Breeding Thin-Billed Prions Use Marine Habitats Ranging from Inshore to Distant Antarctic Waters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Sites
2.2. Field Methods
2.3. Data Analyses
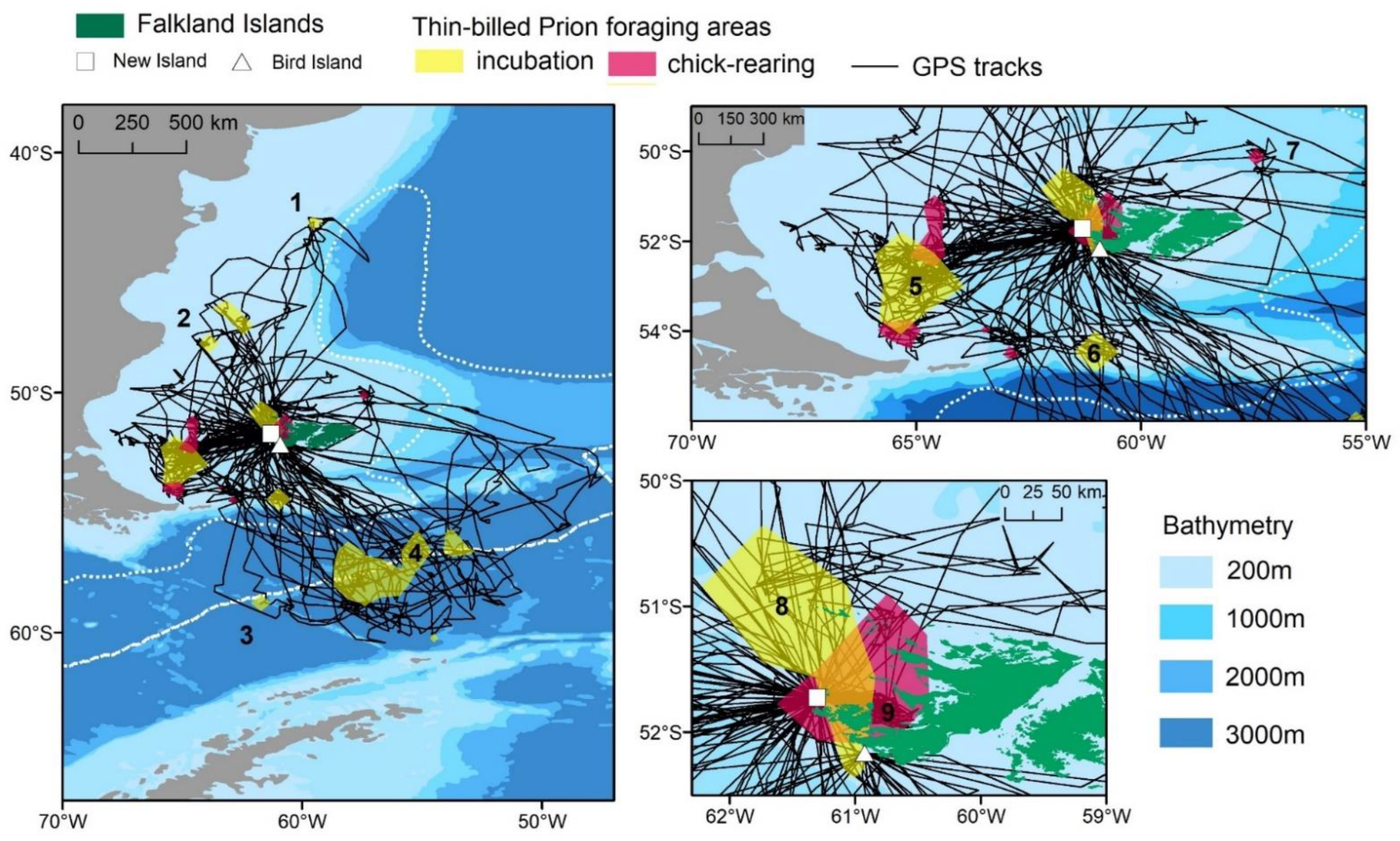
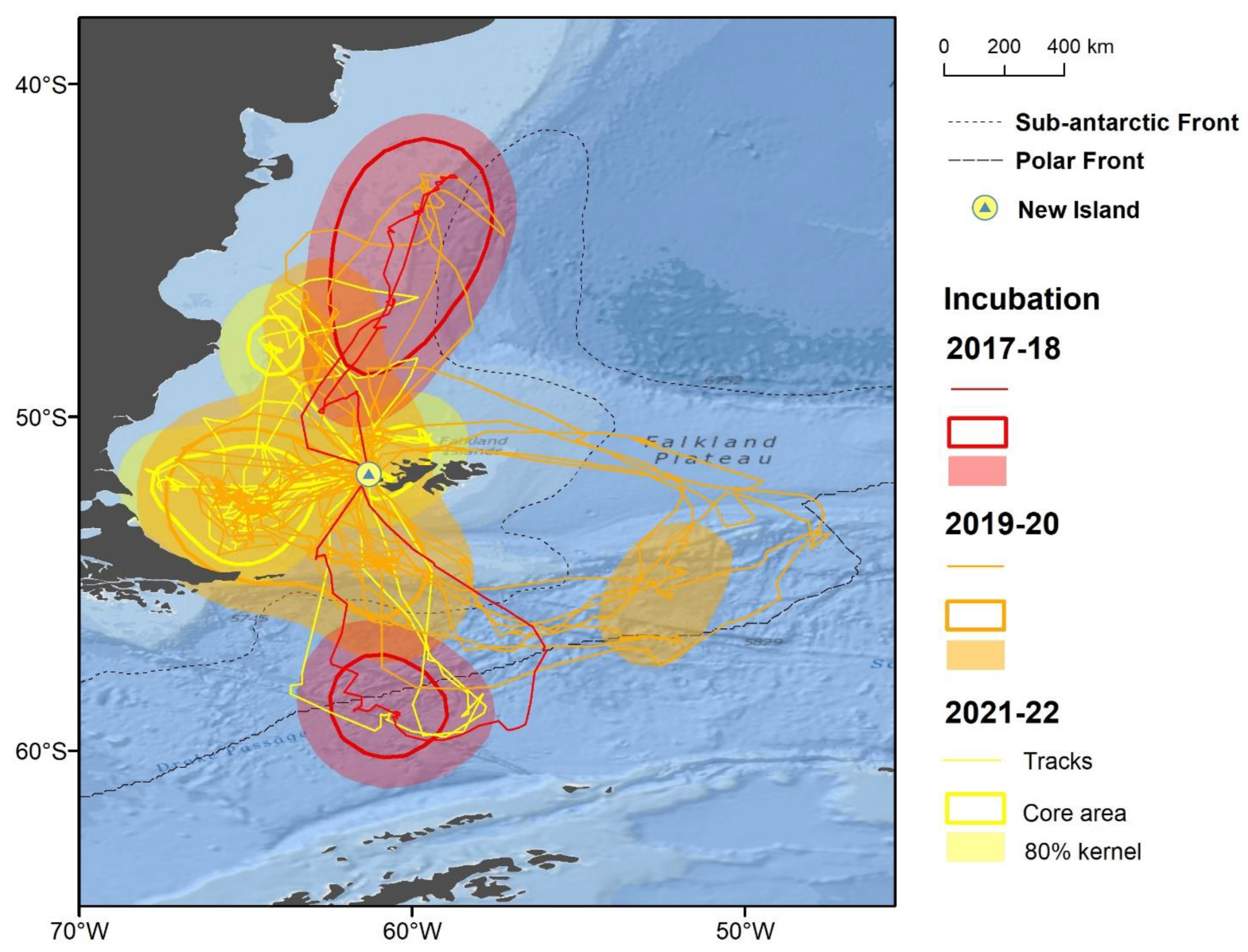
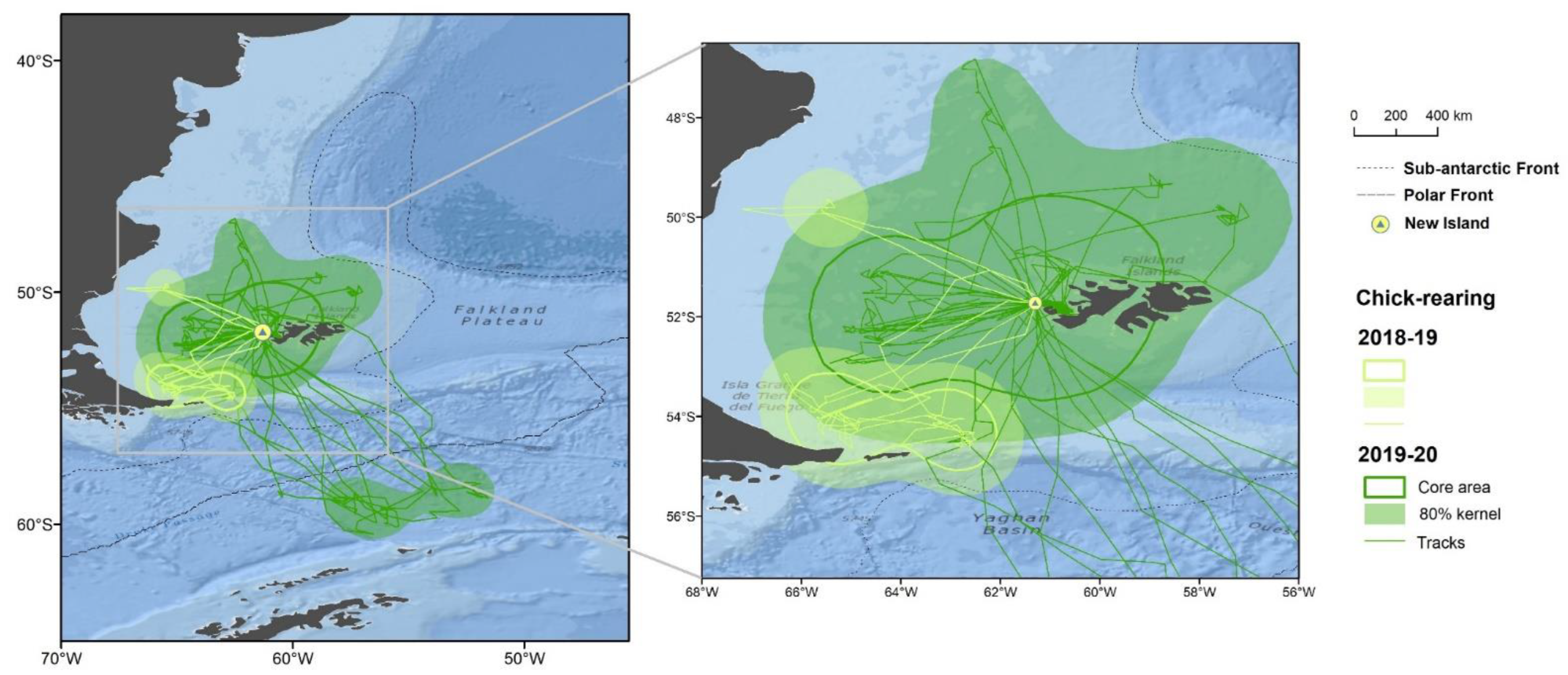
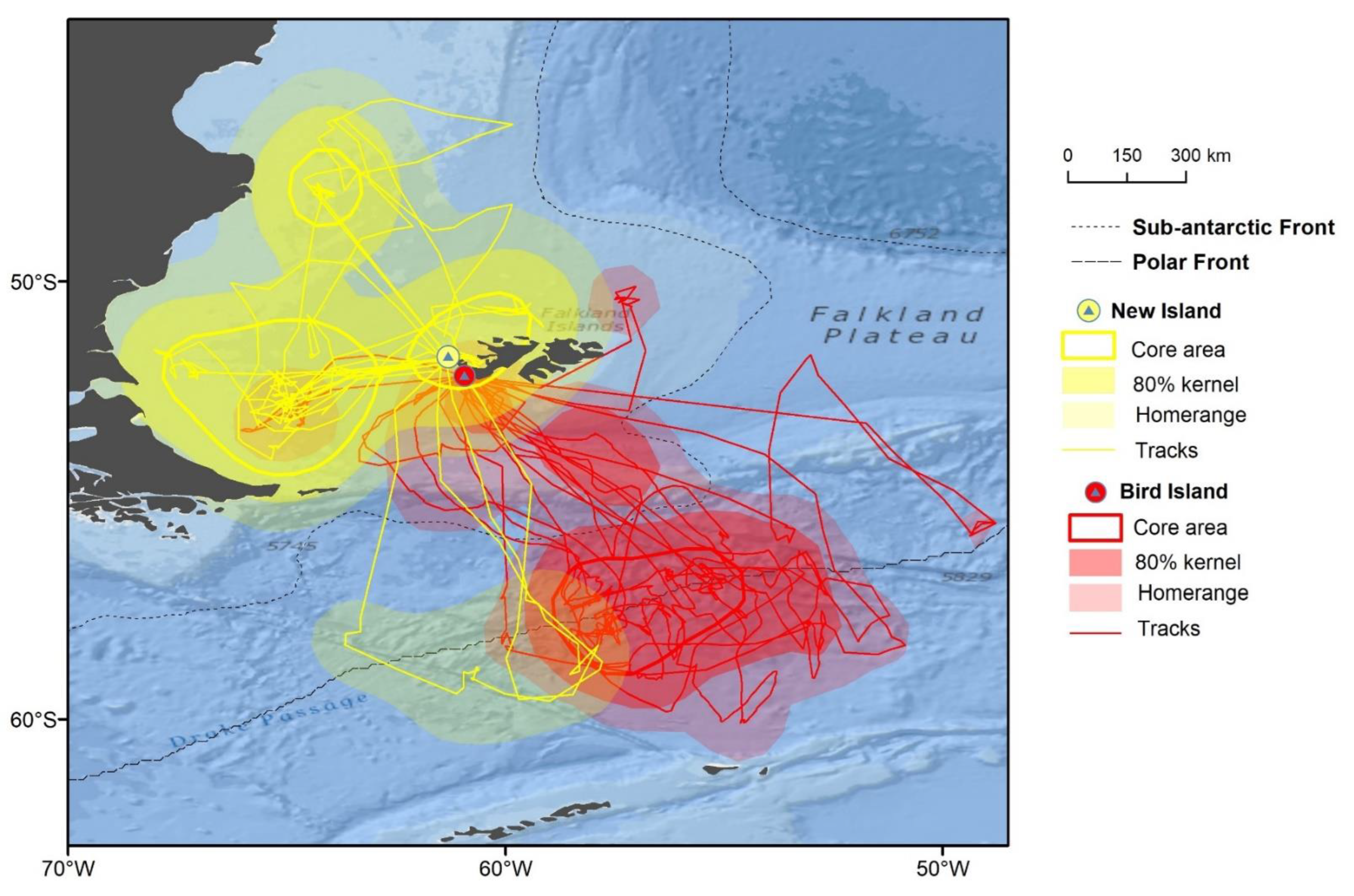
3. Results
3.1. Distribution
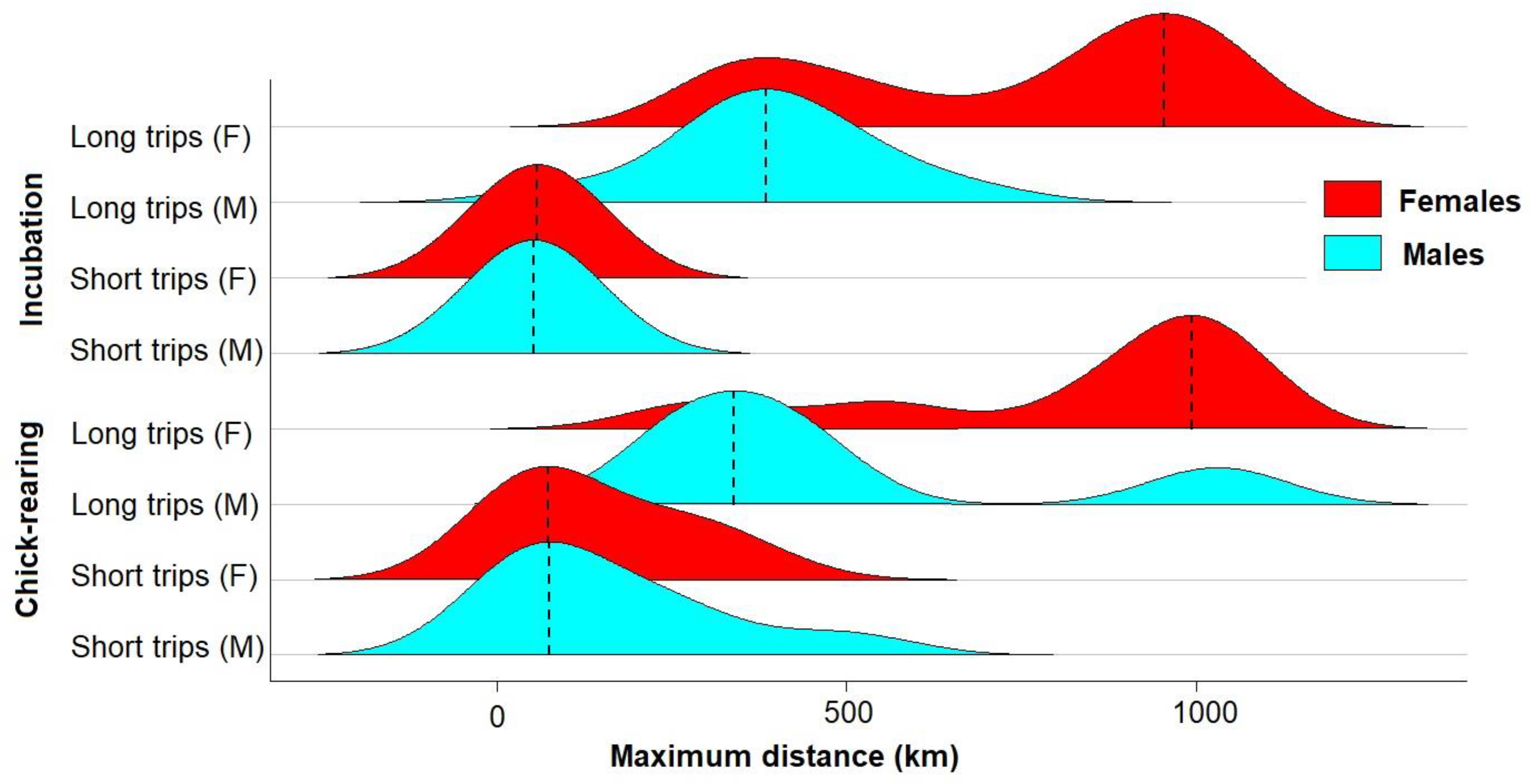
3.2. Trip Parameters
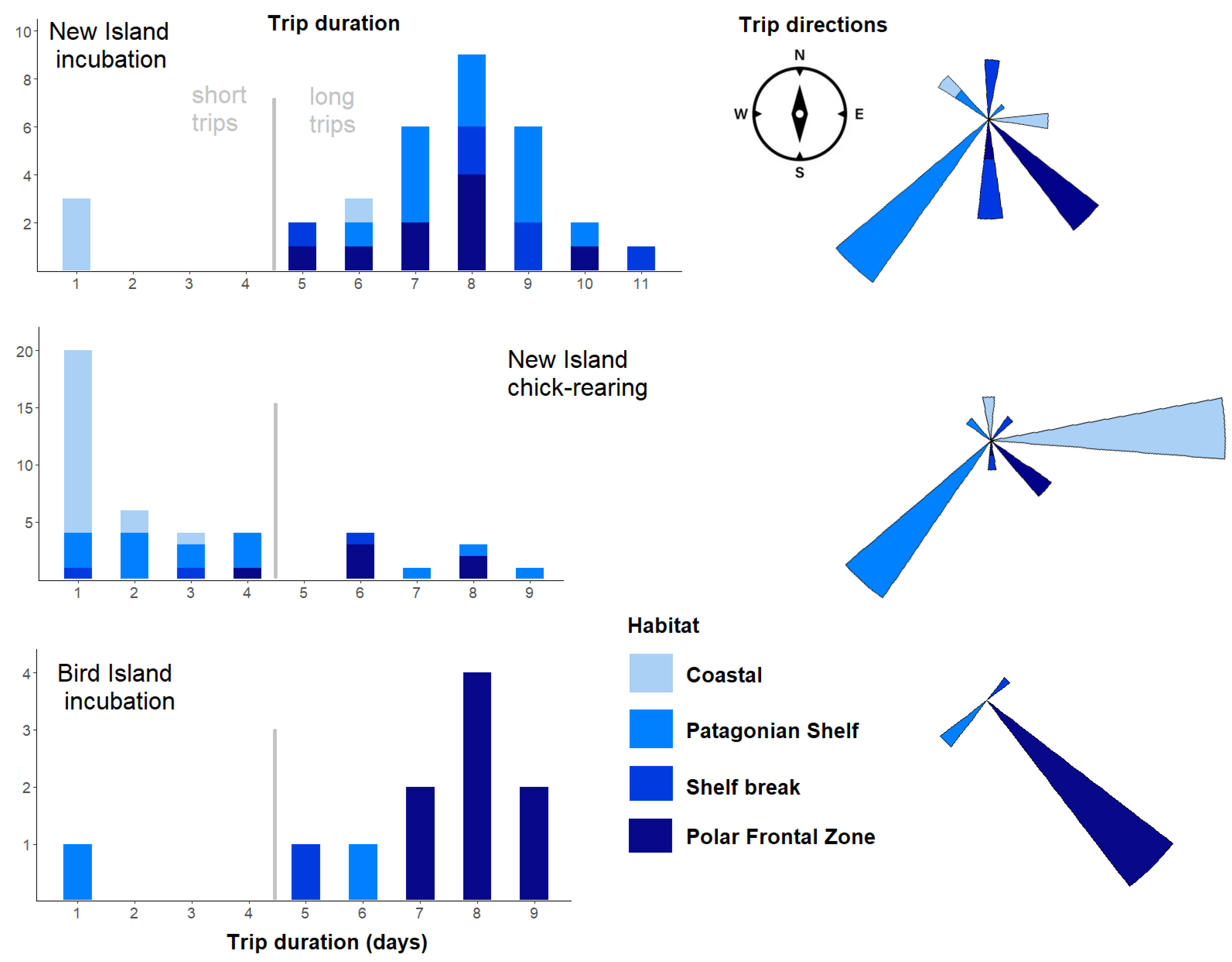
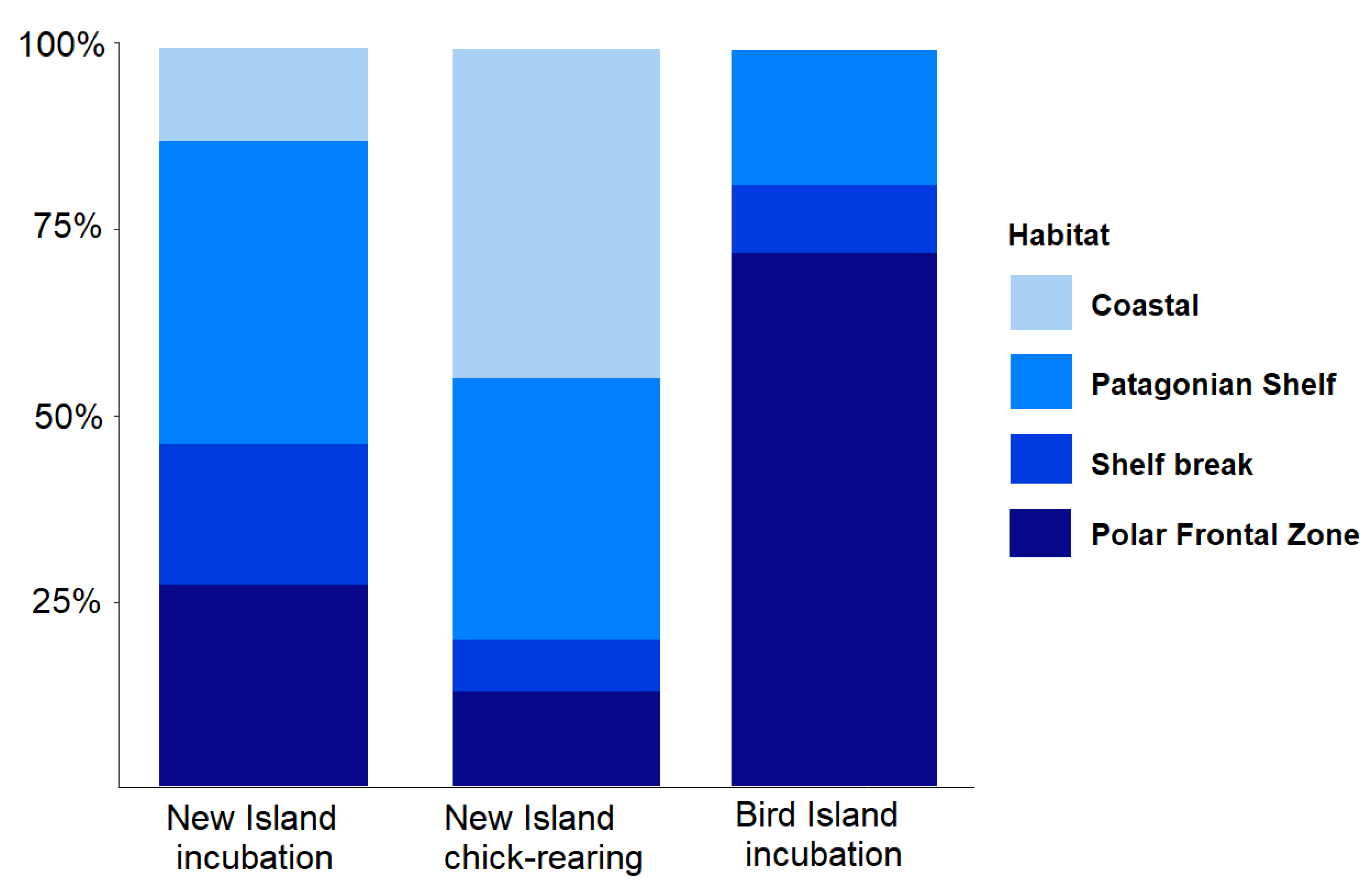
3.3. Foraging Habitat Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolton, M.; Conolly, G.; Carroll, M.; Wakefield, E.D.; Caldow, R. A review of the occurrence of inter-colony segregation of seabird foraging areas and the implications for marine environmental impact assessment. Ibis 2019, 161, 241–259. [Google Scholar] [CrossRef] [Green Version]
- Croxall, J.P.; Prince, P.A. Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biol. J. Linn. Soc. 1980, 14, 103–131. [Google Scholar] [CrossRef] [Green Version]
- Rodhouse, P.G.; Prince, P.A.; Trathan, P.N.; Hatfield, E.M.C.; Watkins, J.L.; Bone, D.G.; Murphy, E.J.; White, M.G. Cephalopods and mesoscale oceanography at the Antarctic Polar Front: Satellite tracked predators locate pelagic trophic interactions. Mar. Ecol. Prog. Ser. 1996, 136, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Guinet, C.; Koudil, M.; Bost, C.A.; Durbec, J.P.; Georges, J.Y.; Mouchot, M.C.; Jouventin, P. Foraging behaviour of satellite-tracked king penguins in relation to sea-surface temperatures obtained by satellite telemetry at Crozet Archipelago, a study during three austral summers. Mar. Ecol. Prog. Ser. 1997, 150, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, A.; Trathan, P.N.; Collins, M. Foraging behaviour of king penguins Aptenodytes patagonicus) in relation to predictable mesoscale oceanographic features in the Polar Front Zone to the north of South Georgia. Prog. Oceanogr. 2010, 86, 232–245. [Google Scholar] [CrossRef]
- Commins, M.L.; Ansorge, I.; Ryan, P.G. Multi-scale factors influencing seabird assemblages in the African sector of the Southern Ocean. Antarct. Sci. 2014, 26, 38–48. [Google Scholar] [CrossRef]
- Rayner, M.J.; Taylor, G.A.; Gaskin, C.P.; Dunphy, B.J. Seasonal activity and unpredicted polar front migration of northern New Zealand Common Diving Petrels Pelecanoides urinatrix. Emu-Austral Ornithol. 2017, 117, 290–298. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Chastel, O.; Ackermann, L.; Chaurand, T.; Cuenot-Chaillet, F.; Hindermeyer, X.; Judas, J. Alternate long and short foraging trips in pelagic seabird parents. Anim. Behav. 1994, 47, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Quillfeldt, P.; Phillips, R.A.; Marx, M.; Masello, J.F. Colony attendance and at-sea distribution of thin-billed prions during the early breeding season. J. Avian Biol. 2014, 45, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Quillfeldt, P.; Weimerskirch, H.; Masello, J.F.; Delord, K.; McGill, R.A.; Furness, R.W.; Cherel, Y. Behavioural plasticity in the early breeding season of pelagic seabirds—A case study of thin-billed prions from two oceans. Mov. Ecol. 2019, 7, 1. [Google Scholar] [CrossRef]
- Quillfeldt, P.; Michalik, A.; Veit-Köhler, G.; Strange, I.J.; Masello, J.F. Inter-annual changes in diet and foraging trip lengths in a small pelagic seabird, the thin-billed prion Pachyptila belcheri. Mar. Biol. 2010, 157, 2043–2050. [Google Scholar] [CrossRef]
- Catry, P.; Campos, A.; Segurado, P.; Silva, M.; Strange, I. Population census and nesting habitat selection of thin-billed prion Pachyptila belcheri on New Island, Falkland Islands. Polar Biol. 2003, 26, 202–207. [Google Scholar] [CrossRef]
- Stokes, A.W.; Catry, P.; Matthiopoulos, J.; Boldenow, M.; Clark, T.J.; Guest, A.; Marengo, I.; Wakefield, E.D. Combining survey and remotely sensed environmental data to estimate the habitat associations, abundance and distribution of breeding thin-billed prions Pachyptila belcheri and Wilson’s storm-petrels Oceanites oceanicus on a South Atlantic tussac island. Polar Biol. 2021, 44, 809–821. [Google Scholar] [CrossRef]
- Strange, I.J. The thin-billed prion, Pachyptila belcheri, at New Island, Falkland Islands. Gerfaut 1980, 70, 411–445. [Google Scholar]
- Quillfeldt, P.; Masello, J.F.; Navarro, J.; Phillips, R.A. Year-round distribution suggests spatial segregation of two small petrel species in the South Atlantic. J. Biogeogr. 2013, 40, 430–441. [Google Scholar] [CrossRef]
- Quillfeldt, P.; Cherel, Y.; Delord, K.; Weimerkirch, H. Cool, cold or colder? Spatial segregation of prions and blue petrels is explained by differences in preferred sea surface temperatures. Biol. Lett. 2015, 11, 20141090. [Google Scholar] [CrossRef] [Green Version]
- Cherel, Y.; Quillfeldt, P.; Delord, K.; Weimerskirch, H. Combination of at-sea activity, geolocation and feather stable isotopes documents where and when seabirds molt. Front. Ecol. Evol. 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Cherel, Y.; Bocher, P.; de Broyer, C.; Hobson, K.A. Food and feeding ecology of the sympatric Thin-billed Pachyptila belcheri and Antarctic P. desolata Prions at Iles Kerguelen, Southern Indian Ocean. Mar. Ecol. Prog. Ser. 2002, 228, 263–281. [Google Scholar] [CrossRef] [Green Version]
- Fridolfsson, A.K.; Ellegren, H.A. simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 December 2021).
- ESRI. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Michelot, T.; Langrock, R.; Patterson, T.A. moveHMM: An R package for the statistical modelling of animal movement data using hidden Markov models. Methods Ecol. Evol. 2016, 7, 1308–1315. [Google Scholar] [CrossRef] [Green Version]
- Calenge, C. The package adehabitat for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Baines, M.; Weir, C.R. Predicting suitable coastal habitat for sei whales, southern right whales and dolphins around the Falkland Islands. PLoS ONE 2020, 15, e0244068. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.E.; Reta, R.; Lutz, V.A.; Segura, V.; Daponte, C. Influence of oceanographic features on the spatial and seasonal patterns of mesozooplankton in the southern Patagonian shelf (Argentina, SW Atlantic). J. Mar. Syst. 2016, 157, 20–38. [Google Scholar] [CrossRef]
- Padovani, L.N.; Viñas, M.D.; Sánchez, F.; Mianzan, H. Amphipod-supported food web: Themisto gaudichaudii, a key food resource for fishes in the southern Patagonian Shelf. J. Sea Res. 2012, 67, 85–90. [Google Scholar] [CrossRef]
- Baylis, A.M.; Tierney, M.; Orben, R.A.; Warwick-Evans, V.; Wakefield, E.; Grecian, W.J.; Wakefield, E.; Reisinger, R.; Trathan, P.; Boersma, D.; et al. Important at-sea areas of colonial breeding marine predators on the southern Patagonian Shelf. Sci. Rep. 2019, 9, 8517. [Google Scholar] [CrossRef] [Green Version]
- Rodhouse, P.G.; Symon, C.; Hatfield, E.M.C. Early life cycle of cephalopods in relation to the major oceanographic features of the southwest Atlantic Ocean. Mar. Ecol. Prog. Ser. 1992, 89, 183–195. [Google Scholar] [CrossRef]
- Takahashi, K.T.; Hosie, G.W.; Odate, T. Intra-annual seasonal variability of surface zooplankton distribution patterns along a 110° E transect of the Southern Ocean in the austral summer of 2011/12. Polar Sci. 2017, 12, 46–58. [Google Scholar] [CrossRef]
- Catard, A.; Weimerskirch, H.; Cherel, Y. Exploitation of distant Antarctic waters and close shelf-break waters by white-chinned petrels rearing chicks. Mar. Ecol. Prog. Ser. 2000, 194, 249–261. [Google Scholar] [CrossRef]
- Quillfeldt, P.; McGill, R.A.R.; Strange, I.J.; Masello, J.F.; Weiss, F.; Brickle, P.; Furness, R.W. Stable isotope analysis reveals sexual and environmental variability and individual consistency in foraging of Thin-billed prions. Mar. Ecol. Prog. Ser. 2008, 373, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Congdon, B.C.; Krockenberger, A.K.; Smithers, B.V. Dual-foraging and co-ordinated provisioning in a tropical Procellariiform, the wedge-tailed shearwater. Mar. Ecol. Prog. Ser. 2005, 301, 293–301. [Google Scholar] [CrossRef]
- Tarroux, A.; Weimerskirch, H.; Wang, S.H.; Bromwich, D.H.; Cherel, Y.; Kato, A.; Descamps, S. Flexible flight response to challenging wind conditions in a commuting Antarctic seabird: Do you catch the drift? Anim. Behav. 2016, 113, 99–112. [Google Scholar] [CrossRef]
- Ventura, F.; Granadeiro, J.P.; Padget, O.; Catry, P. Gadfly petrels use knowledge of the windscape, not memorized foraging patches, to optimize foraging trips on ocean-wide scales. Proc. R. Soc. B 2020, 287, 20191775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quillfeldt, P.; Strange, I.J.; Segelbacher, G.; Masello, J.F. Male and female contributions to provisioning rates of thin-billed prions, Pachyptila belcheri, in the South Atlantic. J. Ornithol. 2007, 148, 367–372. [Google Scholar] [CrossRef]
- Pinet, P.; Jaquemet, S.; Phillips, R.A.; Le Corre, M. Sex-specific foraging strategies throughout the breeding season in a tropical, sexually monomorphic small petrel. Anim. Behav. 2012, 83, 979–989. [Google Scholar] [CrossRef]
- Carreiro, A.R.; Bried, J.; Deakin, Z.; Jones, K.B.; Thomas, R.J.; Symondson, W.O.; Ramos, J.A.; Medeiros, R. First insights into the diet composition of Madeiran and Monteiro’s Storm Petrels (Hydrobates castro and H. monteiroi) breeding in the Azores. Waterbirds 2022, 44, 300–307. [Google Scholar] [CrossRef]
- Kleinschmidt, B.; Burger, C.; Dorsch, M.; Nehls, G.; Heinänen, S.; Morkūnas, J.; Quillfeldt, P. The diet of red-throated divers (Gavia stellata) overwintering in the German Bight (North Sea) analysed using molecular diagnostics. Mar. Biol. 2019, 166, 77. [Google Scholar] [CrossRef]
- Cairns, D.K. The regulation of seabird colony size: A hinterland model. Am. Nat. 1989, 134, 141–146. [Google Scholar] [CrossRef]
- Wakefield, E.D.; Bodey, T.W.; Bearhop, S.; Blackburn, J.; Colhoun, K.; Davies, R.; Dwyer, R.G.; Green, J.A.; Grémillet, D.; Jackson, A.L.; et al. Space partitioning without territoriality in gannets. Science 2013, 341, 68–70. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.; Gilchrist, H.G.; Benjaminsen, S.; Bolton, M.; Bonnet-Lebrun, A.S.; Davoren, G.K.; Descamps, S.; Erikstad, K.E.; Frederiksen, M.; Gaston, A.J.; et al. Foraging range scales with colony size in high-latitude seabirds. Curr. Biol. 2022, 32, 3800–3807. [Google Scholar] [CrossRef]
- Wanless, S.; Harris, M.P. Use of mutually exclusive foraging areas by adjacent colonies of blue-eyed shags (Phalacrocorax atriceps) at South Georgia. Colonial Waterbirds 1993, 16, 176–182. [Google Scholar] [CrossRef]
- Grémillet, D.; Dell’Omo, G.; Ryan, P.G.; Peters, G.; Ropert-Coudert, Y.; Weeks, S.J. Offshore diplomacy, or how seabirds mitigate intra-specific competition: A case study based on GPS tracking of Cape gannets from neighbouring colonies. Mar. Ecol. Prog. Ser. 2004, 268, 265–279. [Google Scholar] [CrossRef]
- Ainley, D.G.; Ribic, C.A.; Ballard, G.; Heath, S.; Gaffney, I.; Karl, B.J.; Barton, K.J.; Wilson, P.R.; Webb, S. Geographic structure of Adelie penguin populations: Overlap in colony-specific foraging areas. Ecol. Monogr. 2004, 74, 159–178. [Google Scholar] [CrossRef] [Green Version]
- Masello, J.F.; Mundry, R.; Poisbleau, M.; Demongin, L.; Voigt, C.C.; Wikelski, M.; Quillfeldt, P. Diving seabirds share foraging space and time within and among species. Ecosphere 2010, 1, 1–28. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, A.; Oka, N.; Iida, T.; Katsumata, N.; Sato, K.; Trathan, P.N. Foraging areas of streaked shearwaters in relation to seasonal changes in the marine environment of the Northwestern Pacific: Inter-colony and sex-related differences. Mar. Ecol. Prog. Ser. 2011, 424, 191–204. [Google Scholar] [CrossRef]
| Island | Period | Deployment Date | N | Recovery Rate | Recovery Date |
|---|---|---|---|---|---|
| New | Incubation 2017–2018 | 1/12–16/12 | 2 | 2 (100%) * | 14/12–30/12 |
| New | Chick rearing 2018–2019 | 25/1–28/1 | 10 | 1 (10%) * | 17/2 |
| New | Incubation 2019–2020 | 4/12–17/12 | 18 | 18 (100%) | 13/12–27/12 |
| New | Chick rearing 2019–2020 | 25/1–28/1 | 9 | 9 (100%) | 4/2–12/2 |
| New | Incubation 2021–2022 | 19/12–28/12 | 30 | 26 (87%) | 27/12–7/1 |
| Bird | Incubation 2021–2022 | 1/12–13/12 | 16 | 13 (81%) | 12/12–22/12 |
| Parameter | Unit | New Island Incubation (N = 32) | New Island Chick-Rearing (N = 43) | Bird Island Incubation (N = 11) | Kruskal Wallis ANOVA (df = 2) or Fisher Test |
|---|---|---|---|---|---|
| Duration | days | 7.1 ± 2.4 a | 2.8 ± 2.4 b | 6.8 ± 2.1 a | χ2 = 34.1, p < 0.001 |
| % Long trips | 91% | 30% | 91% | Fisher test: p < 0.001 | |
| Trip length (all) | km | 1966 ± 984 a | 846 ± 854 b | 2490 ± 977 a | χ2 = 28.2, p < 0.001 |
| Trip length (long) | km | 2155 ± 830 a | 1895 ± 754 a | 2715 ± 700 b | χ2 = 5.4, p = 0.070 |
| Trip length (short) | km | 142 ± 34 | 392 ± 342 | 235 | χ2 = 1.5, p = 0.480 |
| Range (all) | km | 558 ± 308 a | 301 ± 310 b | 781 ± 305 a | χ2 = 21.2, p < 0.001 |
| Range (long) | km | 610 ± 276 a | 655 ± 317 a | 846 ± 237 b | χ2 = 5.4, p = 0.140 |
| Range (short) | km | 55 ± 7 | 147 ± 126 | 135 | χ2 = 1.4, p = 0.500 |
| Travel speed (all) | km/day | 266 ± 104 a | 279 ± 149 a | 349 ± 69 b | χ2 = 4.3, p = 0.110 |
| Travel speed (long) | km/day | 278 ± 101 a | 321 ± 121 b | 363 ± 54 b | χ2 = 5.4, p = 0.070 |
| Travel speed (short) | km/day | 148 ± 39 | 261 ± 156 | 201 | χ2 = 1.1, p = 0.580 |
| Area | Habitat | Direction from Colony | New Island Incubation (N = 32) | New Island Chick-Rearing (N = 43) | Bird Island Incubation (N = 11) |
|---|---|---|---|---|---|
| 1 | Shelf break | North | 3 (9%) | - | - |
| 2 | Patagonian Shelf | North-west | 2 (6%) | 2 (5%) | - |
| 3 | PFZ | South | 2 (6%) | 1 (2%) | - |
| 4 | PFZ | South-east | 7 (22%) | 5 (12%) | 8 (73%) |
| 5 | Patagonian Shelf | South-west | 11 (34%) | 13 (30%) | 2 (18%) |
| 6 | Shelf break | South | 3 (9%) | 1 (2%) | - |
| 7 | Shelf break | North-east | - | 2 (5%) | 1 (9%) |
| 8 | Patagonian Shelf | North | 1 (3%) | - | - |
| 9 | Coastal (Queen Charlotte Bay, Jason Islands) | East, North-east | 3 (9%) | 19 (44%) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quillfeldt, P.; Bange, A.; Boutet, A.; Orben, R.A.; Baylis, A.M.M. Breeding Thin-Billed Prions Use Marine Habitats Ranging from Inshore to Distant Antarctic Waters. Animals 2022, 12, 3131. https://doi.org/10.3390/ani12223131
Quillfeldt P, Bange A, Boutet A, Orben RA, Baylis AMM. Breeding Thin-Billed Prions Use Marine Habitats Ranging from Inshore to Distant Antarctic Waters. Animals. 2022; 12(22):3131. https://doi.org/10.3390/ani12223131
Chicago/Turabian StyleQuillfeldt, Petra, Andreas Bange, Aude Boutet, Rachael A. Orben, and Alastair M. M. Baylis. 2022. "Breeding Thin-Billed Prions Use Marine Habitats Ranging from Inshore to Distant Antarctic Waters" Animals 12, no. 22: 3131. https://doi.org/10.3390/ani12223131






