Would the Cephalic Development in the Purebred Arabian Horse and Its Crosses Indicate a Paedomorphic Process?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
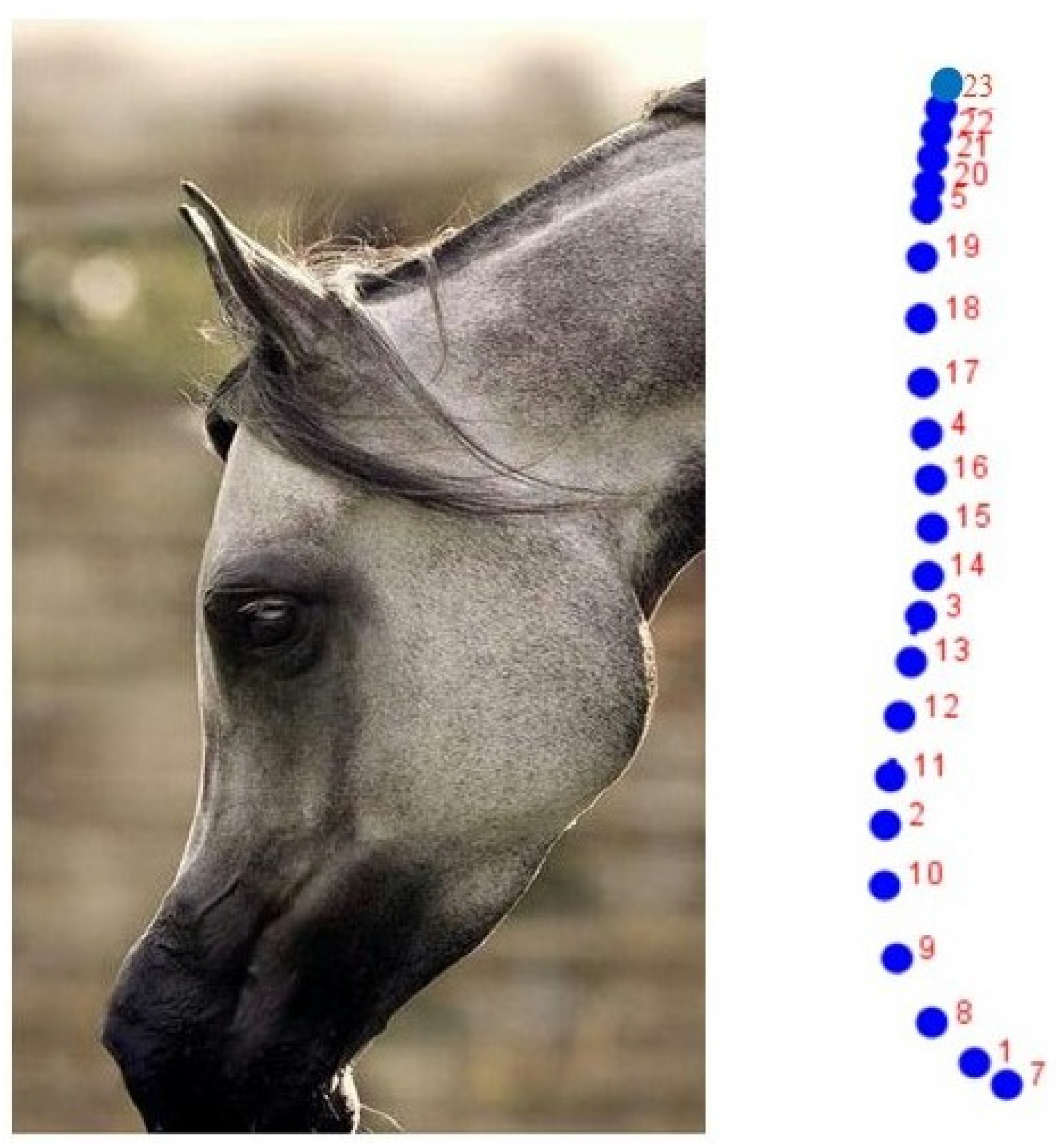
2.1. Sample
2.2. Geometric Morphometrics
3. Results
3.1. Principal Component Analysis
3.2. Non-Parametric Multivariate Analysis of Variance
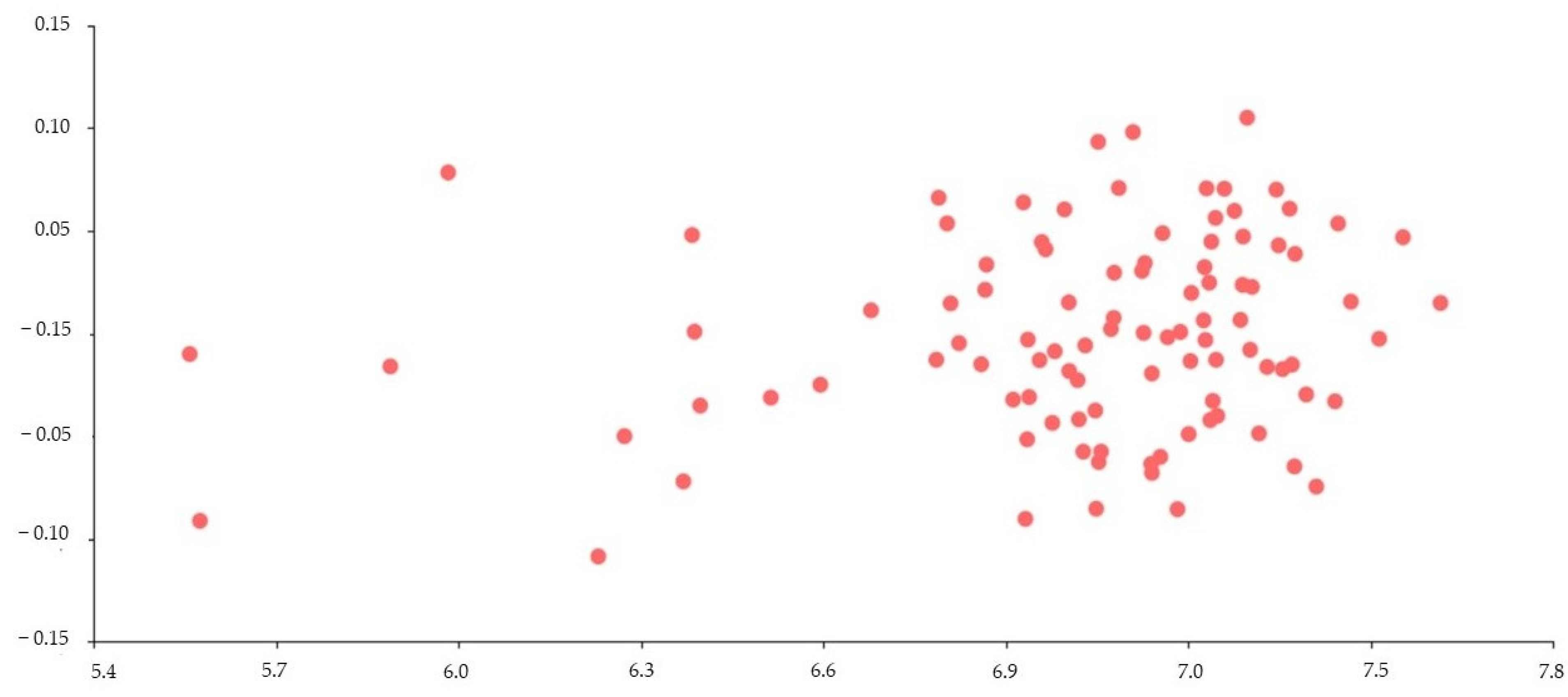
3.3. Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfrey, L.R.; Sutherland, M.R. Paradox of peramorphic paedomorphosis: Heterochrony and human evolution. Amer. J. Phys. Anthropol. 1996, 99, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Heck, L.; Sanchez-Villagra, M.R.; Stange, M. Why the long face? Comparative shape analysis of miniature, pony, and other horse skulls reveals changes in ontogenetic growth. PeerJ 2019, 7, e7678. [Google Scholar] [CrossRef] [PubMed]
- Galán, C. Evolución de la fauna cavernícola: Mecanismos y procesos que explican el origen de las especies troglobias. Bol. SVE 2010, 44, 1–31. [Google Scholar]
- Price, E.O. Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sc. 1999, 65, 245–271. [Google Scholar] [CrossRef]
- Goodwin, D.; Levine, M.; McGreevy, P.D. Preliminary investigation of morphological differences between ten breeds of hors-es suggests selection for paedomorphosis. J. Appl. Anim. Welf 2008, 11, 204–212. [Google Scholar] [CrossRef]
- Skulachev, V.P.; Holtze, S.; Vyssokikh, M.Y.; Bakeeva, L.E.; Skulachev, M.V.; Markov, A.V.; Hildebrandt, T.B.; Sadovnichii, V.A. Neoteny, Prolongation of Youth: From Naked Mole Rats to “Naked Apes” (Humans). Phys. Rev. 2017, 97, 699–720. [Google Scholar] [CrossRef]
- Chemisquy, M.A. Peramorphic males and extreme sexual dimorphism in Monodelphis dimidiata (Didelphidae). Zoomorphology 2015, 134, 587–599. [Google Scholar] [CrossRef]
- Denoël, M.; Joly, P. Neoteny and progenesis as two heterochronic processes involved in paedomorphosis in Triturus alpestris (Amphibia: Caudata). Proc. R. Soc. B Biol. Sci. 2000, 265, 1481–1485. [Google Scholar] [CrossRef]
- Ivanovic, A.; Cvijanovic, M.; Denoël, M.; Slijepcevic, M.; Kalezic, M.L. Facultative paedomorphosis and the pattern of intra- and interspecific variation in cranial skeleton: Lessons from European newts (Ichthyosaura alpestris and Lissotriton vulgaris). Zoomorphology 2014, 133, 99–109. [Google Scholar] [CrossRef]
- Mathiron, A.G.E.; Lena, J.-P.; Baouch, S.; Denoel, M. The ‘male escape hypothesis’: Sex-biased metamorphosis in response to climatic drivers in a facultatively paedomorphic amphibian. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170176. [Google Scholar] [CrossRef]
- Waller, B.M.; Peirce, K.; Caeiro, C.C.; Scheider, L.; Burrows, A.M.; McCune, S.; Kaminski, J. Paedomorphic facial expressions give dogs a selective advantage. PLoS ONE 2013, 8, 82686. [Google Scholar] [CrossRef] [PubMed]
- Parés-Casanova, P.M.; Sofiane, K.; Medina, A. Diferente desarrollo cefálico según tipo de conejo. Rev. Cienc. Vet. 2018, 36, 15–21. [Google Scholar] [CrossRef]
- Liuti, T.; Dixon, P.M. The use of the geometric morphometric method to illustrate shape difference in the skulls of differ-ent-aged horses. Vet. Res. Commun. 2020, 44, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Haussman, S. Cranial Suture Closure in Domestic Dog Breeds and Its Relationships to Skull Morphology. Anat. Rec. 2016, 299, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.A.; Makvandi-Nejad, S.; Chu, E.; Allen, J.J.; Streeter, C.; Gu, E.; McCleery, B.; Murphy, B.A.; Bellone, R.; Sutter, N.B. Morphological variation in the horse: Defining complex traits of body size and shape. Anim. Genet. 2010, 41, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.E.; McGreevy, P.D. Conformation of the Equine Skull: A Morphometric Study. Anat. Hystol. Embryol. 2006, 35, 221–227. [Google Scholar] [CrossRef]
- Parés–Casanova, P.M. El método de Evans & Mcgreevy como herramienta para el estudio aloídico in vivo y post mortem. Una aplicación en caballos. RedVet 2007, 8, 1–7. [Google Scholar]
- Maśko, M.; Wierzbicka, M.; Zdrojkowski, Ł.; Jasinski, T.; Sikorska, U.; Pawlinski, B.; Domino, M. Comparison of Donkey, Pony, and Horse Dorsal Profiles and Head Shapes Using Geometric Morphometrics. Animals 2022, 12, 931. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Toro Ibacache, M.V.; Manriquez, S.G.; Suazo, G.I. Morfometría geométrica y el estudio de las formas biológicas: De la mor-fología descriptiva a la morfología cuantitativa geométrica. Int. J. Morphol. 2010, 28, 977–990. [Google Scholar] [CrossRef]
- Salamanca-Carreño, A.; Parés-Casanova, P.M.; Cantón, C.; Monroy-Ochoa, N.I. Evaluación aloídica de la cabeza. Un ejemplo en el caballo árabe. Actas Iberoam. Conserv. Anim. 2022, 17, 29–33. [Google Scholar]
- Parés-Casanova, P.M.; Cantón, C. Sexual head profile differences in celoid horses assessed by geometric morphometrics. Int. J. Clin. Exp. Med. 2022, 1, 1–3. [Google Scholar]
- Rohlf, F.J. The Tps Series of Software. Hystrix 2015, 26, 9–12. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An Integrated Software Package for Geometric Morphometrics. Mol. Ecol. Res. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST v. 2.17c. Palaeontol. Electron 2001, 4, 1–229. [Google Scholar]
- Borgi, M.; Cirulli, F. Children’s Preferences for Infantile Features in Dogs and Cats. Hum.-Anim. Interact. Bull. 2013, 1, 1–15. [Google Scholar] [CrossRef]
- Sierra Alfranca, I. El concepto de raza: Evolución y realidad. Arch. Zootec. 2001, 50, 547–564. [Google Scholar]
- Künzel, W.; Breit, S.; Oppel, M. Morphometric investigations of breed-specific features in feline skulls and considerations on their functional implications. Anat. Histol. Embryol. 2003, 32, 218–223. [Google Scholar] [CrossRef]
- Sañudo, C. Valoración Morfológica de Los Animales Domésticos; Madrid: Ministerio de Medio Ambiente y Medio Rural y Ma-rino. Fecha De Consult. 2009, 11, 235–266. [Google Scholar]
- Sambraus, H.H. A Colour Atlas of Livestock Breeds; Wolfe Publishing Ltd.: London, UK, 1992. [Google Scholar]

| PC1 (66.60%) | PC2 (14.05%) | |
|---|---|---|
| x1 | −0.2096 | −0.0583 |
| y1 | 0.0995 | −0.3304 |
| x2 | 0.1513 | 0.0858 |
| y2 | 0.0795 | 0.2615 |
| x3 | 0.0527 | 0.0958 |
| y3 | −0.2276 | 0.1520 |
| x4 | −0.0181 | −0.0060 |
| y4 | −0.1562 | 0.1029 |
| x5 | −0.0633 | −0.0620 |
| y5 | −0.1545 | −0.3061 |
| x6 | 0.0232 | −0.0250 |
| y6 | 0.3807 | 0.0658 |
| x7 | −0.1659 | −0.0309 |
| y7 | 0.0063 | −0.3763 |
| x8 | −0.1324 | −0.1124 |
| y8 | 0.1701 | −0.2313 |
| x9 | 0.0381 | −0.1059 |
| y9 | 0.1970 | −0.0598 |
| x10 | 0.1435 | −0.0015 |
| y10 | 0.1430 | 0.1217 |
| x11 | 0.1431 | 0.1201 |
| y11 | 0.0215 | 0.2850 |
| x12 | 0.1141 | 0.1219 |
| y12 | −0.0736 | 0.2288 |
| x13 | 0.0810 | 0.1320 |
| y13 | −0.1572 | 0.1948 |
| x14 | 0.0432 | 0.0699 |
| y14 | −0.2104 | 0.1614 |
| x15 | 0.0259 | 0.0261 |
| y15 | −0.1944 | 0.1480 |
| x16 | 0.0038 | 0.0020 |
| y16 | −0.1746 | 0.1439 |
| x17 | −0.0339 | −0.0006 |
| y17 | −0.1734 | 0.0376 |
| x18 | −0.0510 | −0.0162 |
| y18 | −0.1584 | −0.0862 |
| x19 | −0.0656 | −0.0364 |
| y19 | −0.1499 | −0.2155 |
| x20 | −0.0564 | −0.0628 |
| y20 | −0.0351 | −0.2187 |
| x21 | −0.0384 | −0.0608 |
| y21 | 0.1182 | −0.1223 |
| x22 | −0.0092 | −0.0505 |
| y22 | 0.2684 | −0.0241 |
| x23 | 0.0239 | −0.0244 |
| y23 | 0.3809 | 0.0672 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F | p |
|---|---|---|---|---|---|
| Sex | 3.60 × 1024 | 2 | 1.80 × 1024 | 0.012022 | 0.7078 |
| Age | 7.31 × 1025 | 31 | 2.36 × 1024 | 0.015751 | 0.5212 |
| Interaction | −3.14 × 1026 | 62 | −5.06 × 1024 | −0.033799 | 0.8221 |
| Residual | 4.49 × 1026 | 3 | 1.50 × 1026 | ||
| Total | 2.12 × 1026 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca-Carreño, A.; Parés-Casanova, P.M.; Monroy-Ochoa, N.I.; Vélez-Terranova, M. Would the Cephalic Development in the Purebred Arabian Horse and Its Crosses Indicate a Paedomorphic Process? Animals 2022, 12, 3168. https://doi.org/10.3390/ani12223168
Salamanca-Carreño A, Parés-Casanova PM, Monroy-Ochoa NI, Vélez-Terranova M. Would the Cephalic Development in the Purebred Arabian Horse and Its Crosses Indicate a Paedomorphic Process? Animals. 2022; 12(22):3168. https://doi.org/10.3390/ani12223168
Chicago/Turabian StyleSalamanca-Carreño, Arcesio, Pere M. Parés-Casanova, Néstor Ismael Monroy-Ochoa, and Mauricio Vélez-Terranova. 2022. "Would the Cephalic Development in the Purebred Arabian Horse and Its Crosses Indicate a Paedomorphic Process?" Animals 12, no. 22: 3168. https://doi.org/10.3390/ani12223168
APA StyleSalamanca-Carreño, A., Parés-Casanova, P. M., Monroy-Ochoa, N. I., & Vélez-Terranova, M. (2022). Would the Cephalic Development in the Purebred Arabian Horse and Its Crosses Indicate a Paedomorphic Process? Animals, 12(22), 3168. https://doi.org/10.3390/ani12223168







