Does the Wolf (Canis lupus) Exhibit Human Habituation Behaviours after Rehabilitation and Release into the Wild? A Case Report from Central Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
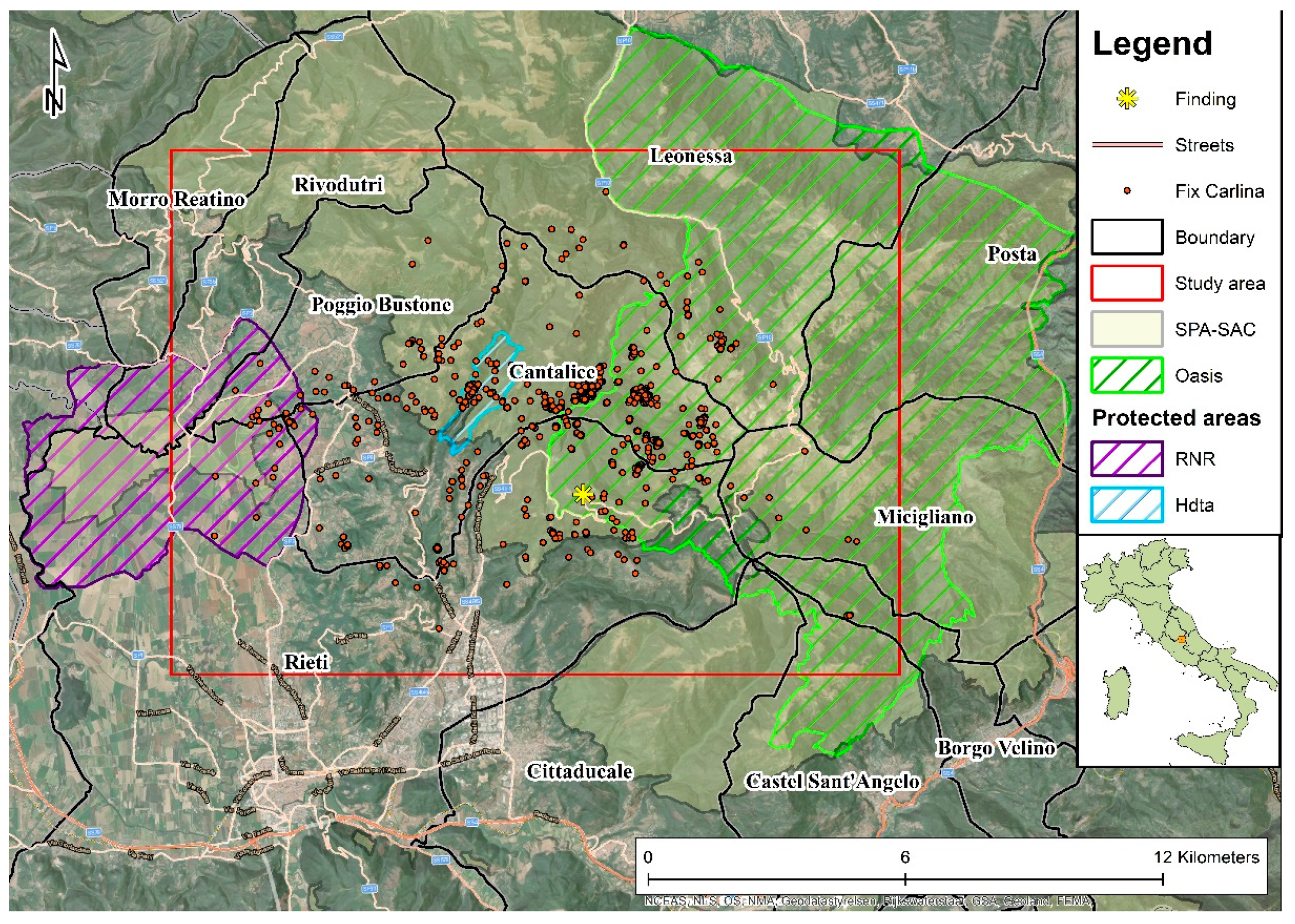
2.1. Study Area
2.2. Application and Setting of the GPS Tracking Collar
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, J.; Olszańska, A.; Morales-Reyes, Z.; Castro, A.A.; Malo, A.F.; Moleón, M.; Sánchez-Zapata, J.A.; Cortés-Avizanda, A.; von Wehrden, H.; Dorresteijn, I.; et al. Human-Carnivore Relations: A Systematic Review. Biol. Conserv. 2019, 237, 480–492. [Google Scholar] [CrossRef]
- Larivière, S.; Jolicoeur, H.; Crête, M. Status and Conservation of the Gray Wolf (Canis lupus) in Wildlife Reserves of Quebec. Biol. Conserv. 2000, 94, 143–151. [Google Scholar] [CrossRef]
- Fernández-Sepúlveda, J.; Martín, C.A. Conservation Status of the World’s Carnivorous Mammals (Order Carnivora). Mamm. Biol. 2022. [Google Scholar] [CrossRef]
- Galaverni, M.; Caniglia, R.; Fabbri, E.; Milanesi, P.; Randi, E. One, No One, or One Hundred Thousand: How Many Wolves Are There Currently in Italy? Mammal Res. 2015, 61, 13–24. [Google Scholar] [CrossRef]
- Cimatti, M.; Ranc, N.; Benítez-López, A.; Maiorano, L.; Boitani, L.; Cagnacci, F.; Čengić, M.; Ciucci, P.; Huijbregts, M.A.J.; Krofel, M.; et al. Large Carnivore Expansion in Europe Is Associated with Human Population Density and Land Cover Changes. Divers. Distrib. 2021, 27, 602–617. [Google Scholar] [CrossRef]
- Marucco, F.; Boitani, L. Wolf Population Monitoring and Livestock Depredation Preventive Measures in Europe. Hystrix 2012, 23, 1–4. [Google Scholar] [CrossRef]
- Dondina, O.; Meriggi, A.; Dagradi, V.; Perversi, M.; Milanesi, P. Wolf Predation on Livestock in an Area of Northern Italy and Prediction of Damage Risk. Ethol. Ecol. Evol. 2015, 27, 200–219. [Google Scholar] [CrossRef]
- Gervasi, V.; Salvatori, V.; Catullo, G.; Ciucci, P. Assessing Trends in Wolf Impact on Livestock through Verified Claims in Historical vs. Recent Areas of Occurrence in Italy. Eur. J. Wildl. Res. 2021, 67, 82. [Google Scholar] [CrossRef]
- Randi, E.; Lucchini, V. Detecting Rare Introgression of Domestic Dog Genes into Wild Wolf (Canis lupus) Populations by Bayesian Admixture Analyses of Microsatellite Variation. Conserv. Genet. 2002, 3, 31–45. [Google Scholar] [CrossRef]
- Montana, L.; Caniglia, R.; Galaverni, M.; Fabbri, E.; Randi, E. A New Mitochondrial Haplotype Confirms the Distinctiveness of the Italian Wolf (Canis lupus) Population. Mamm. Biol. 2017, 84, 30–34. [Google Scholar] [CrossRef]
- Musto, C.; Caniglia, R.; Fabbri, E.; Galaverni, M.; Romagnoli, N.; Pinna, S.; Berti, E.; Naldi, M.; Bologna, E.; Molinari, L.; et al. Conservation at the Individual Level: Successful Rehabilitation and Post-Release Monitoring of an Italian Wolf (Canis lupus italicus) Injured in a Car Accident. Vet. Arh. 2020, 90, 205–212. [Google Scholar] [CrossRef]
- La Morgia, V.; Marucco, F.; Aragno, P.; Salvatori, V.; Gervasi, V.; De Angelis, D.; Fabbri, E.; Caniglia, R.; Velli, E.; Avanzinelli, E.; et al. Stima della Distribuzione e Consistenza del Lupo a Scala Nazionale 2020/2021. Relazione Tecnica Realizzata nell’Ambito della Convenzione ISPRA-Ministero della Transizione Ecologica “Attività di Monitoraggio Nazionale nell’Ambito del Piano di Azione del Lupo”; Istituto Superiore per la Ricerca e la Protezione Ambientale: Roma, Italy, 2022; pp. 4–16. Available online: https://www.isprambiente.gov.it/it/attivita/biodiversita/monitoraggio-nazionale-del-lupo/file-monitoraggio/report-nazionale-lupo-20_21.pdf (accessed on 15 November 2022).
- Lovari, S.; Sforzi, A.; Scala, C.; Fico, R. Mortality Parameters of the Wolf in Italy: Does the Wolf Keep Himself from the Door? J. Zool. 2007, 272, 117–124. [Google Scholar] [CrossRef]
- Bassi, E.; Willis, S.G.; Passilongo, D.; Mattioli, L.; Apollonio, M. Predicting the Spatial Distribution of Wolf (Canis lupus) Breeding Areas in a Mountainous Region of Central Italy. PLoS ONE 2015, 10, e124698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musto, C.; Cerri, J.; Galaverni, M.; Caniglia, R.; Fabbri, E.; Apollonio, M.; Mucci, N.; Bonilauri, P.; Maioli, G.; Fontana, M.C.; et al. Men and Wolves: Anthropogenic Causes Are an Important Driver of Wolf Mortality in Human-Dominated Landscapes in Italy. Glob. Ecol. Conserv. 2021, 32, e01892. [Google Scholar] [CrossRef]
- Mörner, T.; Eriksson, H.; Bröjer, C.; Nilsson, K.; Uhlhorn, H.; Ågren, E.; af Segerstad, C.H.; Jansson, D.S.; Gavier-Widén, D. Diseases and Mortality in Free-Ranging Brown Bear (Ursus arctos), Gray Wolf (Canis lupus), and Wolverine (Gulo gulo) in Sweden. J. Wildl. Dis. 2005, 41, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Rio-Maior, H.; Beja, P.; Nakamura, M.; Santos, N.; Brandão, R.; Sargo, R.; Dias, I.; Silva, F.; Álvares, F. Rehabilitation and Post-Release Monitoring of Two Wolves with Severe Injuries. J. Wildl. Manag. 2016, 80, 729–735. [Google Scholar] [CrossRef]
- Ciucci, P.; Reggioni, W.; Maiorano, L.; Boitani, L. Long-Distance Dispersal of a Rescued Wolf from the Northern Apennines to the Western Alps. J. Wildl. Manag. 2009, 73, 1300–1306. [Google Scholar] [CrossRef]
- Blasi, C.; Di Pietro, R.; Filesi, L.; Ercole, S. Le Serie Di Vegetazione Della Regione Lazio; Regione Lazio: Rome, Italy, 1994. [Google Scholar]
- Viola, P.; Adriani, S.; Rossi, C.M.; Franceschini, C.; Primi, R.; Apollonio, M.; Amici, A. Anthropogenic and Environmental Factors Determining Local Favourable Conditions for Wolves during the Cold Season. Animals 2021, 11, 1895. [Google Scholar] [CrossRef]
- Gipson, P.S.; Ballard, W.B.; Nowak, R.M.; Mech, L.D. Accuracy and Precision of Estimating Age of Gray Wolves by Tooth Wear. J. Wildl. Manag. 2000, 64, 752. [Google Scholar] [CrossRef]
- Aeronautica Militare Italiana, Centro Nazionale di Meteorologia e Climatologia Aeronautica, Servizio di Climatologia e Documentazione. Effemeridi Aeronautiche; Aeronautica Militare: Roma, Italy, 2013; pp. 37–38. Available online: https://clima.meteoam.it/Download/EffemeridiAeronautiche_2013.pdf (accessed on 10 October 2022).
- ESRI. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Wall, J. ArcMET—Movement Ecology Tools for ArcGIS. Available online: http://www.movementecology.net/ (accessed on 30 September 2022).
- Mohr, C.O. Table of Equivalent Populations of North American Small Mammals. Am. Midl. Nat. 1947, 37, 223–249. [Google Scholar] [CrossRef]
- Regione Lazio. Carta dell’Uso Del Suolo. Scala 1:25.000; Selca: Firenze, Italy, 2003. [Google Scholar]
- Chirici, G.; Fattori, C.; Cutolo, N.; Tufano, M.; Corona, P.; Barbati, A.; Blasi, C.; Copiz, R.; Rossi, L.; Biscontini, D.; et al. Map of the Natural and Semi-Natural Environments and Forest Types Map for the Latium Region (Italy). Riv. Selvic. Ecol. For. 2014, 11, 65–71. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 10 October 2022).
- Okarma, H.; Ldrzejewski, J.; Schmidt, K.; Sniezko, S.; Bunevich, A.N.; Ldrzejewska, J. Home Ranges of Wolves in Bialowieza Primeval Forest, Poland, Compared with Other Eurasian Popolations. J. Mammal. 1998, 79, 842–852. [Google Scholar] [CrossRef]
- Ciucci, P.; Boitani, L.; Francisci, F.; Andreoli, G. Home Range, Activity and Movements of a Wolf Pack in Central Italy. J. Zool. 1997, 243, 803–819. [Google Scholar] [CrossRef]
- Mancinelli, S.; Falco, M.; Boitani, L.; Ciucci, P. Social, Behavioural and Temporal Components of Wolf (Canis lupus) Responses to Anthropogenic Landscape Features in the Central Apennines, Italy. J. Zool. 2019, 309, 114–124. [Google Scholar] [CrossRef]
- Jedrzejewski, W.; Schmidt, K.; Theuerkauf, J.; Jedrzejewska, B.; Okarma, H. Daily Movements and Territory Use by Radio-Collared Wolves (Canis lupus) in Bialowieza Primeval Forest in Poland. Can. J. Zool. 2001, 79, 1993–2004. [Google Scholar] [CrossRef]
- Kolenosky, G.B.; Johnston, D.H. Radio-Tracking Timber Wolves in Ontario. Am. Zool. 1967, 7, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Kusak, J.; Skrbinšek, A.M.; Huber, D. Home Ranges, Movements, and Activity of Wolves (Canis lupus) in the Dalmatian Part of Dinarids, Croatia. Eur. J. Wildl. Res. 2005, 51, 254–262. [Google Scholar] [CrossRef]
- Torretta, E.; Caviglia, L.; Serafini, M.; Meriggi, A. Wolf Predation on Wild Ungulates: How Slope and Habitat Cover Influence the Localization of Kill Sites. Curr. Zool. 2018, 64, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Schenone, L.A.; Aristarchi, C.L.; Meriggi, A.L.; Animale, B.; Pavia, U.; Botta, P. Ecologia del Lupo in Provincia di Genova: Distribuzione, Consistenza della Popolazione, Alimentazione, Impatto sulla Zootecnia. Hystrix Ital. J. Mammal. 2003, 14, 13–30. [Google Scholar]
- Viola, P.; Girotti, P.; Serafini, D.; Serafini, S.; Venanzi, R.; Tocci, D.; Amici, A. Seasonal Variation in Wild Ungulate Abundance in a Hunting-Ban Beech Forest: A Case Study of Amiata Mountain, Central Italy. Environ. Sci. Proc. 2021, 3, 34. [Google Scholar] [CrossRef]
- Amici, A.; Serrani, F.; Rossi, C.M.; Primi, R. Increase in Crop Damage Caused by Wild Boar (Sus scrofa L.): The “Refuge Effect”. Agron. Sustain. Dev. 2012, 32, 683–692. [Google Scholar] [CrossRef]
- Tolon, V.; Dray, S.; Loison, A.; Zeileis, A.; Fischer, C.; Baubet, E. Responding to Spatial and Temporal Variations in Predation Risk: Space Use of a Game Species in a Changing Landscape of Fear. Can. J. Zool. 2009, 87, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Theuerkauf, J.; Jȩdrzejewski, W.; Schmidt, K.; Okarma, H.; Ruczyński, I.; Śniezko, S.; Gula, R. Daily Patterns and Duration of Wolf Activity in the Białowieża Forest, Poland. J. Mammal. 2003, 84, 243–253. [Google Scholar] [CrossRef]
- Mancinelli, S.; Boitani, L.; Ciucci, P. Determinants of Home Range Size and Space Use Patterns in a Protected Wolf (Canis lupus) Population in the Central Apennines, Italy. Can. J. Zool. 2018, 96, 828–838. [Google Scholar] [CrossRef] [Green Version]
- Barry, T.; Gurarie, E.; Cheraghi, F.; Kojola, I.; Fagan, W.F. Does Dispersal Make the Heart Grow Bolder? Avoidance of Anthropogenic Habitat Elements across Wolf Life History. Anim. Behav. 2020, 166, 219–231. [Google Scholar] [CrossRef]
- Sazatornil, V.; Rodríguez, A.; Klaczek, M.; Ahmadi, M.; Álvares, F.; Arthur, S.; Blanco, J.C.; Borg, B.L.; Cluff, D.; Cortés, Y.; et al. The Role of Human-Related Risk in Breeding Site Selection by Wolves. Biol. Conserv. 2016, 201, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Ciucci, P.; Masi, M.; Boitani, L. Winter Habitat and Travel Route Selection by Wolves in the Northern Apennines, Italy. Ecography 2003, 26, 223–235. [Google Scholar] [CrossRef]
- Mcnay, M.E. A Case History of Wolf-Human Encounters in Alaska and Canada; Alaska Department of Fish and Game Wildlife Technical Bulletin Vol. 13; Alaska Department of Fish and Game: Juneau, AK, USA, 2002; pp. 1–44.
- Nowak, S.; Szewczyk, M.; Tomczak, P.; Całus, I.; Figura, M.; Mysłajek, R.W. Social and Environmental Factors Influencing Contemporary Cases of Wolf Aggression towards People in Poland. Eur. J. Wildl. Res. 2020, 67, 69. [Google Scholar] [CrossRef]





| Category | Typology | Area (ha) | % |
|---|---|---|---|
| Broad-leaved forests | Beech, hop, hornbeam, oak and other mixed woods | 11,510.48 | 55.83 |
| Coniferous forest | Black pine | 671.72 | 3.26 |
| Cultivated lands | Permanent crops and arable lands | 3137.92 | 15.22 |
| Fruit trees | Chestnuts | 243.41 | 1.18 |
| Open areas | Natural grassland and pastures | 2755.63 | 13.37 |
| Scrubland | Bushes and shrubs | 119.60 | 0.58 |
| Principal road | Paved roads | 854.42 | 4.14 |
| Secondary roads | Gravel roads | 269.59 | 1.31 |
| Urban areas | Settlements and human activities | 923.11 | 4.48 |
| Water bodies | Lake and rivers | 129.27 | 0.63 |
| Total | 20,615.14 | 100.00 |
| Category | Typology | Area (ha) | % |
|---|---|---|---|
| Broad-leaved forests | Beech, hop, hornbeam, oak and mixed woods | 4733.30 | 72.36 |
| Coniferous forest | Black pine | 167.57 | 2.56 |
| Cultivated lands | Permanent crops and arable lands | 275.12 | 4.21 |
| Open areas | Natural grassland and pastures | 852.54 | 13.03 |
| Scrubland | Bushes and shrubs | 280.45 | 4.29 |
| Principal road | Paved roads | 31.68 | 0.48 |
| Secondary roads | Gravel roads | 56.80 | 0.87 |
| Urban areas | Settlements and human activities | 140.40 | 2.15 |
| Water bodies | Lake and rivers | 3.27 | 0.05 |
| Total | 6528.96 | 100.00 |
| Daily Distance Travelled (m) | Movement Rate (km/h) | |||||||
|---|---|---|---|---|---|---|---|---|
| Daytime | Nighttime | U Test | Daytime | Nighttime | U Test | |||
| Season | Mean ± SE | Mean ± SE | U | p | Mean ± SE | Mean ± SE | U | p |
| Summer | 2239.0 ± 329.0 | 4409.4 ± 617.5 | 482.0 | <0.01 | 0.25 ± 0.04 | 0.79 ± 0.1 | 10,823.0 | <0.001 |
| Autumn | 595.9 ± 110.3 | 3684.8 ± 468.1 | 315.0 | <0.001 | 0.10 ± 0.01 | 0.53 ± 0.1 | 3074.0 | <0.001 |
| U | 651.0 | 672.0 | / | / | 17,036.0 | 6134.0 | / | / |
| p | <0.001 | 0.311 | / | / | <0.001 | 0.079 | / | / |
| Speed (km/h) | ||||||
|---|---|---|---|---|---|---|
| Daytime | Nighttime | Kruskal–Wallis | ||||
| Season | Mean ± SE | Range | Mean ± SE | Range | H | p |
| Summer | 1.30 ± 0.16 (28) | 0.10–2.73 | 1.37 ± 0.11 (66) | 0.13–3.64 | 0.11 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viola, P.; Girotti, P.; Adriani, S.; Ronchi, B.; Zaccaroni, M.; Primi, R. Does the Wolf (Canis lupus) Exhibit Human Habituation Behaviours after Rehabilitation and Release into the Wild? A Case Report from Central Italy. Animals 2022, 12, 3495. https://doi.org/10.3390/ani12243495
Viola P, Girotti P, Adriani S, Ronchi B, Zaccaroni M, Primi R. Does the Wolf (Canis lupus) Exhibit Human Habituation Behaviours after Rehabilitation and Release into the Wild? A Case Report from Central Italy. Animals. 2022; 12(24):3495. https://doi.org/10.3390/ani12243495
Chicago/Turabian StyleViola, Paolo, Pedro Girotti, Settimio Adriani, Bruno Ronchi, Marco Zaccaroni, and Riccardo Primi. 2022. "Does the Wolf (Canis lupus) Exhibit Human Habituation Behaviours after Rehabilitation and Release into the Wild? A Case Report from Central Italy" Animals 12, no. 24: 3495. https://doi.org/10.3390/ani12243495
APA StyleViola, P., Girotti, P., Adriani, S., Ronchi, B., Zaccaroni, M., & Primi, R. (2022). Does the Wolf (Canis lupus) Exhibit Human Habituation Behaviours after Rehabilitation and Release into the Wild? A Case Report from Central Italy. Animals, 12(24), 3495. https://doi.org/10.3390/ani12243495






