Simple Summary
Skates and rays generally have low fecundity, delayed maturation age and slow growth rates. These life history traits make them very vulnerable to commercial fisheries’ activities, although they are not usually target species. Understanding age and growth is important for stock assessment of the long-nose skate. We believe that determining these can be the first step toward correct management actions. Little is known about the growth characteristics of the long-nose skate in the Northeastern Mediterranean Sea. Our study aimed at comparing three different growth models (the von Bertalanffy, the Robertson (logistic), and Gompertz) and also the absolute and relative growth characteristics of this species. Our results show that the relationship between the age of the long-nose skate and its total length is adequately explained by the Robertson (logistic) growth model, with the Gompertz growth model being the second best.
Abstract
This study aims to determine the age and growth characteristics of Dipturus oxyrinchus living in the Northeastern Mediterranean Sea and to present data that can provide a comparison with previous studies on the same subject. A total of 255 long-nose skates at a total length of 12.2–93.5 cm and weight of 8.34–3828 g were collected as non-target species from a commercial fishing boat. The male−female ratio was determined as 1:1.27. Using the von Bertalanffy equation and the Gompertz or logistic growth models, the growth parameters of Dipturus oxyrinchus were estimated as L∞ = 154.0, K = 0.064, t0 = −1.622; L∞ = 104.0, K = 0.35, I = 4.99; L∞ = 128.40, K = 0.19, I = 4.39 for all individuals, respectively. Maximum absolute growth was calculated as 9.33 cm at 5–6 years of age. Maximum relative growth at 1–2 years of age was estimated as 36.39%. Both absolute and relative growth were minimal in the 11–12 age group. The highest condition factor value was estimated as 0.416 in the 8-year-old group. As a result, the growth data of long-nose skates were obtained for the first time in the Northeastern Mediterranean Sea.
1. Introduction
The long-nosed skate, Dipturus oxyrinchus (L. 1758), is a demersal species that lives at depths of 70−1230 m on sandy or muddy substrates and, rarely, on rocky and pebbly grounds; it is usually found at depths of 200−500 m [1,2,3,4,5,6]. The long-nosed skate ranges from Norway to Senegal, from the Northeast Atlantic to the Faroe Islands, Skagerrak (the strait connecting the Baltic Sea to the North Sea), the Canary Islands, the Madeira Islands and the Mediterranean Sea [3] and has little commercial value [4]. Sizes between 60 and 100 cm are common, but the largest recorded individual was 150 cm [1]. Dipturus oxyrinchus is globally recognized as a Near Threatened (NT) species by the International Union for Conservation of Nature (IUCN) [7]. Long-nosed skates have been studied satisfactorily by researchers during recent years in other parts of the Mediterranean Sea in terms of age, growth, distribution, systematics, length–weight relationships (LWR) and feeding habits. However, no data on growth parameters are available for this species in the Northeastern Mediterranean Sea [8,9,10,11,12]. The presence of juveniles and mature males of the long-nosed skate was previously reported by Başusta and Başusta [13] from the same region. Griffiths et al. [14] concluded that Mediterranean long-nosed skates may have been genetically isolated from other stocks (e.g., Atlantic). This study aimed to determine the growth characteristics of long-nosed skates living in the Northeastern Mediterranean Sea and compare them with the data reported in other studies conducted in the same region.
2. Materials and Methods
2.1. Collecting of Samples
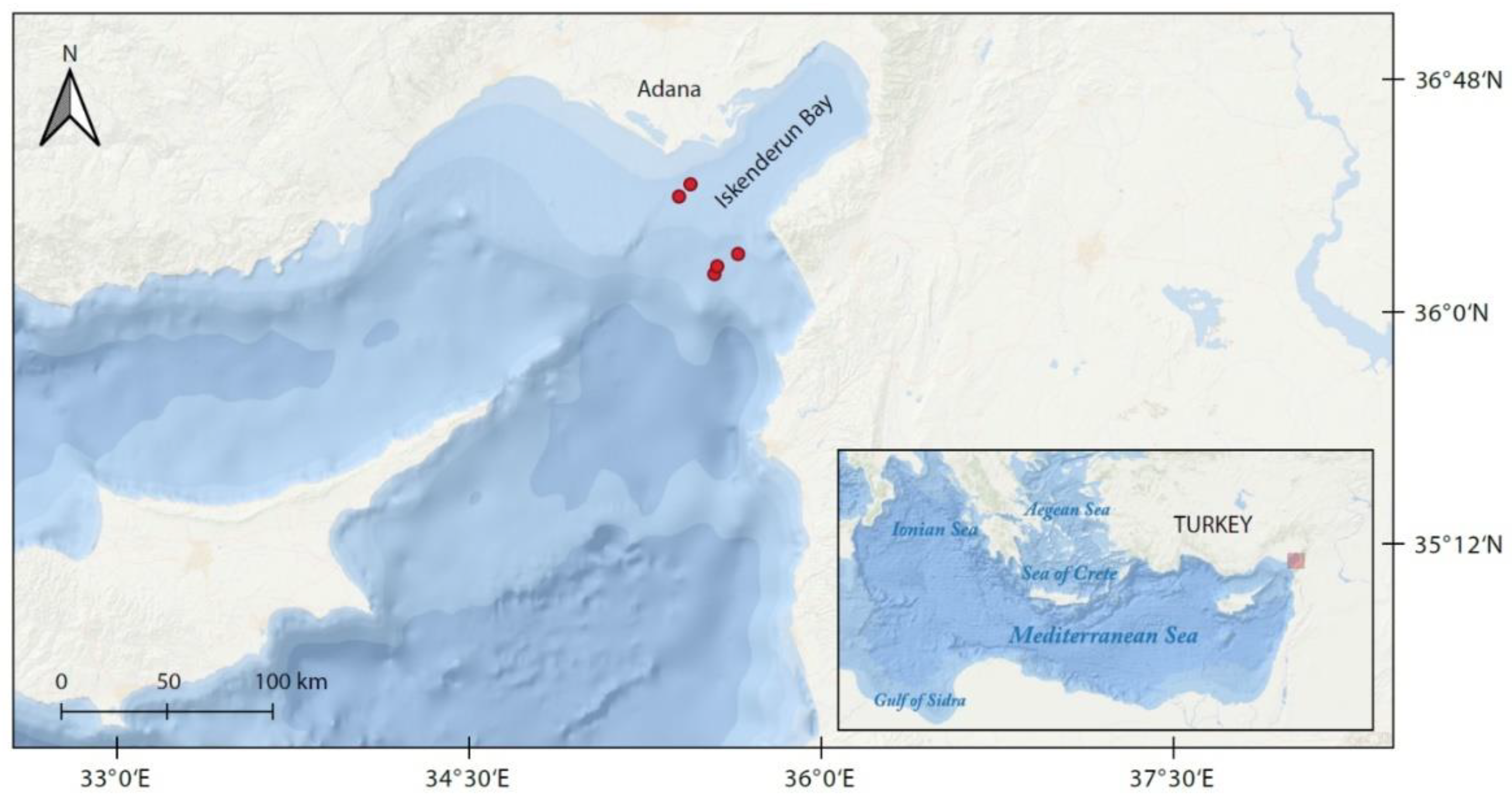
The long-nosed skate individuals were caught by a commercial trawler (F/V NIHAT BABA/31-A-1463) in Iskenderun Bay (Figure 1; 36°29′200′′ N; 35°05′973′′ E–36°07′052′′ N; 35°17′936′′ E–36°07′148′′ N; 35°17′978′′ E–36°13′720′′ N; 35°22′998′′ E–36°13′650′′ N; 35°23′032′′ N–36°16′622′′ E; 35°18′509′′ N) between May 2015 and June 2016. The samples were collected monthly. The bottom trawl gear used was equipped with a 42 mm stretched-mesh size net at the cod-end. Each hauling lasted three hours, with a trawling speed of 2.2–2.9 knots. Approximate sampling depths ranged between 100–150 m, 150–200 m and 200–400 m. After fishing, all samples were transported with ice to the laboratory of the Fisheries Faculty, Firat University. Total length (L) was measured as a straight line from the tip of the rostrum to the end of the tail to the nearest mm and body mass (W) was weighed with a 1 g accuracy for each individual.
Figure 1.
Red dots indicate out of Iskenderun Bay, Turkey, where Dipturus oxyrinchus specimens were collected.
2.2. Processing of Vertebral Centra
A section of 10–12 vertebral centra was removed from the widest portion of the body of 255 long-nosed skate specimens (143 females and 112 males) and subsequently labeled, frozen and stored until further processing. Vertebrae were later thawed and cleaned of excess tissue, rinsed in tap water and then stored in a 70% ethanol solution. Three random vertebrae from each sample were removed from the ethanol and air-dried [15,16]. Smaller centra of less than 5 mm were fixed to a clear glass slide using resin (Crystol bond 509™) and were sanded with a Dremel™ tool to replicate a sagittal cut [17,18,19]. Vertebral sections (0.6 mm thick) were taken using a Ray Tech™ (Littleton, CO, USA) gam saw for large centra >5 mm in diameter [20]. Vertebral cross-sections were mounted on microscope slides using clear resin (Cytoseal 60; Fisher Scientific, Pittsburgh, PA, USA) [21].
2.3. Age Assessment and Verification
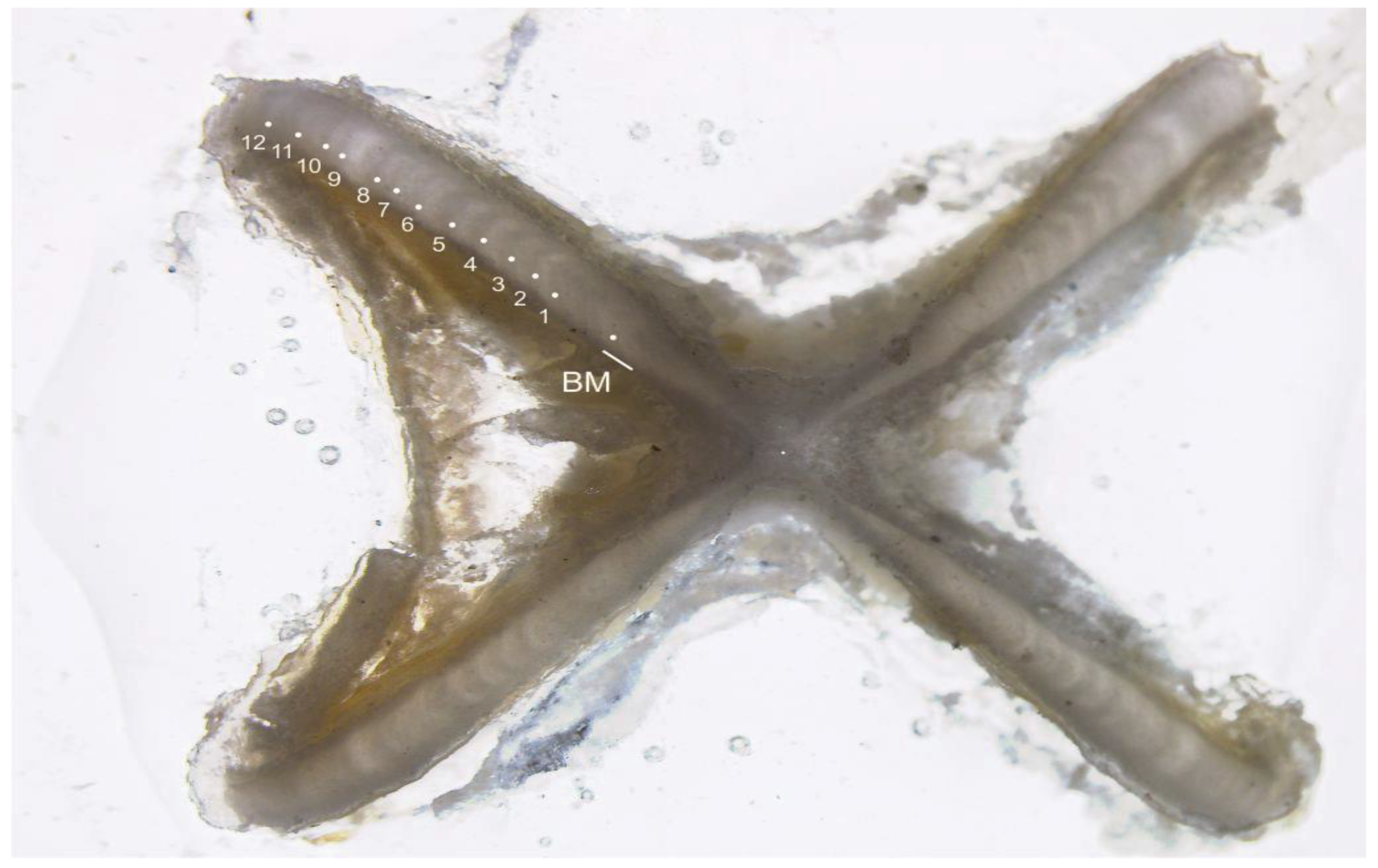
Vertebral cross-sections were examined under a Leica S8 APO™ (Singapore) microscope using LAS software (Version 4.8.0, Leica Microsystems Limited, Heerbrugg, Switzerland). One growth band was defined as an opaque and translucent band pair that traversed the intermedialia and clearly extended into the corpus calcareum (Figure 2) [22,23,24].
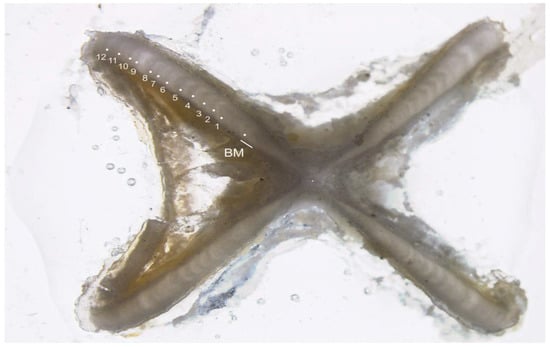
Figure 2.
A vertebral cross-section of an estimated 12-year-old Dipturus oxyrinchus (total length = 93.5 cm, female) (BM, Birth Mark). White dots indicate opaque bands.
Each vertebral cross section was examined by two readers (reader 1 = NB and reader 2 = FVO). Reader 1 made two nonconsecutive band-counts of sampled vertebral cross-sections without prior information of the long-nosed skate’s length or former counts. Reader 2 made two consecutive counts from 50 randomly selected vertebrae sections. Vertebral cross-sections that had an instability of more than two years between each reading were eliminated from further analyses. Count reproducibility was compared by the percent agreement (%PA) and coefficient of variation (%CV) [25], as well as the index of average percent error between the readers (%IAPE) [26]. All were determined using the following mathematical equations:
where R is the number of readings; Xij is the count from the jth fish at the ith reading and Xj is the mean age calculated for the jth fish from i readings.
Pair-wise age-reader comparisons were independently generated by the two readers by making nonconsecutive band counts from a random sample of 50 vertebral sections [27]. All statistical tests were performed with R software version 4.1 [28], with a significance level set at 5%.
2.4. Total Length–Weight Relationship and Growth Modeling
The total length–weight relationship parameters of long-nosed skate were estimated according to the equation given below [29]:
where L = total length (cm); W = body mass (g); a is a constant of proportionality and b is the allometric factor. The deviance of the estimated b values for long-nosed skate from the hypothetical value of 3 (i.e., isometric growth) was tested by a t-test at the 0.01 significance level [29,30].
The absolute length growth (ALG) and the relative length growth (RLG) of long-nosed skate were calculated as:
where Lt is total length at the start of the time interval and Lt+∆t is total length at the end of the time interval (∆t) [30,31].
The values of condition factor were obtained with the formula:
where W, L and b are as defined above [32].
The observed length-at-age data of the long-nosed skate were used as the dependent variable and the age as the independent variable, with the three most-used models to describe the growth of fish. The first model employed was the von Bertalanffy [33] growth equation (VBGM). The VBGM was formulated by Beverton and Holt [34] as:
where Lt is the expected total length at age t years; L∞ is the asymptotic total length; K is the growth coefficient or curvature parameter indicating the rate at which long-nosed skates grow toward their L∞ and t0 is the theoretical age at zero total length.
The second growth model used was that of Gompertz [35], an S-shaped growth model (GGM) [36,37,38]:
where t, Lt and L∞ are the same as in the VBGM; ti is the age at the inflection point of the growth curve, i.e., the age at which the absolute growth rate starts to decrease and G is the instantaneous growth rate coefficient at age ti, where growth becomes asymmetrical.
The Robertson (logistic) model was also used as the last growth model. While t, ti, Lt and L∞ are the same as in the previous models, K is a parameter that affects the rate of exponential growth:
Akaike’s information criterion (AIC) was used to compare the different growth models. A smaller value of the AIC indicates that the observed data are closer to the fitted model [29,39]. The AIC is defined as −2 times the maximum value of the log likelihood () plus 2 times the number of parameters (p) in the model including the estimated variance [40]:
3. Results
3.1. Sample Composition, Sex and Vertebral Analysis
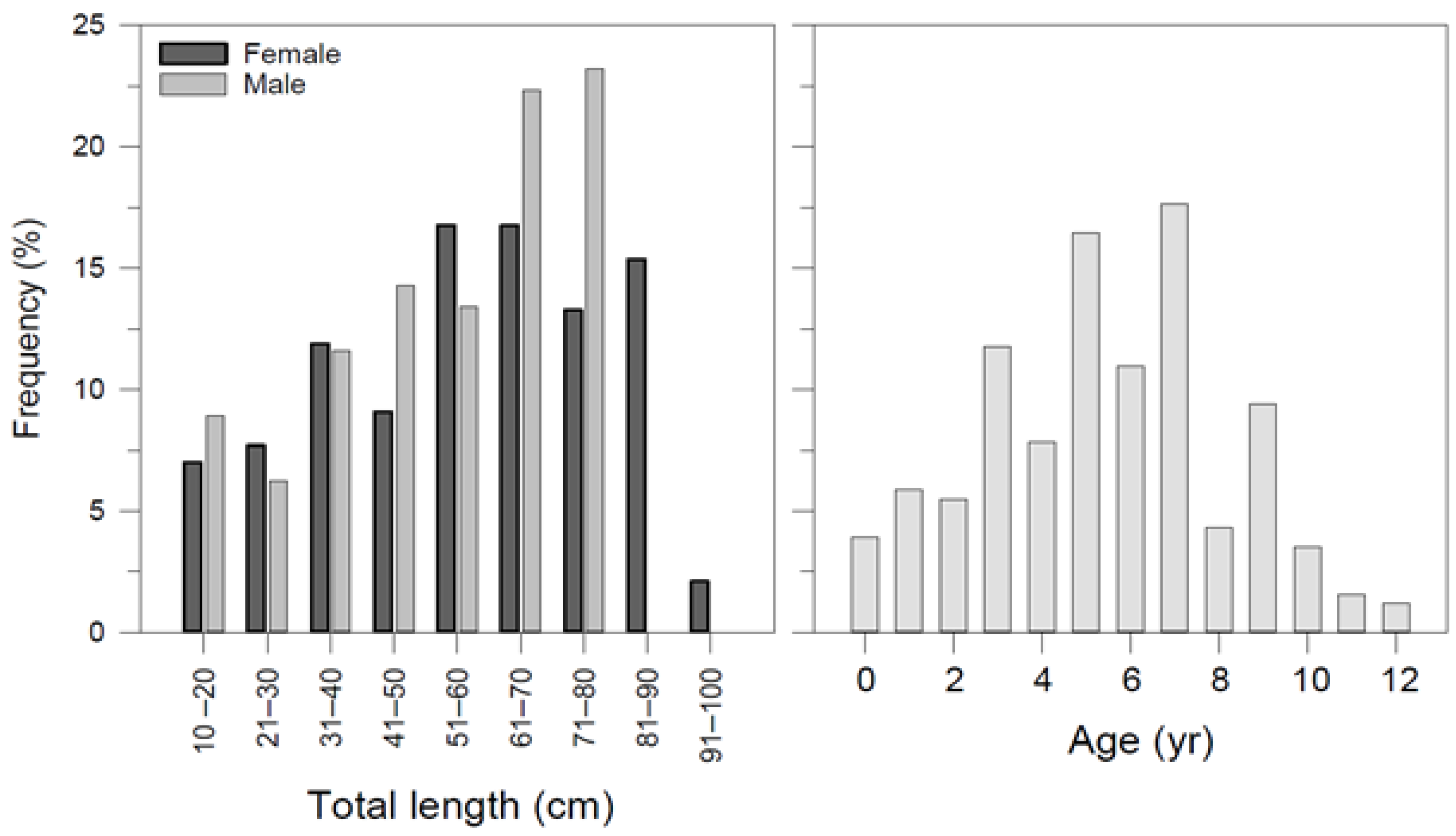
A total of 255 long-nosed skates ranging from 12.2 to 93.5 cm in total length and 8.34–3828 g in weight were collected as bycatch or discard species from a commercial fishing vessel between May 2015 and June 2016 (Table 1). The sex ratio (M/F) was determined as 1:1.27. The ratio of females to males was not statistically different from the expected 1:1 ratio between sexes (p > 0.05). The size frequency based on total length and age group is presented in Figure 3.

Table 1.
Descriptive statistics for Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea.
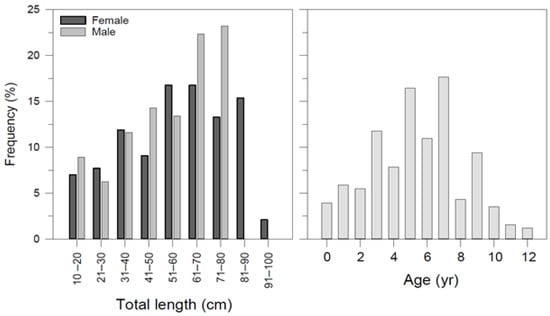
Figure 3.
Frequency distribution of Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea, according to their total length and age.
3.2. Age Estimation, Reading Precision and Age Bias
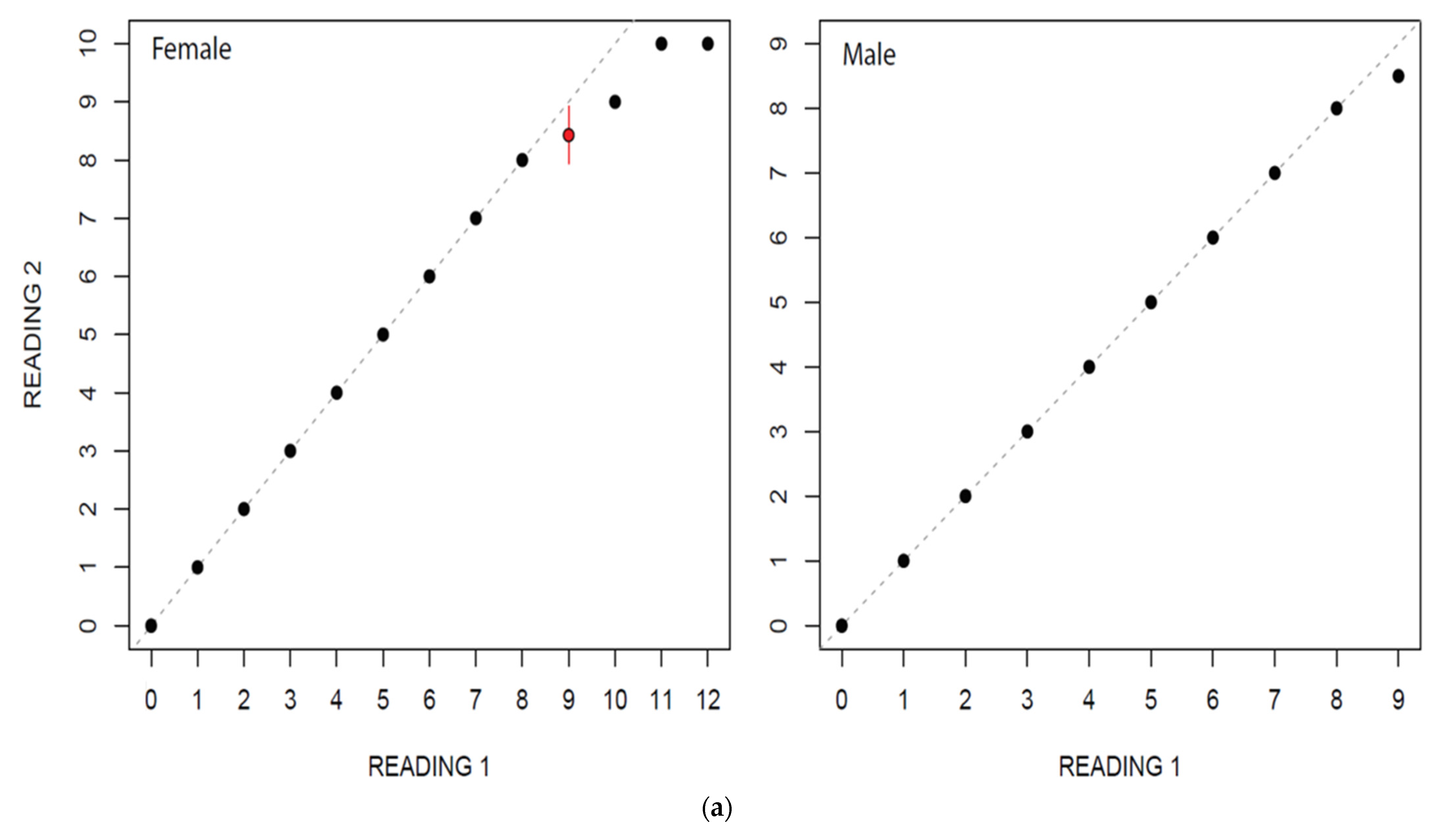
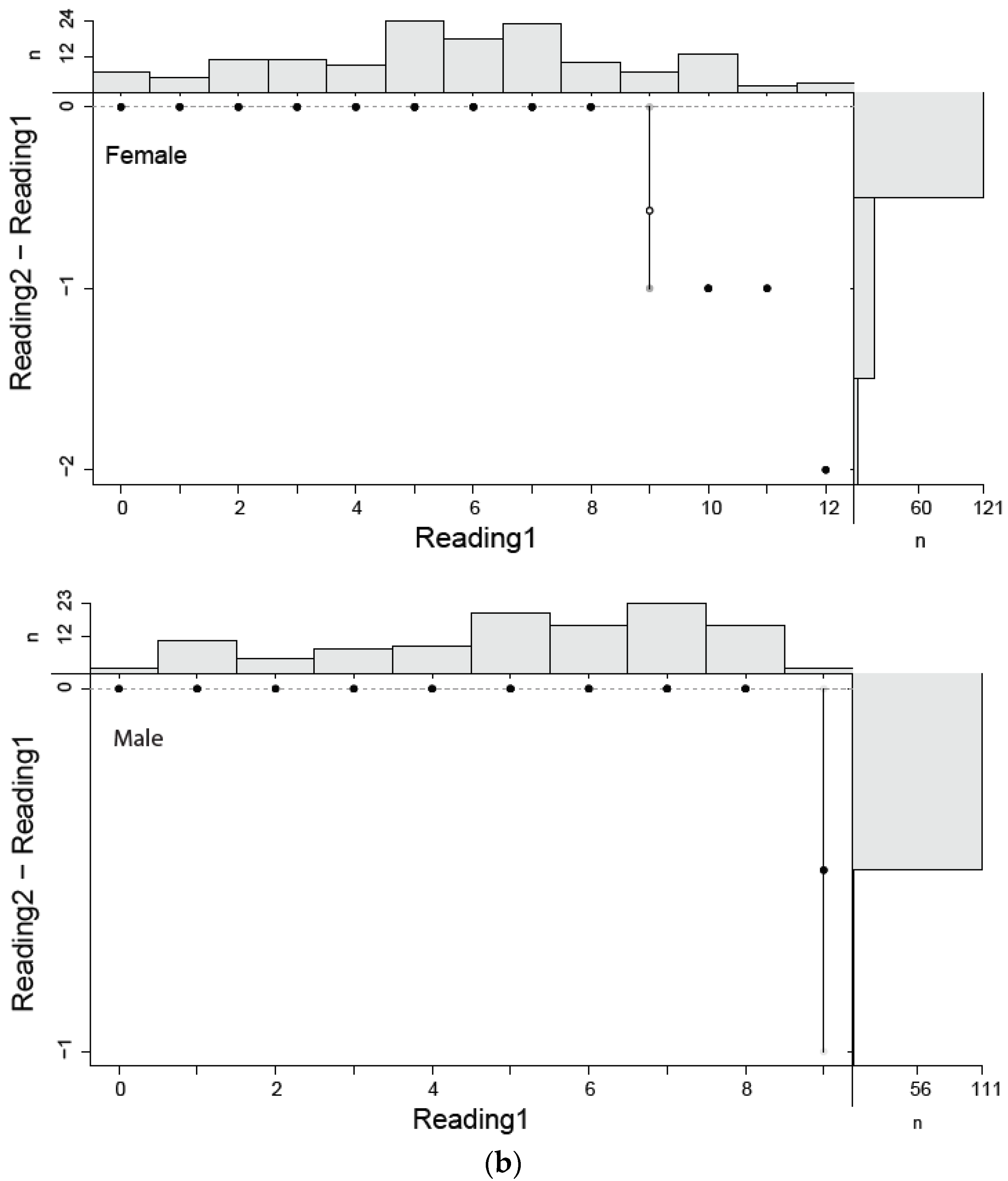
In this study, estimated ages ranged from 0 to 12 for females and from 0 to 9 for males. Age estimation for males and females by two independent readers did not show a considerable variation (Figure 4a,b). The highest PA with lowest IAPE and CV were found for males than female (Table 2).
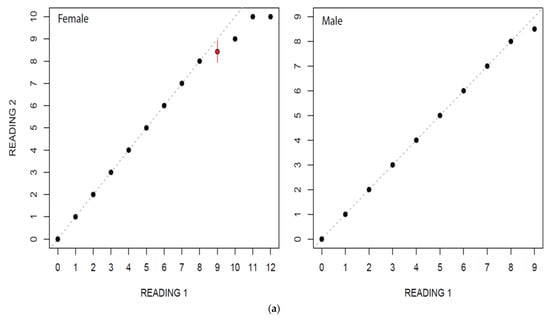
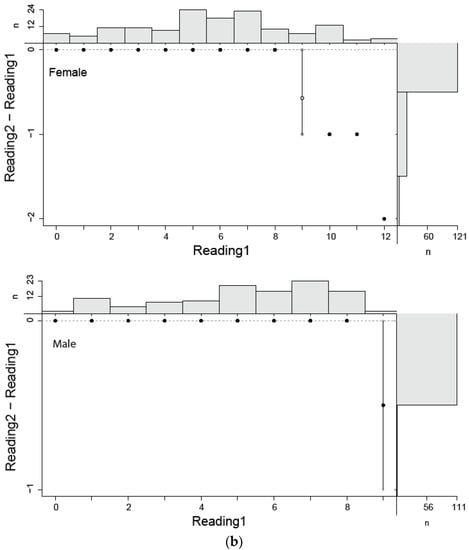
Figure 4.
(a) Age bias graphs for Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea. (b) Age bias plots for two readers for aging Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea. Plots illustrate reference reader’s age estimates on the x-axis; the mean difference (circles) and distribution of the differences between corresponding ages (vertical lines) are represented on the y-axis.

Table 2.
Summary statistics for the coefficient of variation (CV), percentage of agreement (%PA) and index of average percentage error (IAPE) to determine the precision of age readings of Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea.
3.3. Length–Weight Relationships, Growth Patterns and Condition Factor
3.3.1. Length–Weight Relationships
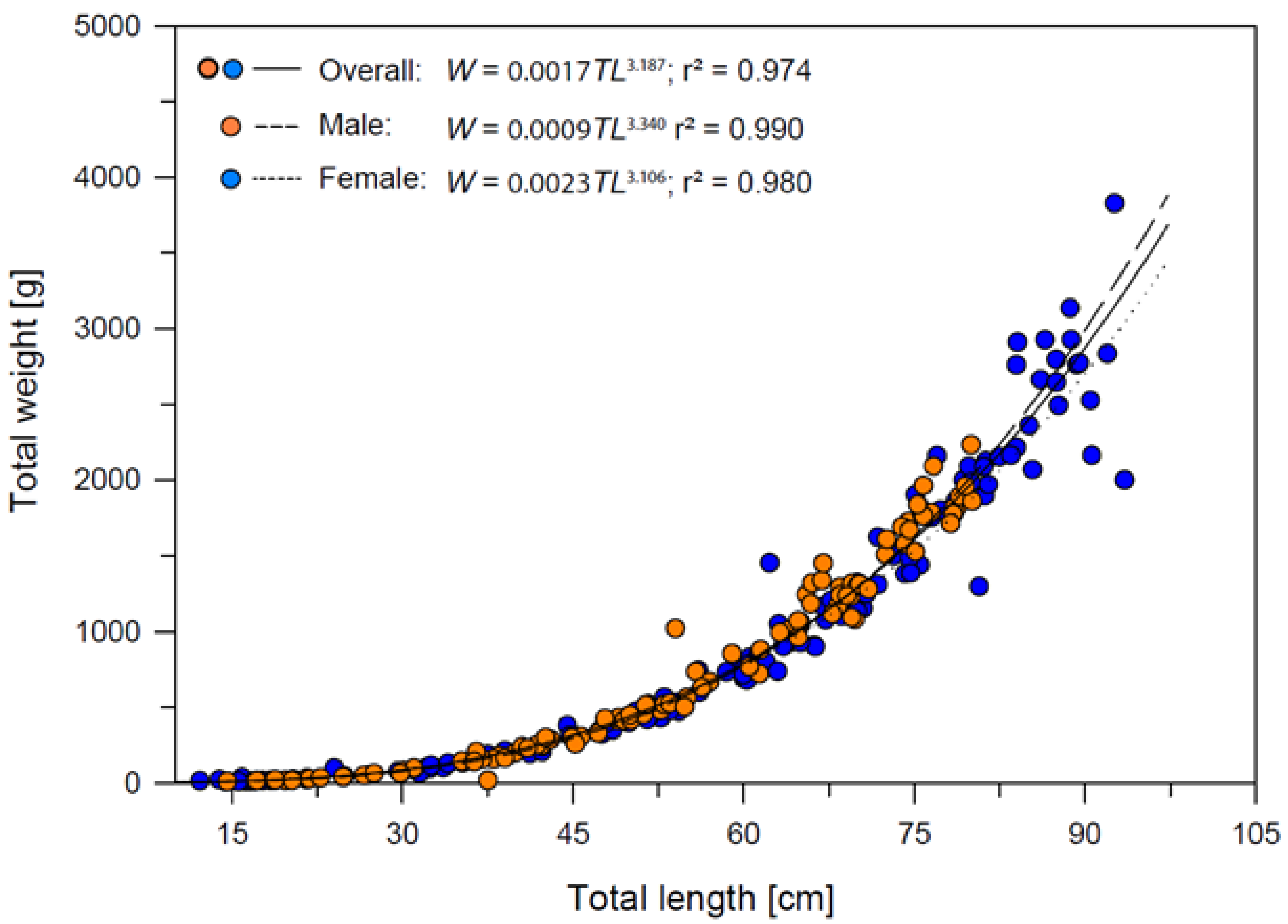
The total length–weight relationships of Dipturus oxyrinchus for female, male and overall are presented in Figure 5. According to these results, positive allometric growth (b > 3) was demonstrated for the overall category. Regression results showed that the length and weight of the long-nosed skate was predicted significantly (r = 0.987, r2 = 0.974, F1,242 = 9092.525 p < 0.001) in all sexes. It is possible to state that 97% of the increase is due to that of the size of Dipturus oxyrinchus in all individuals for the present study. In addition, when t-test results related to the significance of the regression coefficients were analyzed (t-test = 95.355 p < 0.01), the proportion of the individual’s length to weight was found to be important.
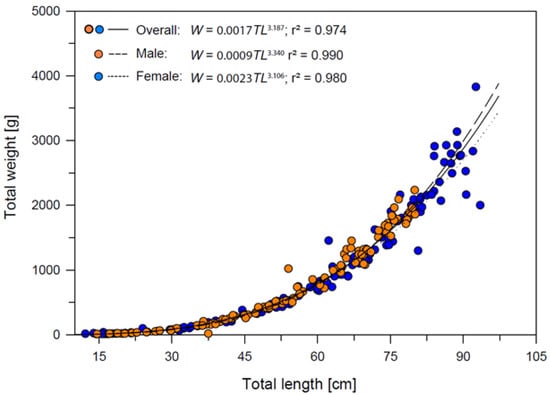
Figure 5.
Total length−weight relationships of Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea.
3.3.2. Growth Characteristics
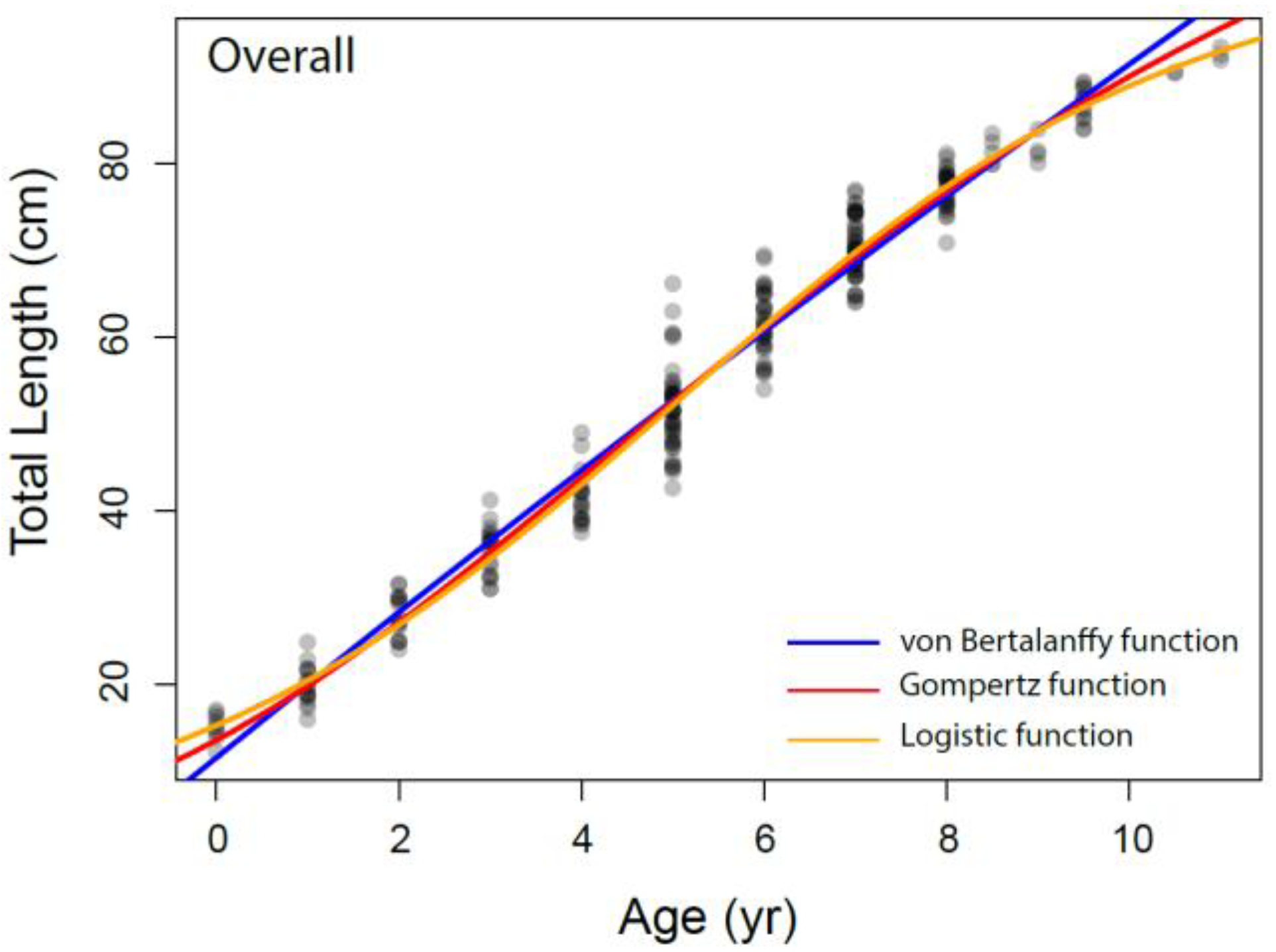
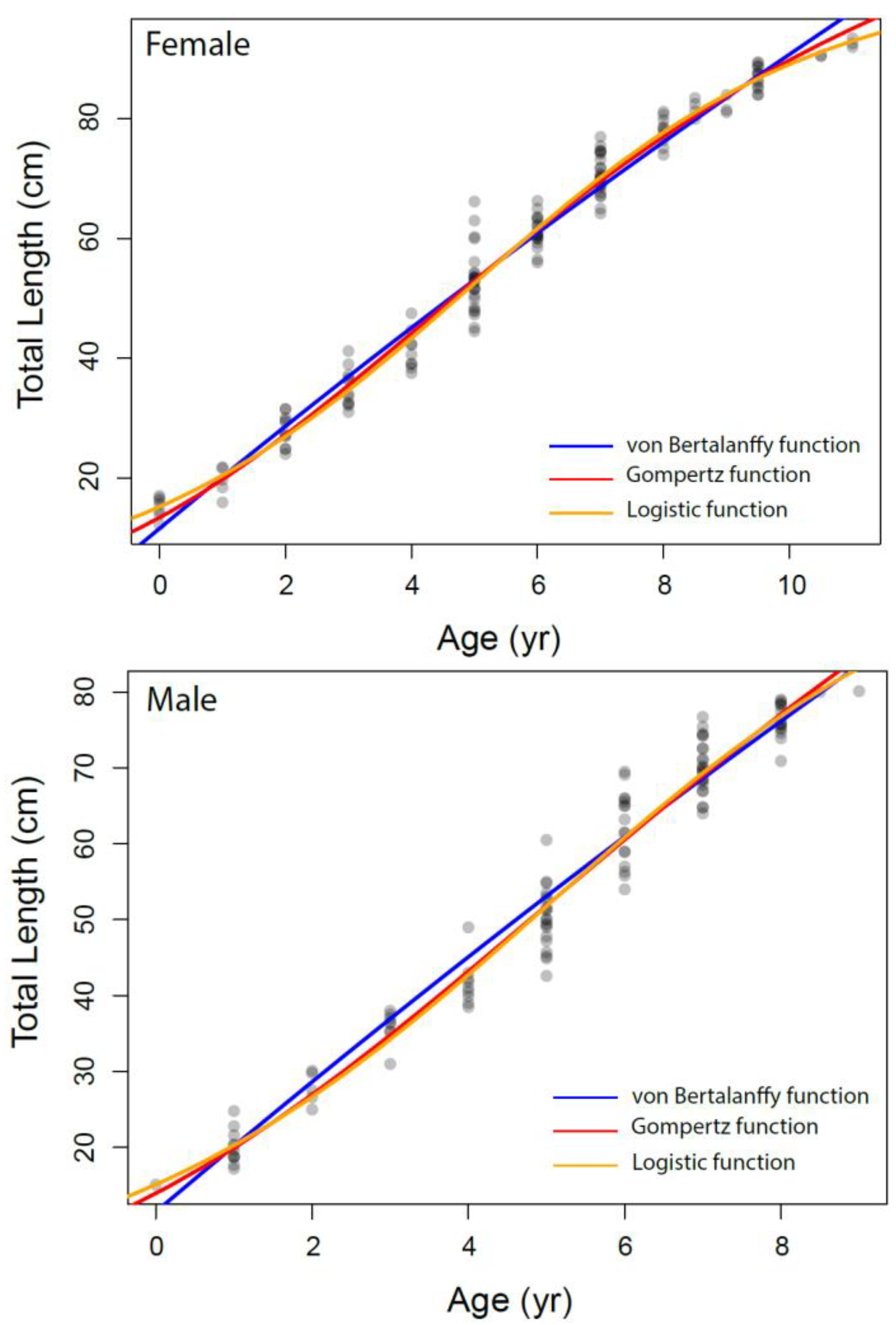
The growth parameters were estimated for overall and each sex separately from the VBGM, the Robertson (logistic) and Gompertz growth model using nonlinear regression analysis, as presented in Table 3 and Figure 6. The relationship between total length and age was adequately described by the Robertson (logistic) growth model followed by the Gompertz growth model. The von Bertalanffy model performed relatively weakly compared to other growth models based on AIC values.

Table 3.
The growth parameters for Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea derived from different growth models.
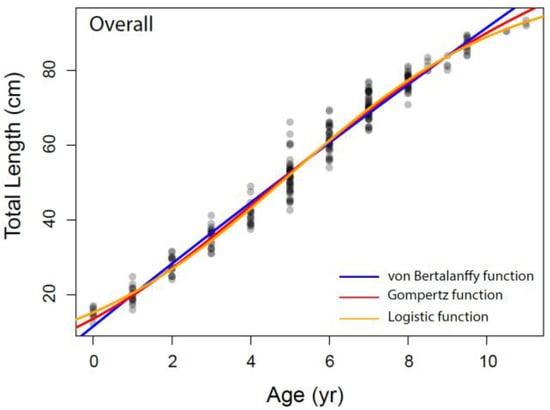
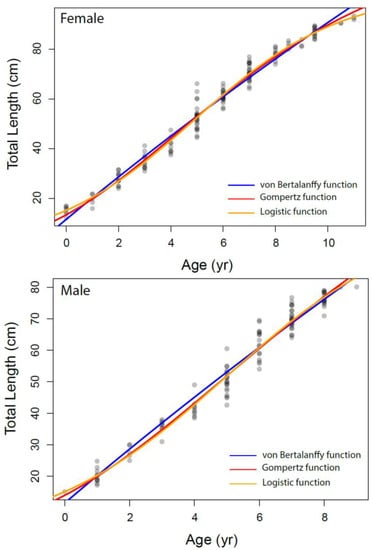
Figure 6.
The different growth models fitted to the data of Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea.
3.3.3. The Relative and Absolute Growth Rates and Condition Factor
The maximum absolute growth was estimated as 9.33 cm with 5–6 years of age. The maximum relative growth was calculated as 36.39% with 1–2 years of age. Both in absolute and relative growth were observed as minimum in the 11–12 age group (Table 4). Average condition factor value of population was estimated as 0.363. The highest and lowest condition factor values were estimated as 0.416 in age group 8 and 0.308 in age group 2, respectively (Table 5).

Table 4.
The relative and absolute growth rates of for Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea.

Table 5.
The condition factor for Dipturus oxyrinchus inhabiting the Northeastern Mediterranean Sea.
4. Discussion
The overall length ranges recorded in our study were found to be smaller than those reported by Alkusairy and Saad [11] for the same species in Syrian waters (34.1–100 cm for females and 34.5–81.6 cm for males). Yigin and Işmen [8] reported a total length of 14.9–100 cm in females in Saros Bay (the North Aegean Sea), while Bellodi et al. [12] reported these values as 15.2–86.5 cm in males and 10.4–117.5 cm in females in Sardinian waters. Finally, Kadri et al. [9] reported 16.5–105 cm in females and 15.5–95 cm in males in the Gulf of Gabès (Southern Tunisia, Central Mediterranean). These values are very close to the values we found in our study. The Robertson (logistic) growth model estimates indicated that the long-nosed skate showed sexual dimorphism with females larger than males. These observations are consistent with other studies for Dipturus oxyrinchus in the Mediterranean Sea (e.g., Yigin and Işmen [8] in the North Aegean Sea, Kadri et al. [9] in Southern Tunisia and Bellodi et al. in Sardinian waters [12]). Sexual dimorphism appears to be a common feature for the Rajidae (e.g., blonde ray Raja brachyura [41] and thornback ray Raja clavata [42]). The percentage of females and males for all samples was 56.07% and 43.93%, respectively. This was not statistically different from the expected 1:1 ratio between the genders. All genders were equally distributed confirming the pattern proposed by Yigin and Işmen [8], Kadri et al. [9] and Bellodi et al. [12]. Growth bands were highly legible and visible in cross-sections, with an easily recognizable birthmark. No staining technique was used to determine the age of Dipturus oxyrinchus, with a maximum age of 12 noted in females and 9 in males. Differences in age determination after age 9 for both sexes are related to the small number of individuals being sampled. The age estimation process ensured a high level of count repeatability among readers (IAPE = 0.53%; %CV = 0.75; %PA = 90.98) and no signs sensitive to bias were detected among readers. These precision values are acceptable [43]. The b parameter of the length–weight relationship of Dipturus oxyrinchus showed positive allometric growth for both genders in the current study. The estimated b values for Dipturus oxyrinchus by region are shown in Table 6, which are very close to our study’s findings. Other b values were reported as 3.539 for the south coasts of Portugal by Borges et al. [44] and 3.40 for North Aegean Sea by Filiz and Bilge [45]. These values differ from those of our study, which may be due to the small sample size of the fish or the fact that samples were made in different seasons. Relative and absolute growth rates and condition factor for Dipturus oxyrinchus were calculated for the first time in our study and therefore no comparison with other studies could be made. Absolute growth rates indicate actual growth between two years (ages) in terms of weight or length. Absolute growth rate decreases with age (t) and provides information about the years (ages) when growth is highest. The way the absolute growth rate is calculated depends strongly on the size the fish has reached. For comparison purposes, the relative growth rate may be more useful. This is used to determine age-related growth rate in natural populations [30].

Table 6.
Length–weight relationship values for Dipturus oxyrinchus from different regions.
Bellodi et al. [12] emphasized that the Gompertz function provided the best fit among the four growth models examined. Additionally, Liu et al. [49] stated that multiple model applications should be tested in elasmobranch age and growth studies. They also indicated that the Robertson (logistic) and Gompertz models provide the best fit for small-sized demersal skates/rays living in deep water. In our study, according to the AIC values, the logistic and Gompertz models were found to be more appropriate in describing the growth parameters of Dipturus oxyrinchus. These results agree with Liu et al. [49] and Bellodi et al. [12]. The logistic parameters determined in our study show that females attain a slightly larger asymptotic TL∞ (103.54 cm) than males (103.23 cm). In addition, the K values of the long-nosed skate were found to be similar for both genders. These growth rates appear to be similar with other skate species of similar size in the Mediterranean Sea. Bellodi et al. [12] suggested that the best growth model was the Gompertz model and estimated L∞ as 127.55 cm for all genders. This result is very close to the asymptotic value (L∞ = 128.40 cm) calculated with the Gompertz model for all individuals in our study. Yigin and Işmen [8] reported that, for both genders, the L∞ was 256.46 cm and the K was 0.04, the t0 value was −1.17 year and the maximum age was 9 years. The above authors [8] found individuals aged up to 9 years and a total length of up to 100 cm in their study. The largest individual captured in our study (93.5 cm) was smaller than that reported by the above researchers, despite being older. This shows that there may be a mistake in reading the older age rings. This leads to an overcalculation of the asymptotic length. For females, Kadri et al. [9] reported the L∞ as 123.9 cm, the K as 0.08, the t0 as −1.26 and the maximum age as 25; for males, the L∞ as 102.1 cm, the K as 0.12, the t0 as −1.18 and the maximum age as 22. These researchers found that the oldest individual was 105 cm in total length and 25 years old. Again, these values were very high compared to those in our study, which therefore indicated to us that mistakes might have been made in the reading of the age rings of the older individuals. These errors may have caused an underestimation of the L∞ value. Considering that the largest Dipturus oxyrinchus caught in nature is 150 cm in TL, the L∞ value should not be lower than this value.
5. Conclusions
Our work ensured basic growth parameters and the best fit among the three growth models for the long-nosed skate and that the Robertson (logistic) growth model was the best model to describe the species growth. Results of the research showed that the long-nosed skate has life history features similar to other Rajidae species in the Mediterranean Sea and are long-lived and slow growing. The present study has provided the first analysis of growth characteristics and data for Dipturus oxyrinchus for conservative management plans in the Northeastern Mediterranean Sea.
Author Contributions
Conceptualization, N.B.; methodology, N.B.; software, F.V.O.; validation, F.V.O.; formal analysis, N.B.; investigation, F.V.O.; resources, F.V.O.; data curation, F.V.O.; writing—original draft preparation, N.B.; writing—review and editing, N.B.; visualization, N.B.; supervision, N.B.; project administration, N.B.; funding acquisition, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Research Projects Coordination Unit of Firat University, grant number: SUF.15.04.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Experimentation Ethics Committee of Firat University, Turkey (protocol code 54/109 and 27.05.2015).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data associated with this research are available and can be obtained by contacting the corresponding author.
Acknowledgments
Specimen collection was permitted through the General Directorate of Fisheries and Aquaculture of the Turkish Ministry of Agriculture and Forestry (permit no: 26.05.2015–2065-42337). A part of this work was presented as Abstract at 11–13 May 2017, at the Ecology International Symposium in Kayseri, Turkey.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Serena, F. Field Identification Guide to the Sharks and Rays of the Mediterranean and Black Sea; Food and Agriculture Organization of The United Nation: Rome, Italy, 2005; p. 97. ISSN 1020-6868. [Google Scholar]
- Ebert, D.A.; Stehmann, M.F.W. Sharks, Batoids, and Chimaeras of the North Atlantic. FAO Species Cat. Fish. Purp. 2013, 7, 1–523. [Google Scholar]
- Stehmann, M.F.W.; Bürkel, D.L. Rajidae. In Fishes of the North-Western Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1984; Volume I, pp. 163–196. [Google Scholar]
- Golani, D.; Öztürk, B.; Başusta, N. Fishes of the Eastern Mediterranean, 1st ed.; Turkish Marine Research Foundation: Istanbul, Turkey, 2006; p. 259. [Google Scholar]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef] [PubMed]
- Last, P.R.; Séret, B.; Stehmann, M.F.W.; Weigmann, S. Skates, Family Rajidae. In Rays of the World; Last, P.R., White, W.T., de Carvalho, M.R., Séret, B., Stehmann, M.F.W., Naylor, G.J.P., Eds.; CSIRO Publishing: Melbourne, Australia, 2016; pp. 204–363. [Google Scholar]
- Ellis, J.; Abella, A.; Serena, F.; Stehmann, M.F.W.; Walls, R. Dipturus oxyrinchus. In The IUCN Red List of Threatened Species; IUCN Global Species Programme Red List Unit: UK, 2015; p. e.T63100A48908629. Available online: https://www.iucnredlist.org/species/63100/48908629 (accessed on 7 November 2022).
- Yigin, C.; Işmen, A. Age, growth, reproduction and feed of long-nosed skate, Dipturus oxyrinchus (Linnaeus 1758) in Saros Bay, the north Aegean Sea. J. Appl. Ichthyol. 2010, 26, 913–919. [Google Scholar] [CrossRef]
- Kadri, H.; Marouani, S.; Bradai, M.N.; Bouaïn, A.; Morize, E. Age, growth, longevity, mortality and reproductive biology of Dipturus oxyrinchus, (Chondrichthyes: Rajidae) off the Gulf of Gabès (Southern Tunisia, central Mediterranean). J. Mar. Biol. Assoc. U. K. 2014, 95, 569–577. [Google Scholar] [CrossRef]
- Eronat, E.G.T.; Özaydın, O. Length-weight relationship of cartilaginous fish species from Central Aegean Sea (Izmir Bay and Sığacık Bay). Ege J. Fish Aqua. Sci. 2014, 31, 119–125. [Google Scholar] [CrossRef]
- Alkusairy, H.H.; Saad, A.A. Some morphological and biological aspects of long-nosed skate, Dipturus oxyrinchus (Elasmobranchii: Rajiformes: Rajidae), in Syrian marine waters (Eastern Mediterranean). Acta Ichthyol. Piscat. 2017, 47, 371–383. [Google Scholar] [CrossRef]
- Bellodi, A.; Porcu, C.; Cannas, R.; Cau, A.; Marongiu, M.F.; Mulas, A.; Vittori, S.; Follesa, M.C. Life-history traits of the long-nosed skate Dipturus oxyrinchus. J. Fish Biol. 2017, 90, 867–888. [Google Scholar] [CrossRef]
- Başusta, N.; Başusta, A. Occurence of adult male and juveniles of Dipturus Oxyrinchus from North-Eastern Mediterranean Sea. Ecol. Life Sci. 2019, 14, 40–42. [Google Scholar] [CrossRef]
- Griffiths, A.M.; Sims, D.W.; Johnson, A.; Lynghammar, A.; McHugh, M.; Bakken, T.; Genner, M.J. Levels of connectivity between longnose skate (Dipturus oxyrinchus) in the Mediterranean Sea and the north-eastern Atlantic Ocean. Conserv. Genet. 2011, 12, 577–582. [Google Scholar] [CrossRef]
- Martin, L.K.; Cailliet, G.M. Age and growth determination of the bat ray, Myliobatis california Gill, in central California. Copeia 1988, 3, 762–773. [Google Scholar] [CrossRef]
- Duman, O.V.; Başusta, N. Age and growth characteristics of marbled electric ray Torpedo marmorata (Risso, 1810) inhabiting Iskenderun Bay, North-eastern Mediterranean Sea. Turk. J. Fish. Aquat. Sci. 2013, 13, 551–559. [Google Scholar] [CrossRef]
- Başusta, N.; Demirhan, S.A.; Çiçek, E.; Başusta, A.; Kuleli, T. Age and growth of the common guitarfish, Rhinobatos rhinobatos (Linnaeus, 1758), in Iskenderun Bay (northeastern Mediterranean, Turkey). J. Mar. Biol. Assoc. U. K. 2008, 88, 837–842. [Google Scholar] [CrossRef]
- Başusta, N.; Aslan, E. Age and growth of bull ray Aetomylaeus bovinus (Chondrichthyes: Myliobatidae) from the northeastern Mediterranean coast of Turkey. Cah. Biol. Mar. 2018, 59, 107–114. [Google Scholar] [CrossRef]
- Girgin, H.; Başusta, N. Testing staining techniques to determine age and growth of Dasyatis pastinaca (Linnaeus, 1758) captured in Iskenderun Bay, northeastern Mediterranean. J. Appl. Ichthyol. 2016, 32, 595–601. [Google Scholar] [CrossRef]
- Başusta, N.; Sulikowski, J.A. The oldest estimated age for roughtail stingray (Dasyatis centroura; Mitchill, 1815) from the Mediterranean Sea. J. Appl. Ichthyol. 2012, 28, 641–642. [Google Scholar] [CrossRef]
- Başusta, N.; Başusta, A.; Çiçek, E.; Cicia, A.M.; Sulikowski, J.A. First Estimates of Age and Growth of the Lusitanian Cownose Ray (Rhinoptera marginata) from the Mediterranean Sea. J. Mar. Sci. Eng. 2022, 10, 685. [Google Scholar] [CrossRef]
- Sulikowski, J.A.; Morin, M.D.; Suk, S.H.; Howell, W.H. Age and growth estimates of the winter skate (Leucoraja ocellata) in the western gulf of Maine. Fish. Bull. 2003, 101, 405–413. [Google Scholar]
- Sulikowski, J.A.; Kneebone, J.; Elzey, S.; Jurek, J.; Danley, P.D.; Howell, W.H.; Tsang, P.C.W. Age and growth estimates of the thorny skate (Amblyraja radiata) in the western Gulf of Maine. Fish. Bull. 2005, 103, 161–168. [Google Scholar]
- Goldman, K.J.; Cailliet, G.M.; Andrews, A.H.; Natanson, L.J. Assessing the age and growth of chondrichthyan fishes. In Biology of Sharks and Their Relatives, 2nd ed.; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2012; pp. 423–451. [Google Scholar]
- Chang, W.Y.B. A statistical method for evaluating the reproducibility of age determination. Can. J. Fish. Aquat. Sci. 1982, 39, 1208–1210. [Google Scholar] [CrossRef]
- Beamish, R.J.; Fournier, D.A. A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- Natanson, L.J.; Mello, J.J.; Campana, S.E. Validated age and growth of the porbeagle shark, Lamna nasus, in the western North Atlantic Ocean. Fish. Bull. 2002, 100, 266–278. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.1; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Quinn, T.J., II; Deriso, R.B. Quantitative Fish Dynamics; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Tıraşın, E.M. Balık populasyonlarının büyüme parametrelerinin araştırılması. Turk. J. Zool. 1993, 17, 29–82. (In Turkish) [Google Scholar]
- Richer, W.E. Computation and Interpretation of Biological Statistics of Fish Populations; Bullettin of the Fisheries Research Board Canada: Ottowa, ON, Canada, 1975; Volume 191, p. 382. [Google Scholar]
- Le Cren, C.D. The length-weight relationship and seasonal cycle in gonad weight and condition in Perch, Perca fluviatilis. J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Fisheries Investigations, Series 2; Springer Science+Business Media, B.V.: London, UK, 1957. [Google Scholar]
- Gompertz, B. On the nature of the function expressive of the law of human mortality and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. Lond. 1825, 115, 515–585. [Google Scholar]
- Ricker, W.E. Growth rates and Models. In Bioenergetics and Growth; Hoar, W.S., Randal, D.J., Brett, J.R., Eds.; Academic Press: Cambridge, MA, USA, 1979; Fish ISSN:1307-3311 Physiology; Volume VIII, pp. 677–743. [Google Scholar]
- Smart, J.J.; Chin, A.; Tobin, A.J.; Simpfendorfer, C.A. Multimodel approaches in 420 shark and ray growth studies: Strengths, weaknesses and the future. Fish Fish. 2016, 17, 955–971. [Google Scholar] [CrossRef]
- Tjørve, K.M.C.; Tjørve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef]
- Başusta, N.; Başusta, A.; Tıraşın, E.M.; Sulikowski, J.A. Age and growth of the blackchin guitarfish Glaucostegus cemiculus (Geoffroy Saint-Hilaire, 1817) from Iskenderun Bay (Northeastern Mediterranean). J. Appl. Ichthyol. 2019, 36, 880–887. [Google Scholar] [CrossRef]
- Akaike, H. Information theory as an extension of the maximum likelihood principle. In Proceedings of the Second International Symposium on Information Theory, Armenia, USSR, 2–8 September 1971; Petrov, B.N., Csaki, F., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Porcu, C.; Bellodi, A.; Cannas, R.; Marongiu, M.F.; Mulas, A.; Follesa, M.C. Life-history traits of a commercial ray, Raja brachyura from the central western Mediterranean Sea. Medit. Mar. Sci. 2015, 16, 90–102. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Nolan, C.P.; Jeal, F. Age, growth and maturity of the commercial ray species from the Irish Sea. J. Northwest Atl. Fish. Sci. 2005, 35, 47–66. [Google Scholar] [CrossRef]
- Campana, S.E. Accuracy, precision and quality control in age determination, including of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Borges, T.C.; Olim, S.; Erzini, K. Weight-length relationship for fish species discarded in commercial fisheries of the Algarve (southern Portugal). J. Appl. Ichthyol. 2003, 19, 394–396. [Google Scholar] [CrossRef]
- Filiz, H.; Bilge, G. Length-weight relationships of 24 fish species from the North Aegean Sea, Turkey. J. Appl. Ichthyol. 2004, 20, 431–432. [Google Scholar] [CrossRef]
- Geraci, M.L.; Ragonese, S.; Scannella, D.; Falsone, F.; Gancitano, V.; Mifsud, J.; Gambin, M.; Said, A.; Vitale, S. Batoid abundances, spatial distribution, and life history traits in the Strait of Sicily (Central Mediterranean Sea): Bridging a knowledge gap through three decades of survey. Animals 2021, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Barría, C.; Navarro, J.; Coll, M.; Fernancez-Arcaya, U.; Sáez-Liante, R. Morphological parameters of abundant and threatened chondrichthyans of the northwestern Mediterranean Sea. J. Appl. Ichthyol. 2015, 31, 114–119. [Google Scholar] [CrossRef]
- Işmen, A.; Özen, Ö.; Altınağaç, U.; Özekinci, U.; Ayaz, A. Weight–length relationships of 63 fish species in Saros Bay, Turkey. J. Appl. Ichthyol. 2007, 23, 707–708. [Google Scholar] [CrossRef]
- Liu, K.M.; Wu, C.B.; Joung, S.J.; Tsai, W.P.; Su, K.Y. Multi-model approach on growth estimation and association with life history trait for elasmobranchs. Front. Mar. Sci. 2021, 8, 591692. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).