Pathological and Molecular Characterization of a Duck Plague Outbreak in Southern China in 2021
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Case Report and Sample Collection
2.3. Histopathologic Examination
2.4. DNA Extraction and Virus Detection
2.5. Virus Titration
2.6. DPV Isolation
2.7. Amplification of Partial Virulence Sequences
2.8. Phylogenetic Analysis
2.9. Bioinformatics Analysis
2.10. Statistical Analysis
3. Results
3.1. Gross Lesions
3.2. Virus Detection
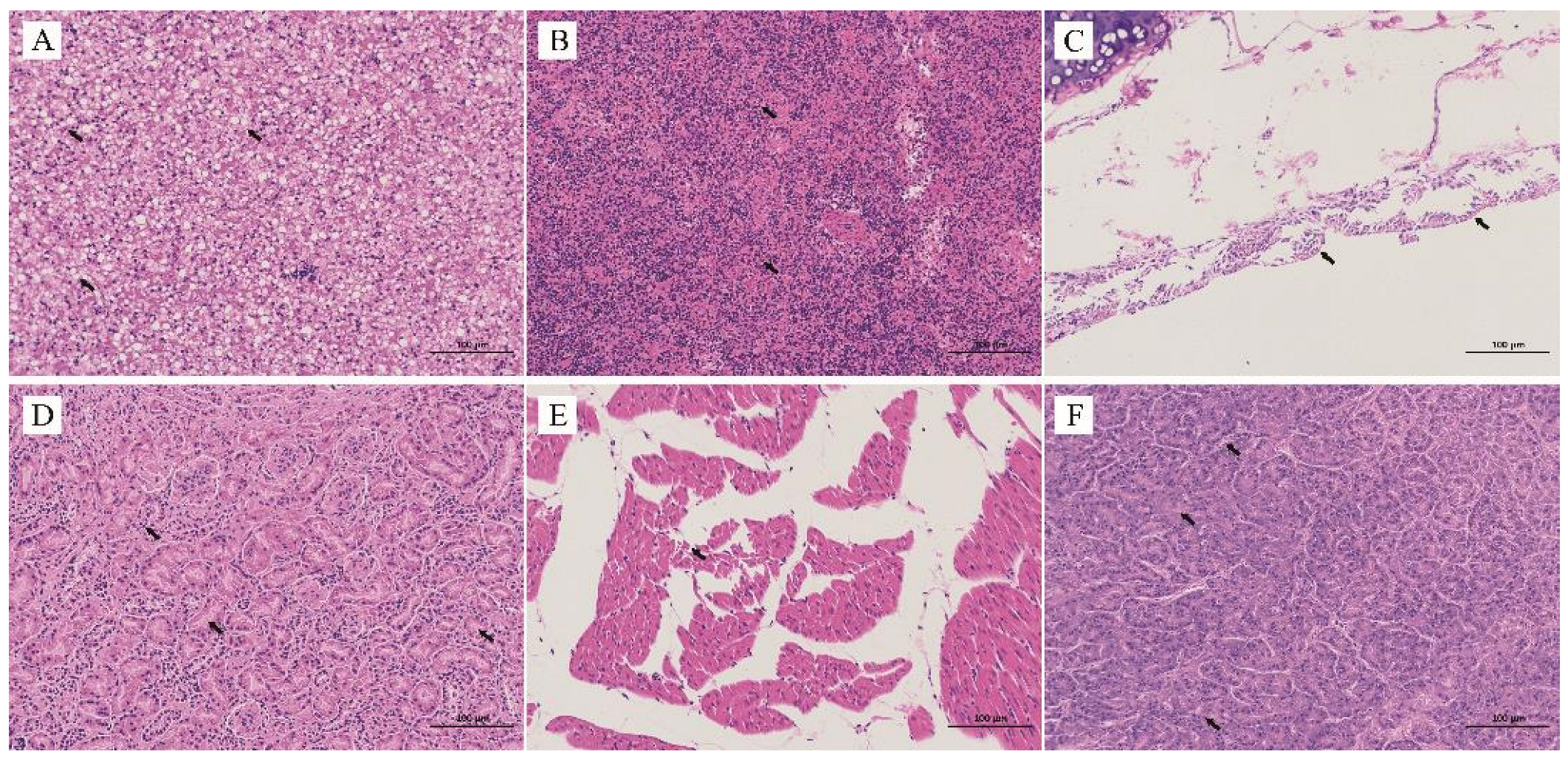
3.3. Histopathological Analysis
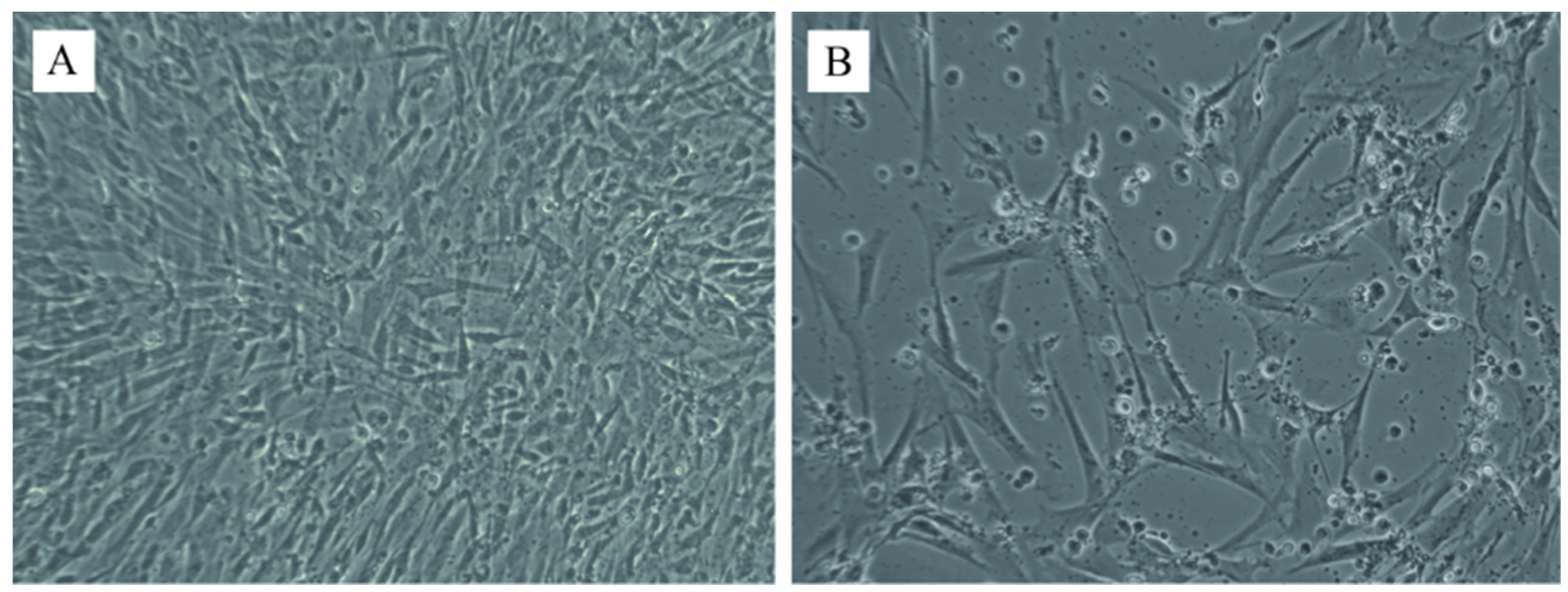
3.4. DPV Isolation
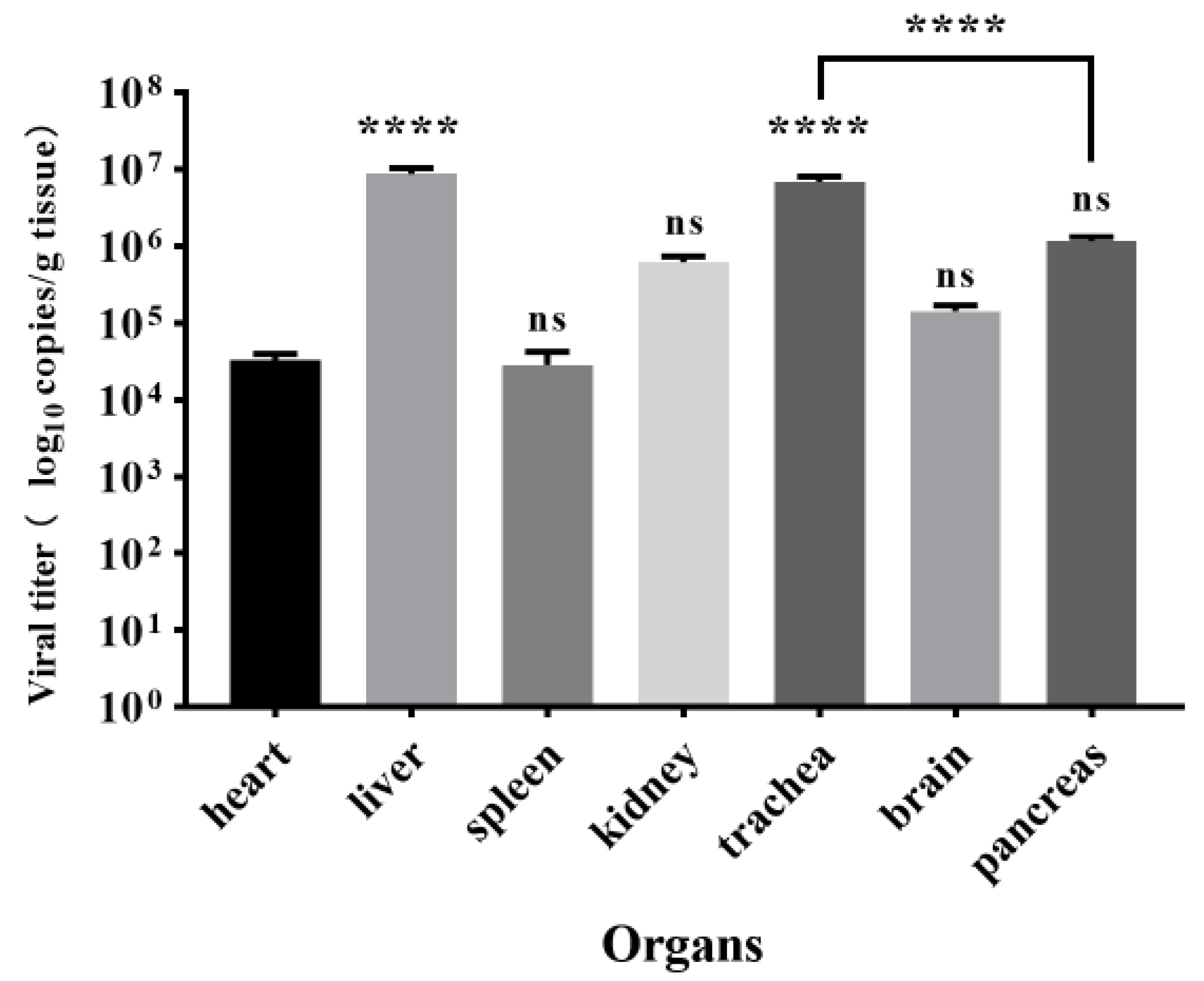
3.5. Viral Load in Tissues
3.6. Sequence Analysis of Virulence Genes
3.6.1. Analysis of the UL2 Gene
3.6.2. Analysis of the UL12 Gene
3.6.3. Analysis of the UL41 Gene
3.6.4. Analysis of the UL47 Gene
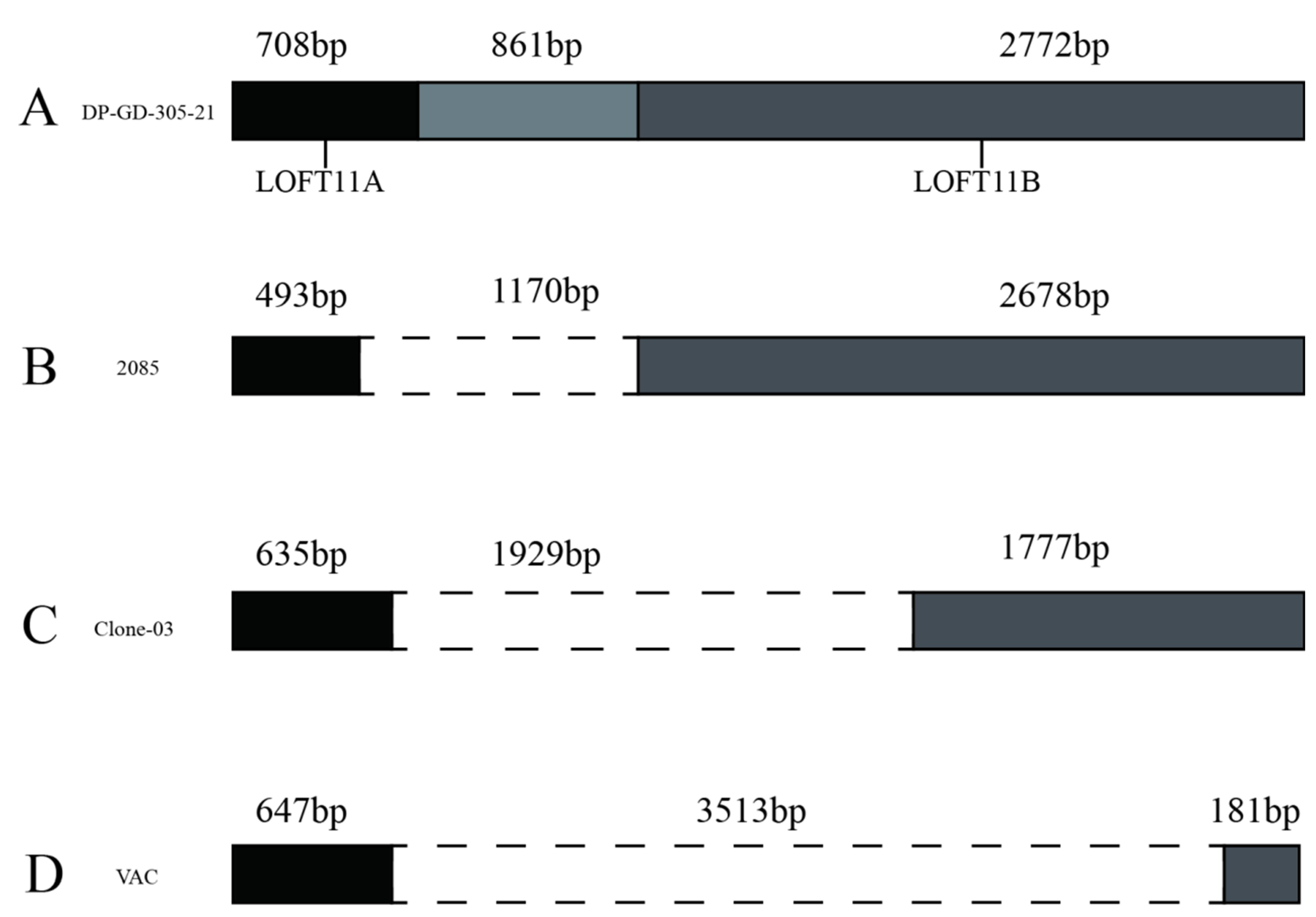
3.6.5. Analysis of the LORF11 Gene
3.7. Prediction of the Protein Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leibovitz, L.; Hwang, J. Duck plague on the American continent. Avian Dis. 1968, 12, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Sanchez-Cordon, P.J.; Nunez, A.; Gomez-Villamandos, J.C. Histopathological and ultrastructural changes associated with herpesvirus infection in waterfowl. Avian Pathol. 2002, 31, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, H.; Shah, M.; Pathak, M.; Barman, N.N.; Koul, M.; Gupta, A.; Sahariah, P.J.; Neher, S.; Das, S.K.; Gogoi, S.M.; et al. Pathodynamics of Circulating Strains of Duck Enteritis Virus: A Step Forward to Understand Its Pathogenesis. Avian Dis. 2020, 64, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Pazhanivel, N.; Rajeswar, J.; Ramprabhu, R.; Manoharan, S.; Bala, M.A.; Balachandran, C.; Kumanan, K.; Prathaban, S.; Saahithya, R. Duck plague outbreak in a Chara-Chemballi duck farm. Iran. J. Vet. Res. 2019, 20, 308–312. [Google Scholar]
- Li, N.; Hong, T.; Li, R.; Guo, M.; Wang, Y.; Zhang, J.; Liu, J.; Cai, Y.; Liu, S.; Chai, T.; et al. Pathogenicity of duck plague and innate immune responses of the Cherry Valley ducks to duck plague virus. Sci. Rep. 2016, 6, 32183. [Google Scholar] [CrossRef]
- Wang, G.; Qu, Y.; Wang, F.; Hu, D.; Liu, L.; Li, N.; Yue, R.; Li, C.; Liu, S. The comprehensive diagnosis and prevention of duck plague in northwest Shandong province of China. Poult. Sci. 2013, 92, 2892–2898. [Google Scholar] [CrossRef]
- Baudet, A.E.R.F. Mortality in ducks in the Netherlands caused by a filtrable virus; fowl plague. Tijdschr. Diergeneeskd. 1923, 50, 455–459. [Google Scholar]
- Woźniakowski, G.; Samorek-Salamonowicz, E. First survey of the occurrence of duck enteritis virus (DEV) in free-ranging Polish water birds. Arch. Virol. 2014, 159, 1439–1444. [Google Scholar] [CrossRef]
- Islam, M.M.; Islam, J.; Islam, M.S.; Ahamed, T.; Islam, M.R.; Khatun, M.M.; Islam, M.A. Duck virus enteritis (duck plague) outbreak in an Australian black swan (Cygnus atratus) flock at safari park in Bangladesh: A case report. J. Adv. Vet. Anim. Res. 2021, 8, 557–562. [Google Scholar] [CrossRef]
- Aasdev, A.; Pawar, S.D.; Mishra, A.; Dubey, C.K.; Patil, S.S.; Gogoi, S.M.; Bora, D.P.; Barman, N.N.; Raut, A.A. First complete genome characterization of duck plague virus from India. VirusDisease 2021, 32, 789–796. [Google Scholar] [CrossRef]
- Dhama, K.; Kumar, N.; Saminathan, M.; Tiwari, R.; Karthik, K.; Kumar, M.A.; Palanivelu, M.; Shabbir, M.Z.; Malik, Y.S.; Singh, R.K. Duck virus enteritis (duck plague)—A comprehensive update. Vet. Q. 2017, 37, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. Research of duck plague. South China J. Agron. 1959, 1, 67–78. [Google Scholar]
- Liu, R.; Chen, C.; Huang, Y.; Cheng, L.; Lu, R.; Fu, G.; Shi, S.; Chen, H.; Wan, C.; Fu, Q.; et al. Microbiological identification and analysis of waterfowl livers collected from backyard farms in southern China. J. Vet. Med. Sci. 2018, 80, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, M.; Cheng, A.; Jia, R.; Yang, Q.; Wu, Y.; Liu, M.; Zhao, X.; Chen, S.; Zhang, S.; et al. Duck plague virus gE serves essential functions during the virion final envelopment through influence capsids budding into the cytoplasmic vesicles. Sci. Rep. 2020, 10, 5658. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hoper, D.; Beer, M.; Osterrieder, N. Complete genome sequence of virulent duck enteritis virus (DEV) strain 2085 and comparison with genome sequences of virulent and attenuated DEV strains. Virus Res. 2011, 160, 316–325. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Li, Q.; Li, H.; Xia, Y.; Guo, X.; Yu, K.; Yang, H. Complete genome sequence of an attenuated duck enteritis virus obtained by in vitro serial passage. Genome Announc. 2013, 1, e00685-13. [Google Scholar] [CrossRef]
- He, T.; Wang, M.; Cheng, A.; Yang, Q.; Jia, R.; Wu, Y.; Huang, J.; Tian, B.; Liu, M.; Chen, S.; et al. DPV UL41 gene encoding protein induces host shutoff activity and affects viral replication. Vet. Microbiol. 2021, 255, 108979. [Google Scholar] [CrossRef]
- Xie, L.; Xie, Z.; Huang, L.; Wang, S.; Huang, J.; Zhang, Y.; Zeng, T.; Luo, S. A polymerase chain reaction assay for detection of virulent and attenuated strains of duck plague virus. J. Virol. Methods 2017, 249, 66–68. [Google Scholar] [CrossRef]
- Huang, J.; Jia, R.; Wang, M.; Shu, B.; Yu, X.; Zhu, D.; Chen, S.; Yin, Z.; Chen, X.; Cheng, A. An attenuated duck plague virus (DPV) vaccine induces both systemic and mucosal immune responses to protect ducks against virulent DPV infection. Clin. Vaccine Immunol. 2014, 21, 457–462. [Google Scholar] [CrossRef]
- Yang, X.; Qi, X.; Cheng, A.; Wang, M.; Zhu, D.; Jia, R.; Chen, X. Intestinal mucosal immune response in ducklings following oral immunisation with an attenuated Duck enteritis virus vaccine. Vet. J. 2010, 185, 199–203. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Y.; Cui, L.; Ma, B.; Mu, X.; Li, Y.; Zhang, Z.; Li, D.; Wei, W.; Gao, M.; et al. Duck enteritis virus glycoprotein D and B DNA vaccines induce immune responses and immunoprotection in Pekin ducks. PLoS ONE 2014, 9, e95093. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Huang, K.; Wei, Y.; Chen, H.; Liu, Z.; Jin, M. Construction of a highly efficient CRISPR/Cas9-mediated duck enteritis virus-based vaccine against H5N1 avian influenza virus and duck Tembusu virus infection. Sci. Rep. 2017, 7, 1478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Niu, Y.; Lu, T.; Yin, H.; Zhang, Y.; Xu, L.; Wang, Y.; Chen, H. Metagenomic Analysis of the Jinding Duck Fecal Virome. Curr. Microbiol. 2018, 75, 658–665. [Google Scholar] [CrossRef]
- Suo, H.Y.; Tang, C.; Yue, H.; Tang, M.; Jing, B.; Han, P. Establishment of TaqMan real-time fluorescent quantitative PCR for detection of duck plague virus. Chin. J. Prev. Vet. Med. 2006, 28, 332–335. [Google Scholar]
- Wang, J.; Zhang, C.; Xu, M.; Wang, Z.; Hou, J. Sequencing and analysis of several genes of a virulent duck enteritis virus strain LH2011. Jiangsu J. Agric. Sci. 2013, 29, 1086–1091. [Google Scholar]
- Panickan, S.; Dandapat, S.; Kumar, J.; Mahendran, M.; Nandi, S.; Punnoose, P. Molecular Characterization and Pathogenicity of an Indian Isolate of Duck Enteritis Virus Recovered from a Natural Outbreak. Indian J. Anim. Res. 2020, 55, 636–641. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Hamed, M.F.; Matter, A.A.; Abou El-Azm, K.I. Molecular and pathological characterization of duck enteritis virus in Egypt. Transbound. Emerg. Dis. 2019, 66, 217–224. [Google Scholar] [CrossRef]
- Pathak, M. Pathology of Wild Strain (DP/As-Km/0019) of Duck Plague Virus Infection Revived in Ducklings. J. Anim. Res. 2019, 9, 831–835. [Google Scholar] [CrossRef]
- Campagnolo, E.R.; Banerjee, M.; Panigrahy, B.; Jones, R.L. An outbreak of duck viral enteritis (duck plague) in domestic Muscovy ducks (Cairina moschata domesticus) in Illinois. Avian Dis. 2001, 45, 522–528. [Google Scholar] [CrossRef]
- Kumar, J.; Dandapat, S.; Panickan, S.; Kumar, A.; Singh, M.; Bindu, S.; Dhama, K. Expression profiles of toll like receptors, MHC and cytokine genes along with viral load in organs of ducklings infected with an Indian isolate of duck enteritis virus. Microb. Pathog. 2022, 165, 105502. [Google Scholar] [CrossRef]
- Aravind, S.; Kamble, N.M.; Gaikwad, S.S.; Shukla, S.K.; Dey, S.; Mohan, C.M. Adaptation and growth kinetics study of an Indian isolate of virulent duck enteritis virus in Vero cells. Microb. Pathog. 2015, 78, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, A.; Wang, M.; Zhu, D.; Jia, R.; Chen, S.; Zhou, Y.; Chen, X. Comparative genomic analysis of duck enteritis virus strains. J. Virol. 2012, 86, 13841–13842. [Google Scholar] [CrossRef] [PubMed]
- Bogani, F.; Chua, C.N.; Boehmer, P.E. Reconstitution of uracil DNA glycosylase-initiated base excision repair in herpes simplex virus-1. J. Biol. Chem. 2009, 284, 16784–16790. [Google Scholar] [CrossRef]
- Niu, Y.; Su, S.; Chen, X.; Zhao, L.; Chen, H. Biological characteristic and cytokines response of passages duck plague virus in ducks. Virus Res. 2021, 295, 198320. [Google Scholar] [CrossRef] [PubMed]



| ORF | Amino Acid Position(s) | Amino Acid | ||||||
|---|---|---|---|---|---|---|---|---|
| DP-GD-305-21 | CHv (JQ647509.1) | CV (JQ673560.1) | SD (JQ673560.1) | LH2011 (MZ574076.1) | 2085 (JF999965.1) | DP-AS-Km-19 (MZ574076.1) | ||
| UL12 | 368 | S | A | A | A | A | A | A |
| UL41 | 125 | - * | D | D | - | - | - | D |
| 303 | Y | Y | Y | Y | C | C | C | |
| UL47 | 113 | H | R | R | H | H | H | H |
| 149 | - | D | D | - | - | - | - | |
| 404 | A | V | V | V | V | V | V | |
| 599 | A | T | T | A | A | A | A | |
| 727 | F | L | L | L | L | L | L | |
| LORF11 | 2 | - | E | E | E | A | E | E |
| 435 | S | P | P | P | P | - | - | |
| 486 | K | N | N | K | N | - | - | |
| 713 | I | V | V | I | V | I | I | |
| 745 | R | H | H | R | H | R | R | |
| 789 | Y | - | - | Y | - | Y | Y | |
| 869 | L | R | L | L | L | L | L | |
| 894 | R | H | H | R | H | R | R | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Guo, J.; Yuan, S.; Cheng, Q.; Zhang, X.; Liu, Z.; Wang, C.; Li, Z.; Hou, B.; Huang, S.; et al. Pathological and Molecular Characterization of a Duck Plague Outbreak in Southern China in 2021. Animals 2022, 12, 3523. https://doi.org/10.3390/ani12243523
Liang Z, Guo J, Yuan S, Cheng Q, Zhang X, Liu Z, Wang C, Li Z, Hou B, Huang S, et al. Pathological and Molecular Characterization of a Duck Plague Outbreak in Southern China in 2021. Animals. 2022; 12(24):3523. https://doi.org/10.3390/ani12243523
Chicago/Turabian StyleLiang, Zhipeng, Jinyue Guo, Sheng Yuan, Qing Cheng, Xinyu Zhang, Zhun Liu, Congying Wang, Zhili Li, Bo Hou, Shujian Huang, and et al. 2022. "Pathological and Molecular Characterization of a Duck Plague Outbreak in Southern China in 2021" Animals 12, no. 24: 3523. https://doi.org/10.3390/ani12243523





