google Scholar] [CrossRef]
- Deng, D.F.; Wang, C.; Lee, S.; Bai, S.; Hung, S.S.O. Feeding Rates Affect Heat Shock Protein Levels in Liver of Larval White Sturgeon (Acipenser transmontanus). Aquaculture 2009, 287, 223–226. [Google Scholar] [CrossRef]
- Caldarone, E.M.; Wagner, M.; St. Onge-Burns, J.; Buckley, L.J. Protocol and Guide for Estimating Nucleic Acids in Larval Fish Using a Fluorescence Microplate Reader; Ref. Doc. 01-11; Northeast Fisheries Science Center: Woods Hole, MA, USA, 2001; 22p. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Heldrich, K., Ed.; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Anderson, E.R. Artificial Propagation of Lake Sturgeon Acipenser fulvescens (Rafinesque), Under Hatchery Conditions in Michigan; Fisheries Report 1898; Michigan Department of Natural Resources, Fisheries Division: Ann Arbor, MI, USA, 1984. [Google Scholar]
- Ćeskleba, D.G.; AveLallement, S.; Thuemler, T.F. Artificial Spawning and Rearing of Lake Sturgeon, Acipenser fulvescens, in Wild Rose State Fish Hatchery, Wisconsin, 1982-1983. Environ. Biol. Fish. 1985, 14, 79–85. [Google Scholar] [CrossRef]
- Hung, S.S.O. Recent Advances in Sturgeon Nutrition. Anim. Nutr. 2017, 3, 191–204. [Google Scholar] [CrossRef]
- Clemmesen, C. Importance and Limits of RNA/DNA Ratios as a Measure of Nutritional Condition in Fish Larvae. In Survival Strategies in Early Life Stages of Marine Resources: Proceedings of the International Workshop, Tsukuba, Japan, 11–15 November 1996; Watanabe, Y., Yamashita, Y.A., Oozeki, Y., Eds.; Balkema: Rotterdam, The Netherlands, 1996; pp. 67–82. ISBN 90-5410-637-9. [Google Scholar]
- Chung, K.S.; Deb, M.S.; Donaldson, E.M. RNA:DNA Ratio as Physiological Condition of Rainbow Trout Fry Fasted and Fed. Ital. J. Zool. 1998, 65, 517–519. [Google Scholar] [CrossRef]
- Kono, N.; Tsukamoto, Y.; Zenitani, H. RNA/DNA Ratio for Diagnosis of the Nutritional Condition of Japanese Anchovy Engraulis japonicus Larvae During the Firstfeeding Stage. Fish. Sci. 2003, 69, 1096–1102. [Google Scholar] [CrossRef]
- Zehra, S.; Khan, M.A. Dietary Leucine Requirement of Fingerling Catla catla (Hamilton) Based on Growth, Feed Conversion Ratio, RNA/DNA Ratio, Leucine Gain, Blood Indices and Carcass Composition. Aquac. Int. 2014, 23, 577–595. [Google Scholar] [CrossRef]
- Barnes, C.; Sweeting, C.J.; Jennings, S.; Barry, J.T.; Polunin, N.V.C. Effect of Temperature and Ration Size on Carbon and Nitrogen Stable Isotope Trophic Fractionation. Funct. Ecol. 2007, 21, 356–362. [Google Scholar] [CrossRef]
- Abrantes, K.G.; Semmens, J.M.; Lyle, J.M.; Peter, D. Normalisation Models for Accounting for Fat Content in Stable Isotope Measurements in Salmonid Muscle Tissue. Mar. Biol. 2012, 159, 57–64. [Google Scholar] [CrossRef]
- Hoffman, J.; Sierszen, M.; Cotter, A. Fish Tissue Lipid-C:N Relationships for Correcting ä13C Values and Estimating Lipid Content in Aquatic Food Web Studies. Rapid Commun. Mass Spectrom. 2015, 29, 2069–2077. [Google Scholar] [CrossRef]
- D’Abramo, L.R. Challenges in Developing Successful Formulated Feed for Culture of Larval Fish and Crustaceans. In Avances en Nutrición Acuícola VI. Memorias del VI Simposium Internacional de Nutrición Acuícola. Cancún, Quintana Roo, México, 3–6 September 2002; Cruz-Suárez, L.E., Ricque-Marie, D., Tapia-Salazar, M., Gaxiola-Cortés, M.G., Simoes, N., Eds.; Universidad Autónoma de Nuevo León: Monterrey, NL, México, 2002. [Google Scholar]
- Tocher, D.R. Fatty Acid Requirements in Ontogeny of Marine and Freshwater Fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Yoon, G.R.; Laluk, A.; Bouyoucos, I.A.; Anderson, W.G. Effects of Dietary Shifts on Ontogenetic Development of Metabolic Rates in Age 0 Lake Sturgeon (Acipenser fulvescens). Physiol. Biochem. Zool. 2022, 95, 135–151. [Google Scholar] [CrossRef]
- McKenzie, D.; Piraccini, G.; Papini, N. Oxygen Consumption and Ventilatory Reflex Responses are Influenced by Dietary Lipids in Sturgeon. Fish. Physiol. Biochem. 1997, 16, 365–379. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Lund, L.; Pedersen, P.B. Essential Fatty Acids Influence Metabolic Rate and Tolerance of Hypoxia in Dover Sole (Solea solea) Larvae and Juveniles. Mar. Biol. 2008, 154, 1041–1051. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Piraccini, G.; Piccolella, M.; Steffensen, J.F.; Bolis, C.L.; Taylor, E.W. Effects of Dietary Fatty Acid Composition on Metabolic Rate and Responses to Hypoxia in the European Eel, Anguilla anguilla. Fish. Physiol. Biochem. 2000, 22, 281–296. [Google Scholar] [CrossRef]
- Babaei, S.S.; Abedian-Kenari, A.; Nazari, R.; Gisbert, E. Developmental Changes of Digestive Enzymes in Persian sturgeon (Acipenser persicus) During Larval Ontogeny. Aquaculture 2011, 318, 138–144. [Google Scholar] [CrossRef]
- Sanz, A.; Llorente, J.I.; Furné, M.; Carmona, M.R.; Domezain, A.; Hidalgo, M.C. Digestive Enzymes During Ontogeny of the Sturgeon Acipenser naccarii: Intestine and Pancreas Development. J. Appl. Ichthyol. 2011, 27, 1139–1146. [Google Scholar] [CrossRef]
- Ghasemi, N.; Imani, A.; Noori, F.; Shahrooz, R. Ontogeny of Digestive Tract of Stellate Sturgeon (Acipenser stellatus) from Hatching to Juvenile Stage: Digestive Enzymes Activity, Stomach and Proximal Intestine. Aquaculture 2020, 519, 734–751. [Google Scholar] [CrossRef]
- Gwangseok, R.Y.; Amjad, H.; Weinrauch, A.M.; Laluk, A.; Suh, M.; Anderson, W.G. Long-Term Effects of EPA and DHA Enriched Diets on Digestive Enzyme Activity, Aerobic Scope, Growth and Survival in Age-0 Lake Sturgeon (Acipenser fulvescens). Aquaculture 2022, 552, 737972. [Google Scholar]

| Treatment | Feeding Rate (% BW) during the 4 Weeks | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Artemia (A) | 10 | 7.5 | 6.5 | 4.8 |
| Commercial diet (B) | 20 | No more feeding | ||
| Lab diet (L) | 20 | No more feeding | ||
| A + B | A: 5, B: 10 | A: 3.75, B: 7.5 | A: 3.25, B: 6.5 | A: 2.4, B: 4.8 |
| A + L | A: 5, L: 10 | A: 3.75, L: 7.5 | A: 3.25, L: 6.5 | A: 2.4, L: 4.8 |
| Test Diet | |||
|---|---|---|---|
| Analysis | Artemia nauplii | Commercial Feed (Otohime B) | Lab-Made Diet |
| Proximate composition (g/kg, dry matter basis) | |||
| Moisture | 946 | 65 | 110 |
| Protein | 513 | 551 | 491 |
| Lipid | 164 | 180 | 139 |
| Ash | 98 | 129 | 76 |
| Major fatty acid (g/kg of total fat) | |||
| 16:0 | 113.4 | 176.6 | 187 |
| 16:1 | 31.9 | 41.8 | 49.7 |
| 18:0 | 40.2 | 22.8 | 56.2 |
| 18:1 n-9 | 187.0 | 100.4 | 138.3 |
| 18:2 n-6 | 56.3 | 53.1 | 218.1 |
| 18:3 n-3 | 308.9 | 44.2 | 47 |
| 20:5 n-3 | 14.9 | 123.3 | 54.5 |
| 22:6 n-3 | 0.3 | 107.8 | 38.1 |
| Dietary Treatment | Feeding Duration (Week) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Feeding Rate 8 | ||||||
| A-R 1 | 10 A | 5 A | 3 A | 3 A | 9 B | 9 B |
| A2 2 | 20 A | 10 A | 9 B | 9 B | ||
| A3 3 | 20 A | 10 A | 6 A | 9 B | 6 B | 6 B |
| A4 4 | 20 A | 10 A | 6 A | 6 A | 6 B | 6 B |
| AB2 5 | 10 A + 10 B | 5 A + 10 B | 9 B | 9 B | ||
| AB3 6 | 10 A + 10 B | 5 A + 10 B | 3 A + 6 B | 9 B | 6 B | 6 B |
| AB4 7 | 10 A + 10 B | 5 A + 10 B | 3 A + 6 B | 3 A + 6 B | 6 B | 6 B |
| Treatment 2 | |||||
|---|---|---|---|---|---|
| Measurement | A | B | L | A + B | A + L |
| Survival (%) | 100.0 ± 0.0 A | 78.2 ± 2.7 B | 80.9 ± 7.2 B | 99.1 ± 0.9 A | 99.1 ± 0.9 A |
| Final body weight (mg) | 137 ± 3 A | 93 ± 6 C | 81 ± 1 C | 121 ± 3 AB | 103 ± 8 BC |
| Weight gain 3 (%) | 120.2 ± 4.3 A | 49.3 ± 10.2 C | 29.9 ± 1.9 C | 95.1 ± 5.6 AB | 66.4 ± 12.9 BC |
| Feed conversion ratio 4 | 0.4 ± 0.0 C | 2.6 ± 0.7 AB | 3.6 ± 0.2 A | 0.8 ± 0.1 C | 1.2 ± 0.3 BC |
| Protein efficiency ratio 5 | 4.74 ± 0.17 A | 0.74 ± 0.15 C | 0.53 ± 0.03 C | 2.07 ± 0.12 B | 1.65 ± 0.32 B |
| Treatment 2 | |||
|---|---|---|---|
| A | A + B | A + L | |
| Growth performance | |||
| Survival (%) | 96.7 ± 0.7 | 94.7 ± 2.9 | 92.7 ± 1.8 |
| Final body weight (mg) | 560 ± 20 A | 599 ± 27 A | 389 ± 22 B |
| Weight gain (%) | 803.4 ± 31.8 A | 866.8 ± 43.6 A | 527.3 ± 35.4 B |
| Condition factor | 0.34 ± 0.01 | 0.34 ± 0.00 | 0.33 ± 0.00 |
| Feed conversion ratio | 0.5 ± 0.0 C | 0.7 ± 0.0 B | 0.8 ± 0.0 A |
| Protein efficiency ratio | 3.83 ± 0.11 A | 2.55 ± 0.10 B | 2.22 ± 0.08 B |
| Proximate composition | |||
| Moisture (g/kg) | 895 ± 3 A | 880 ± 3 B | 900 ± 3 A |
| Crude protein (g/kg) | 78 ± 1 | 83 ± 1 | 76 ± 3 |
| Crude ash (g/kg) | 14 ± 1 A | 13 ± 0 AB | 11 ± 1 B |
| C/N ratio | 3.74 ± 0.05 B | 4.25 ± 0.13 A | 3.70 ± 0.02 B |
| RNA/DNA ratio | 1.12 ± 0.09 | 1.19 ± 0.08 | 1.26 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Zhai, S.; Deng, D.-F.; Li, Y.; Blaufuss, P.C.; Eggold, B.T.; Binkowski, F. Feeding Strategies for Adapting Lake Sturgeon (Acipenser fulvescens) Larvae to Formulated Diets at Early Life Stages. Animals 2022, 12, 3128. https://doi.org/10.3390/ani12223128
Lee S, Zhai S, Deng D-F, Li Y, Blaufuss PC, Eggold BT, Binkowski F. Feeding Strategies for Adapting Lake Sturgeon (Acipenser fulvescens) Larvae to Formulated Diets at Early Life Stages. Animals. 2022; 12(22):3128. https://doi.org/10.3390/ani12223128
Chicago/Turabian StyleLee, Seunghyung, Shaowei Zhai, Dong-Fang Deng, Yuquan Li, Patrick Christopher Blaufuss, Bradley T. Eggold, and Fred Binkowski. 2022. "Feeding Strategies for Adapting Lake Sturgeon (Acipenser fulvescens) Larvae to Formulated Diets at Early Life Stages" Animals 12, no. 22: 3128. https://doi.org/10.3390/ani12223128





