Quercetin Ameliorates Lipopolysaccharide-Induced Duodenal Inflammation through Modulating Autophagy, Programmed Cell Death and Intestinal Mucosal Barrier Function in Chicken Embryos
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chinese Herbal Medicines with Antidiarrheal Effects and Key Active Ingredients
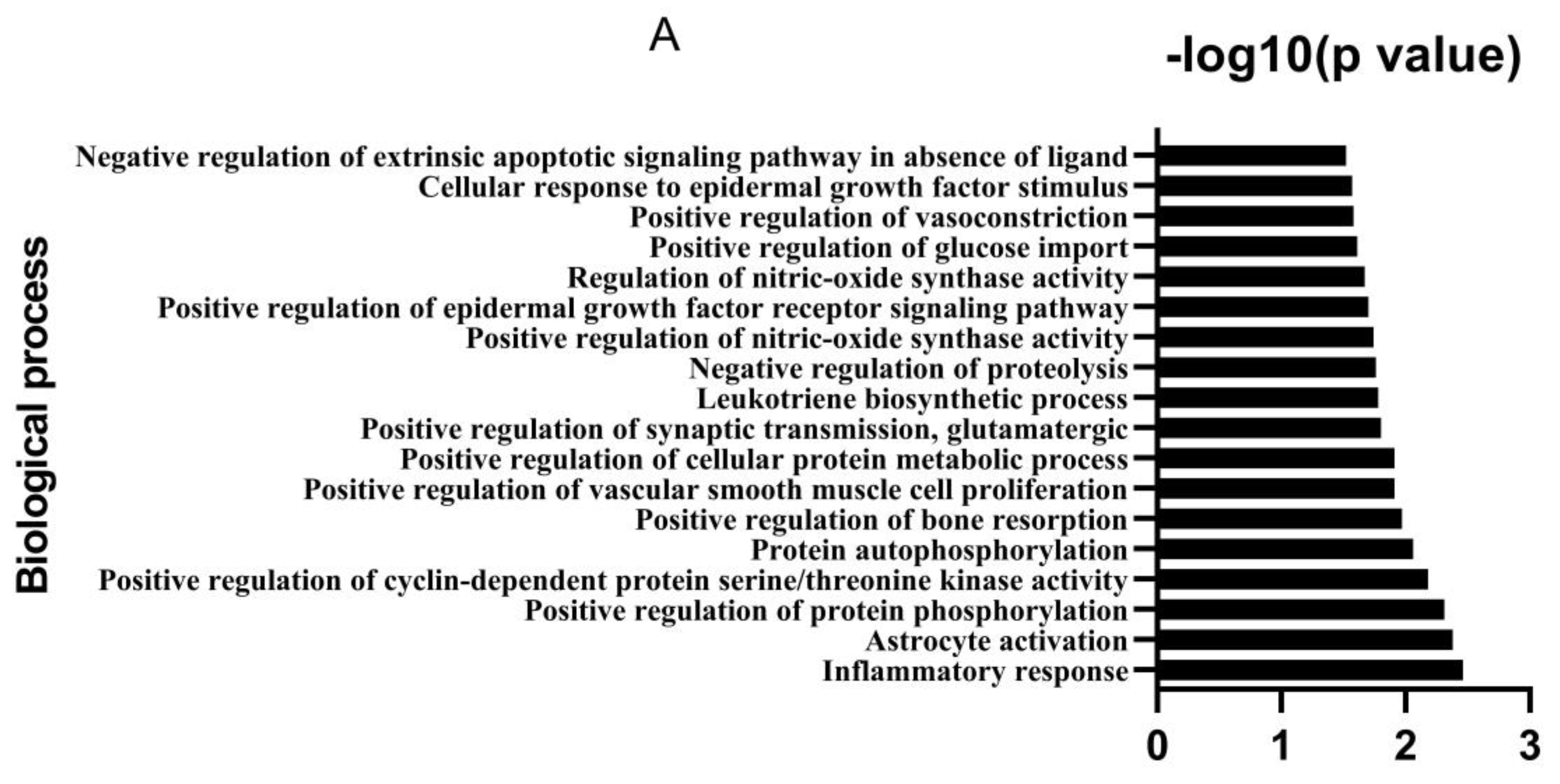
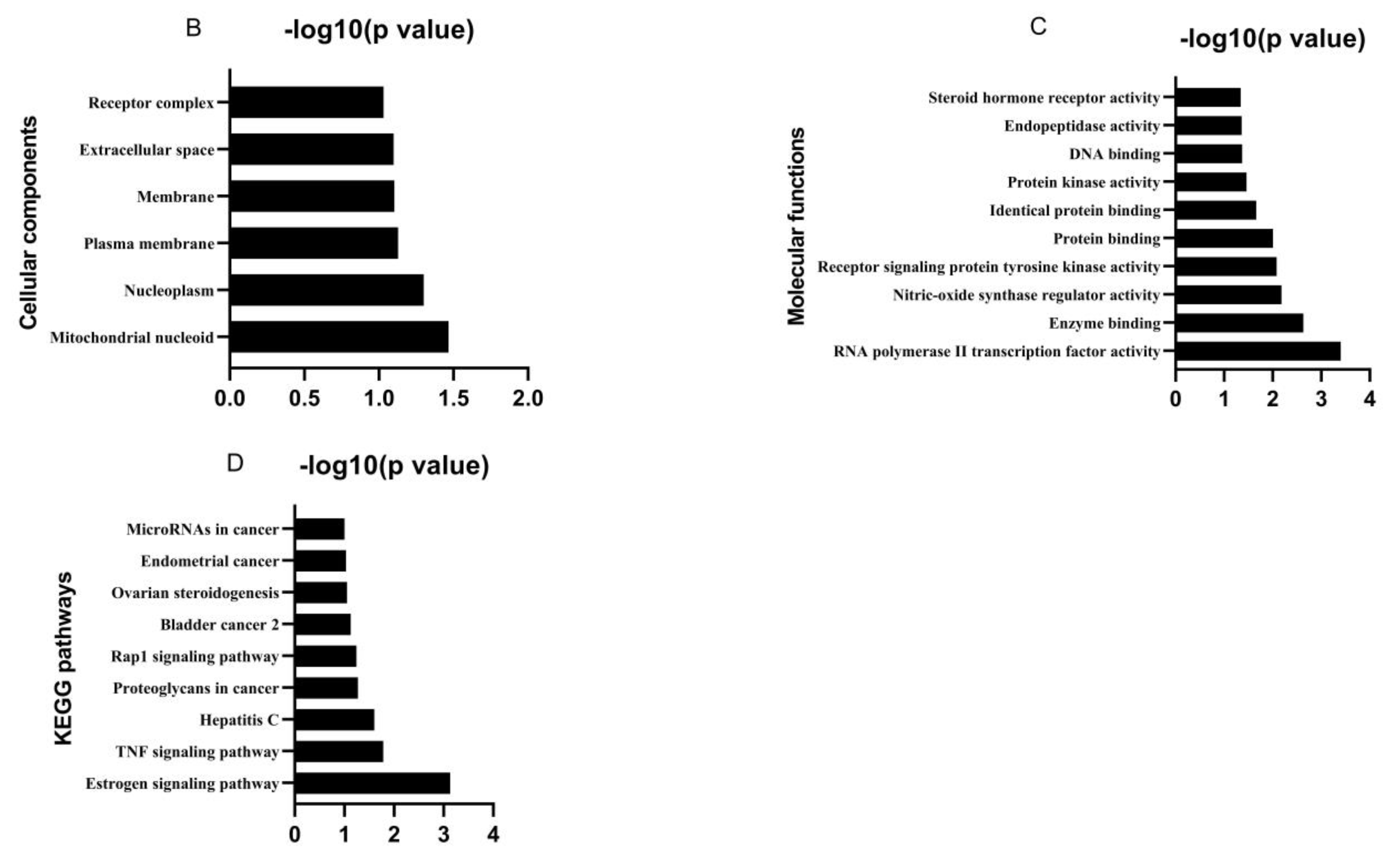
2.2. Analysis of Potential Targets and Signaling Pathways
2.3. Reagents, Chicken Embryos and Experimental Design
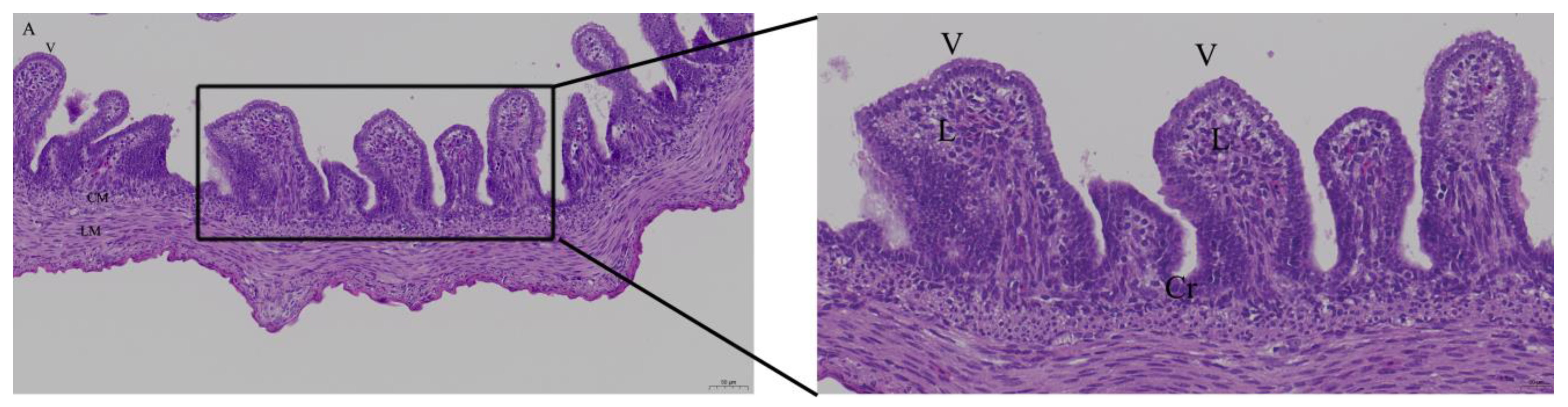
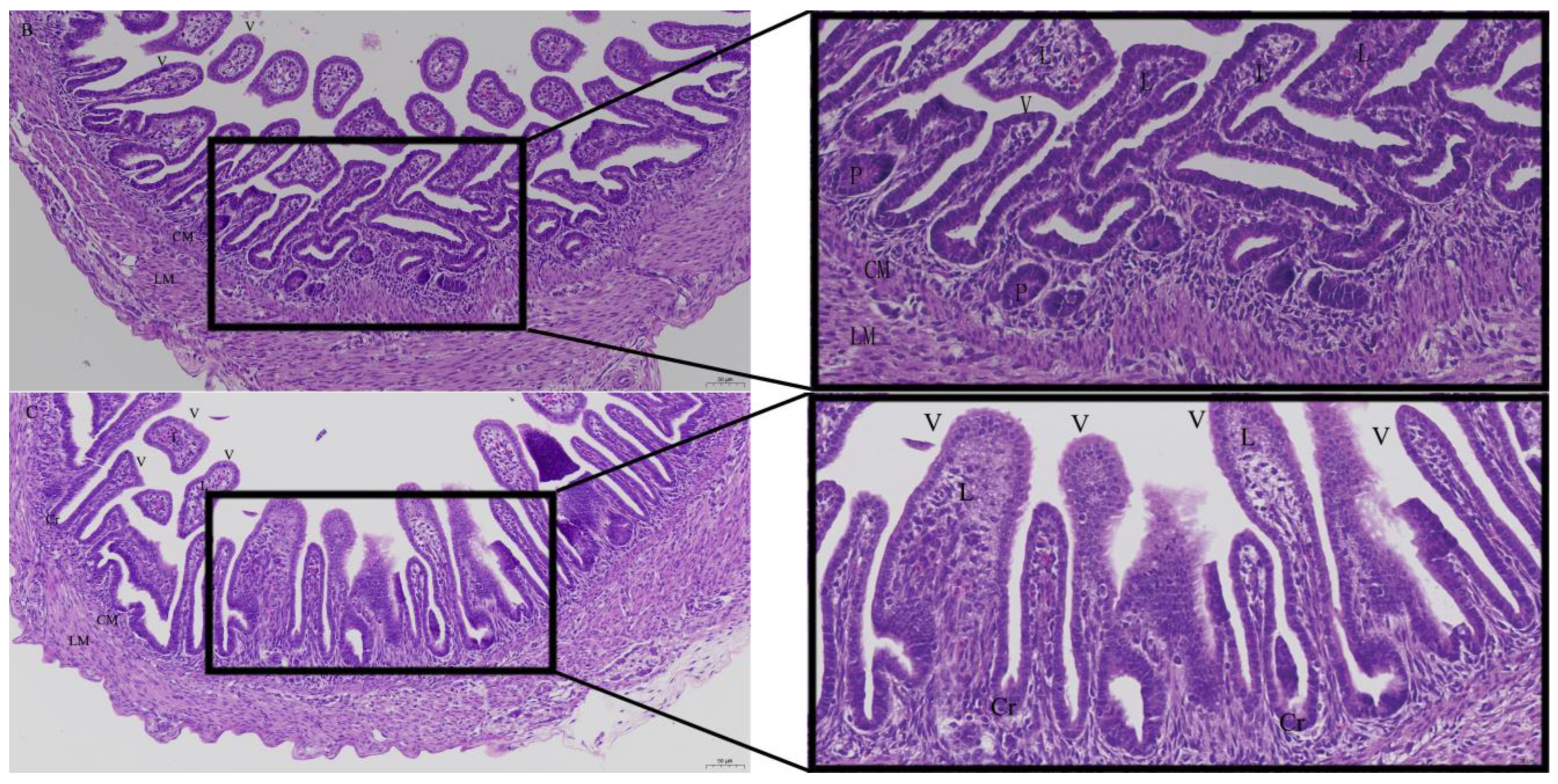
2.4. Histology
2.5. qPCR
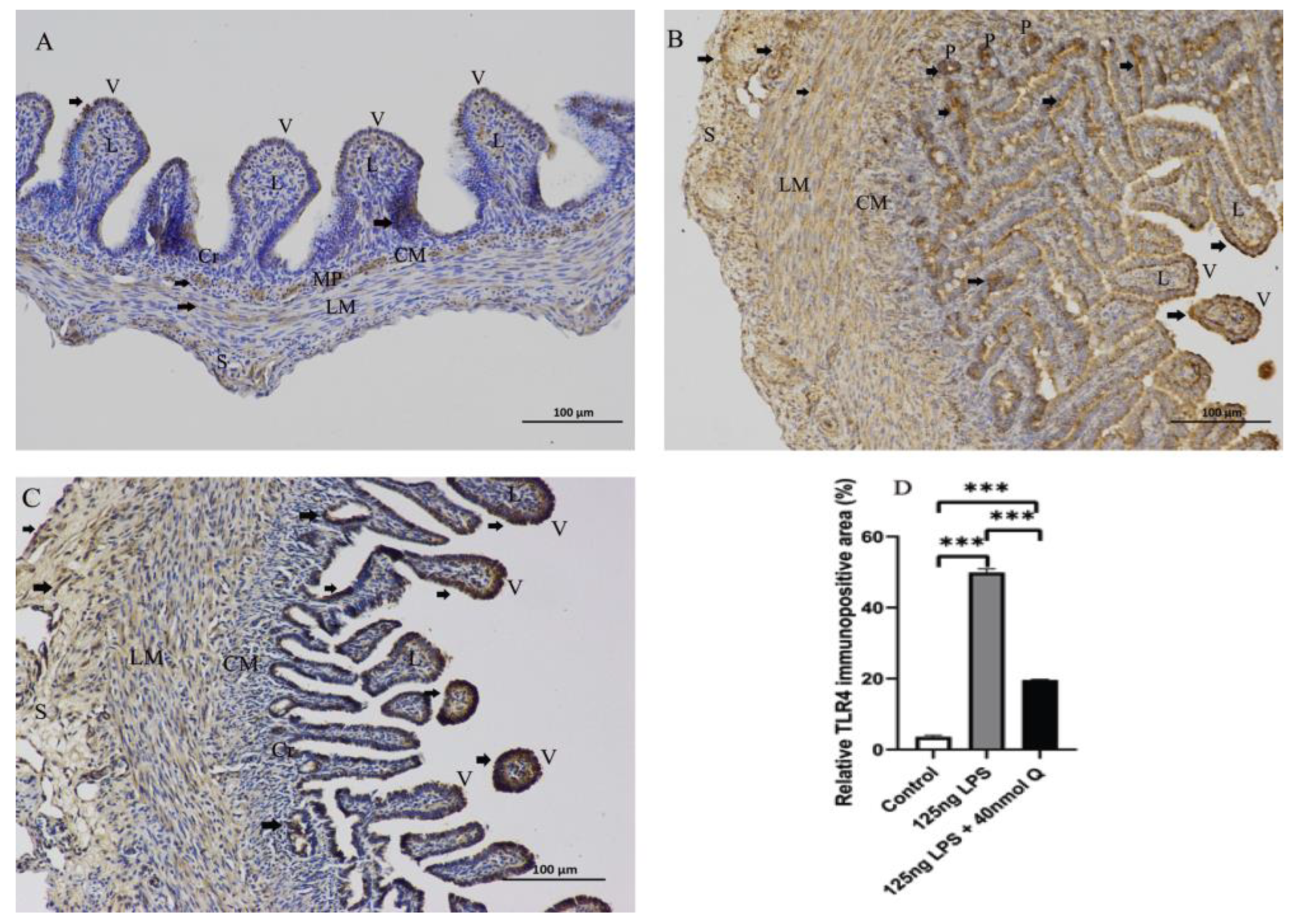
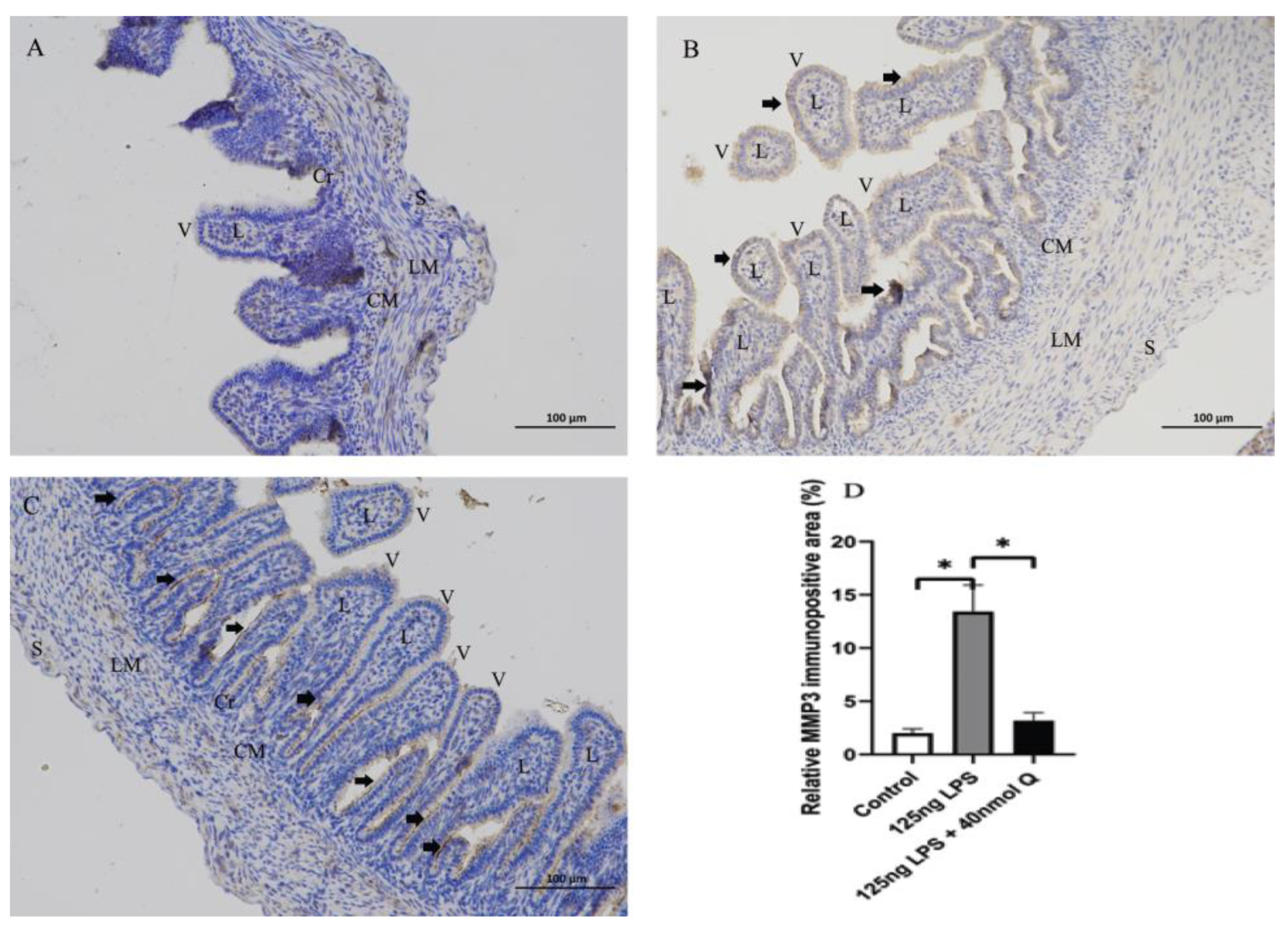
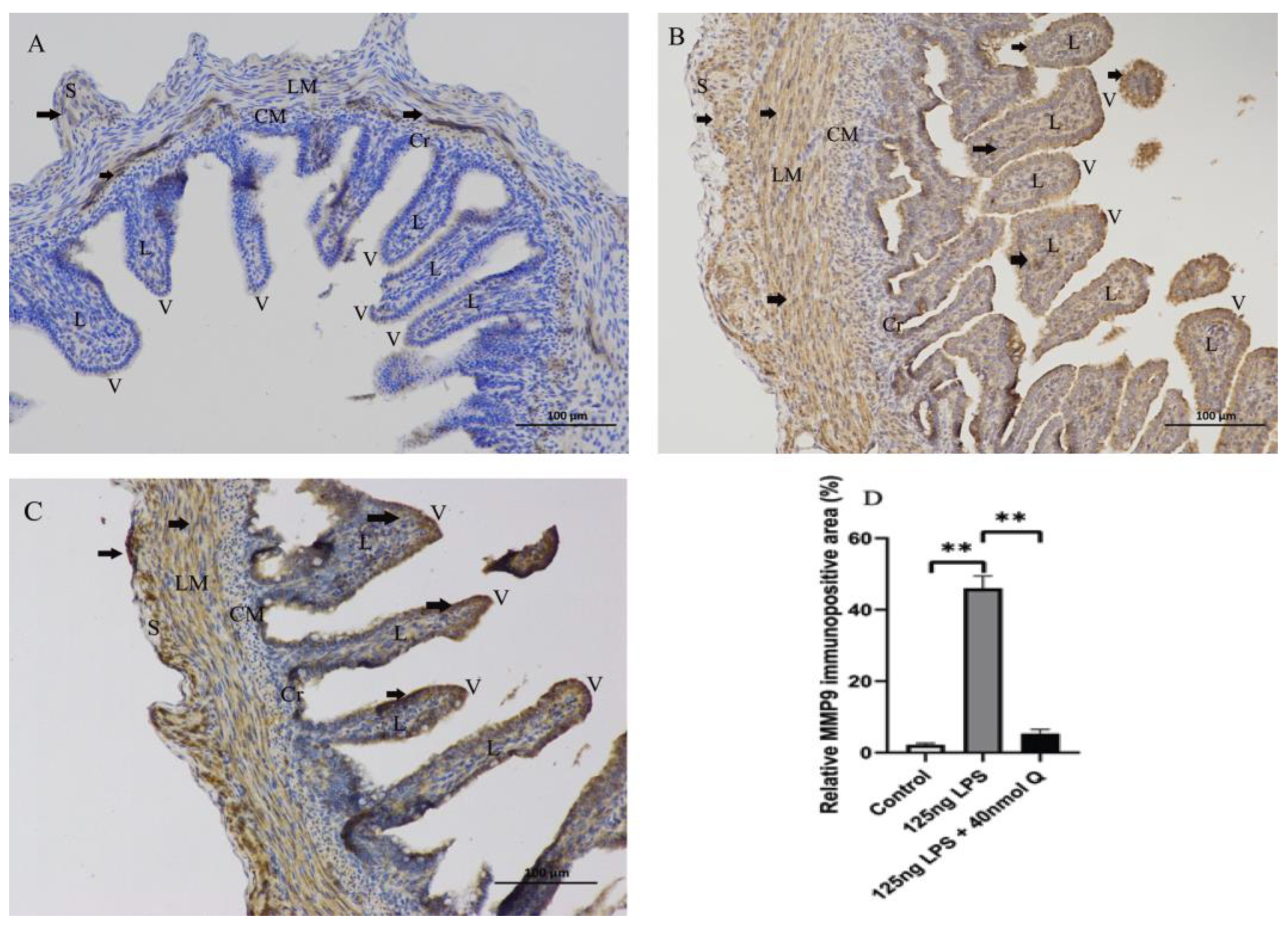
2.6. Immunohistochemistry Investigation
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Bioactive Ingredients and Targets of Chinese Herbal Medicines
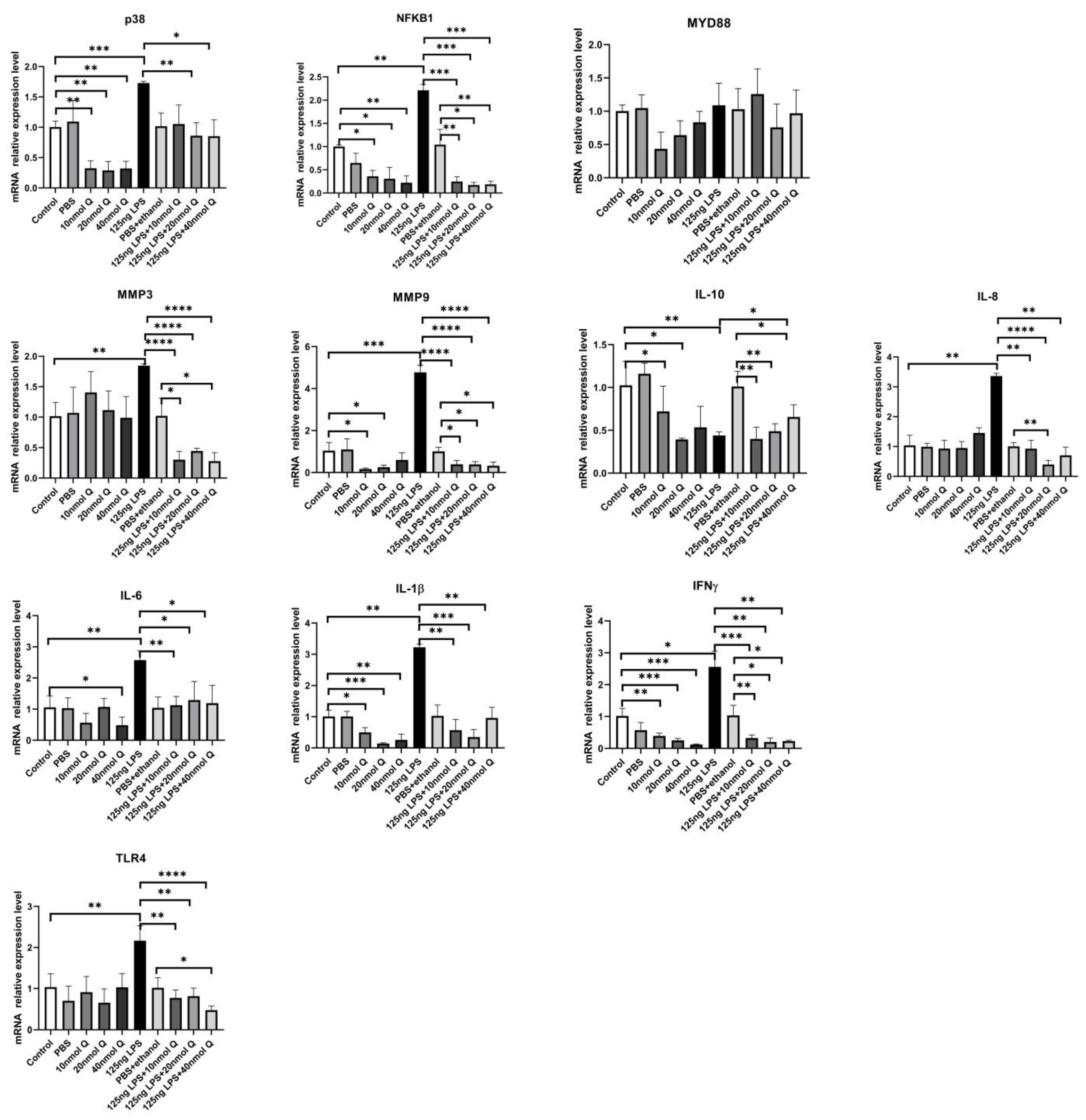
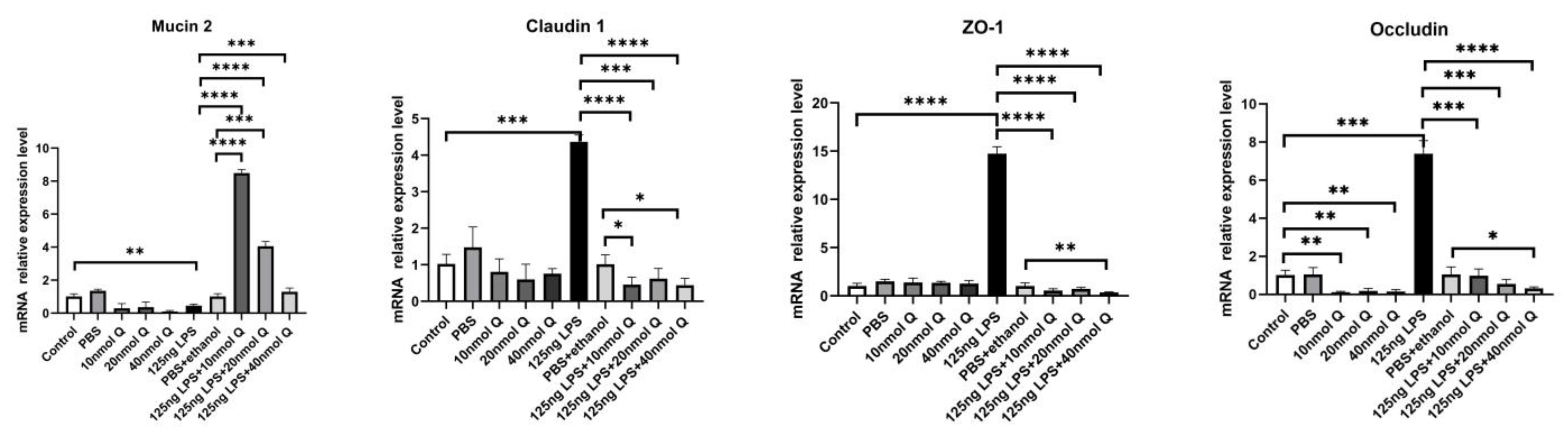
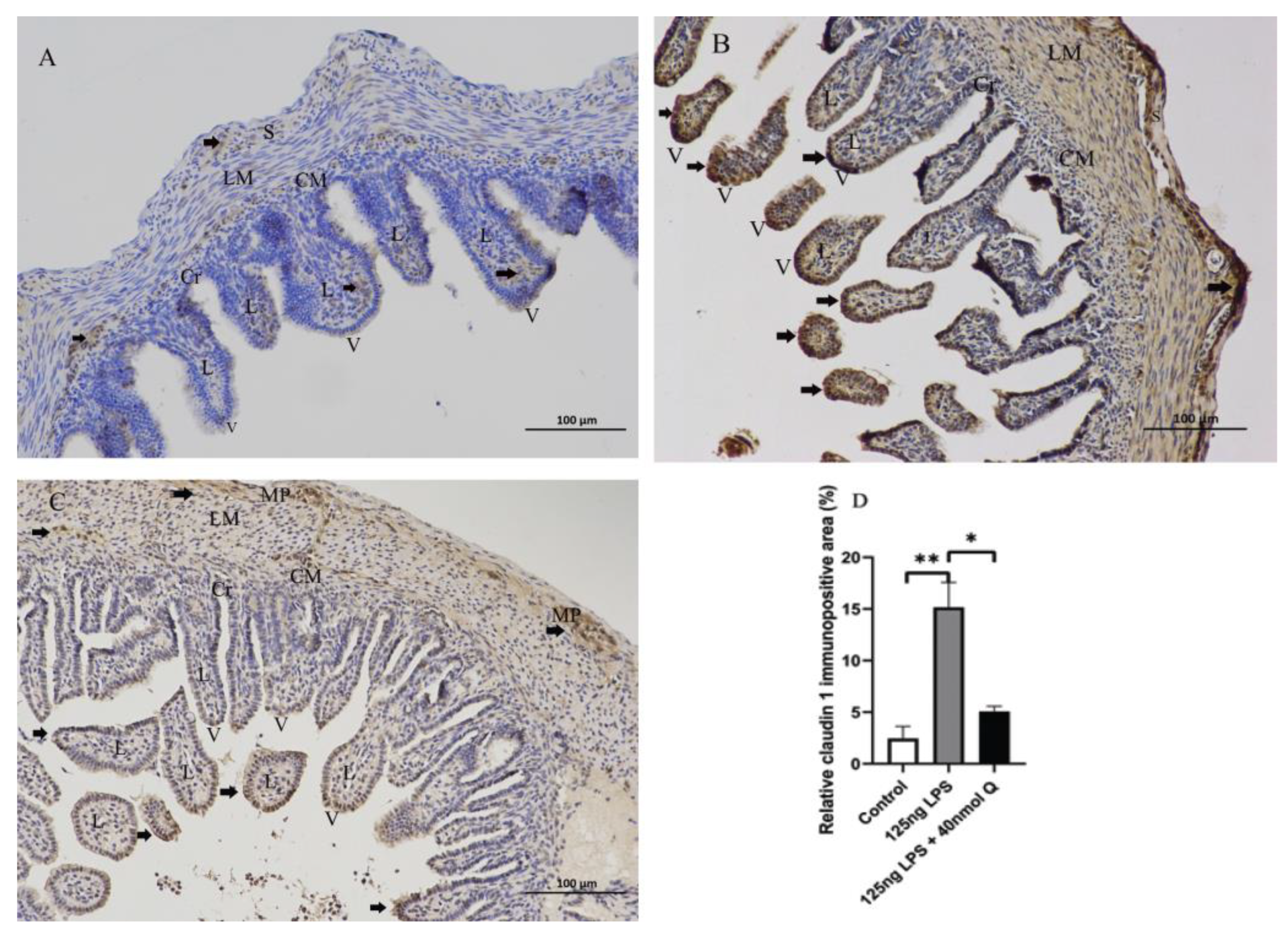
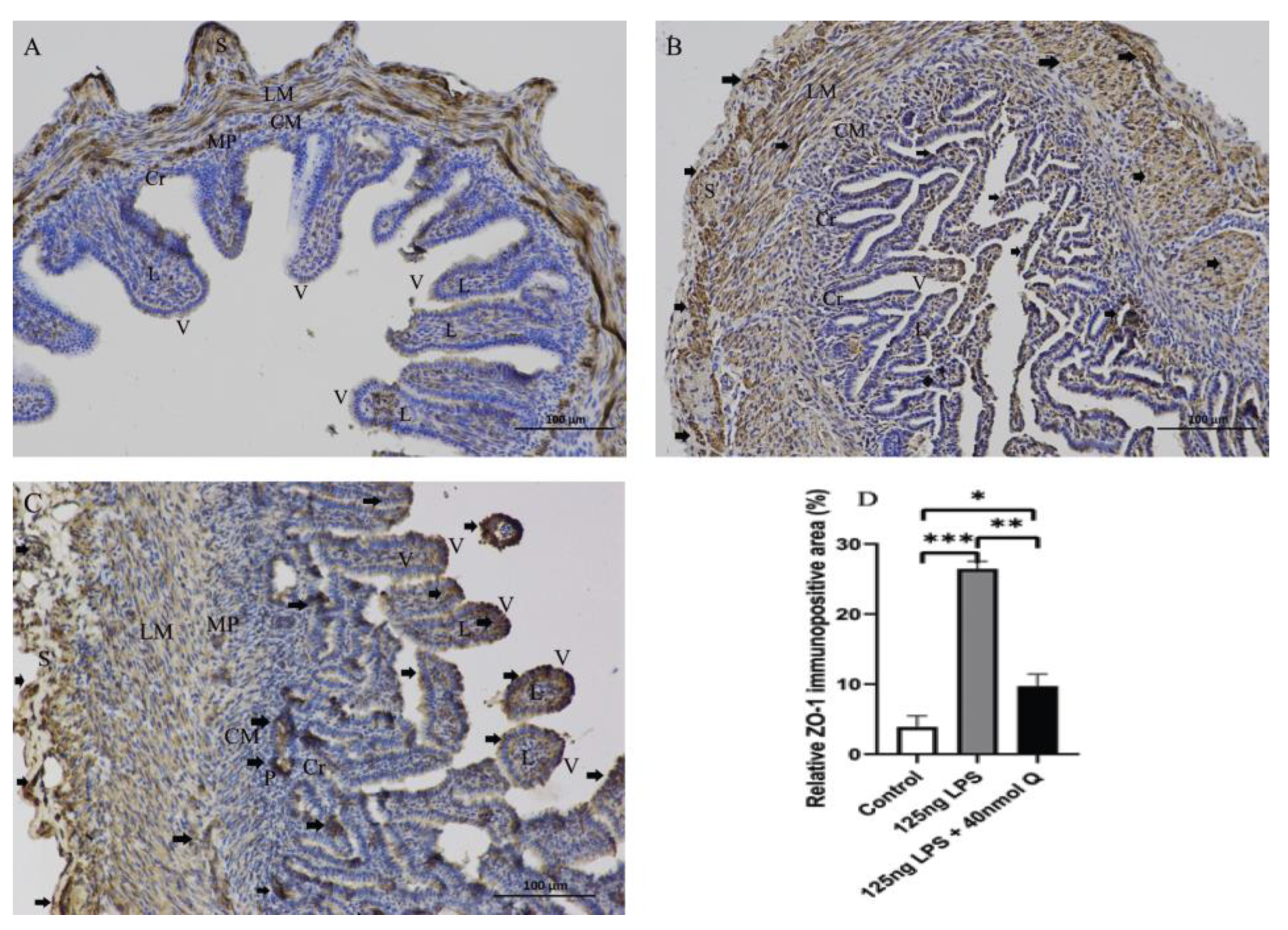
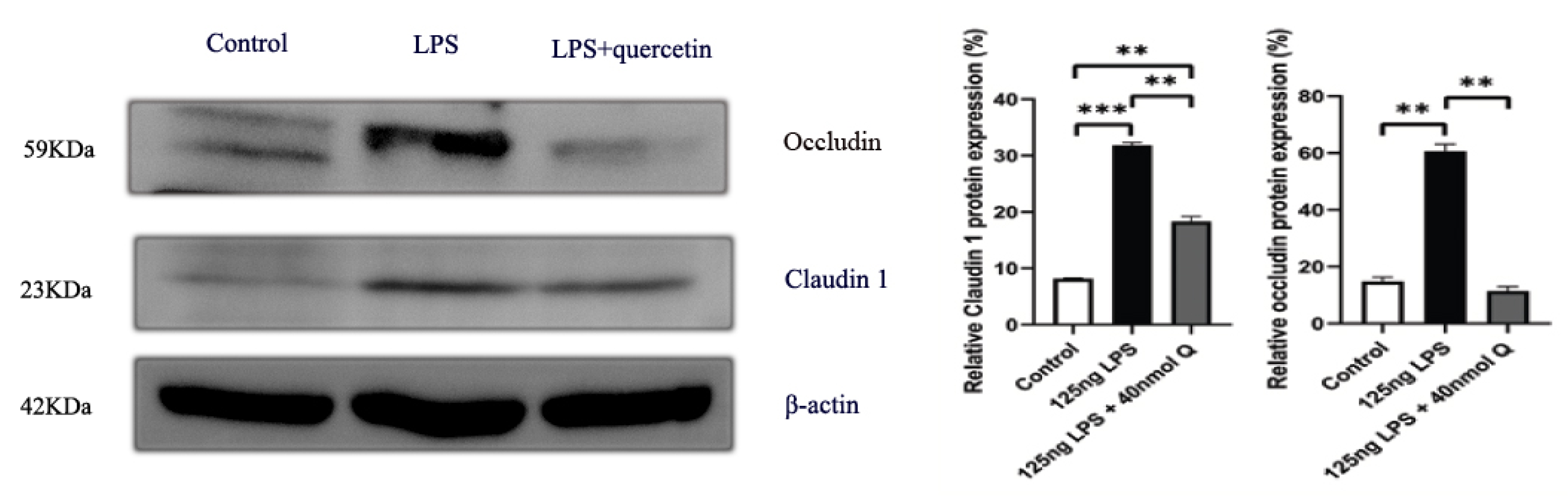
3.2. Effects of Quercetin on Intestinal Mucosa after LPS Induction in Chicken Embryos
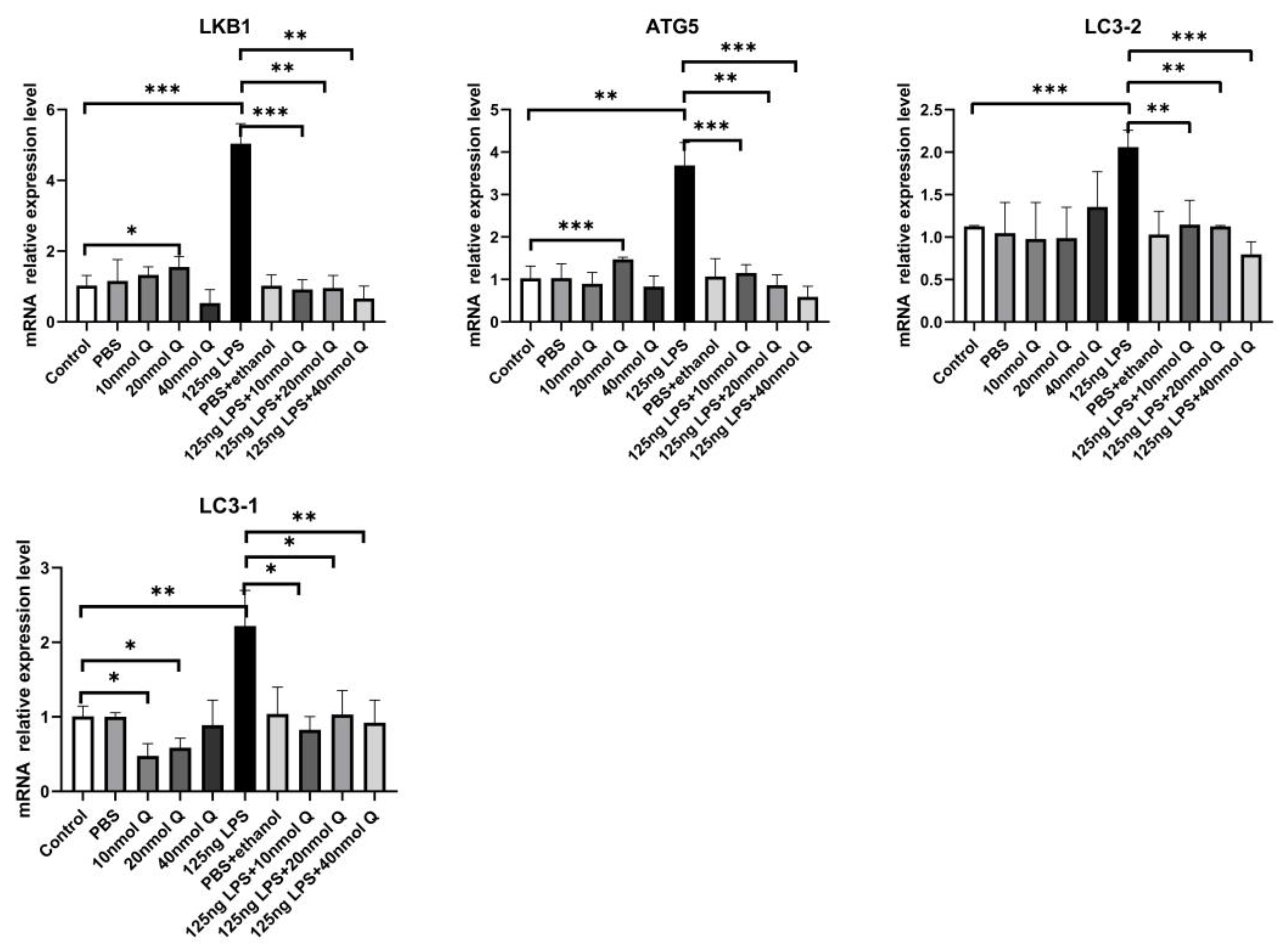
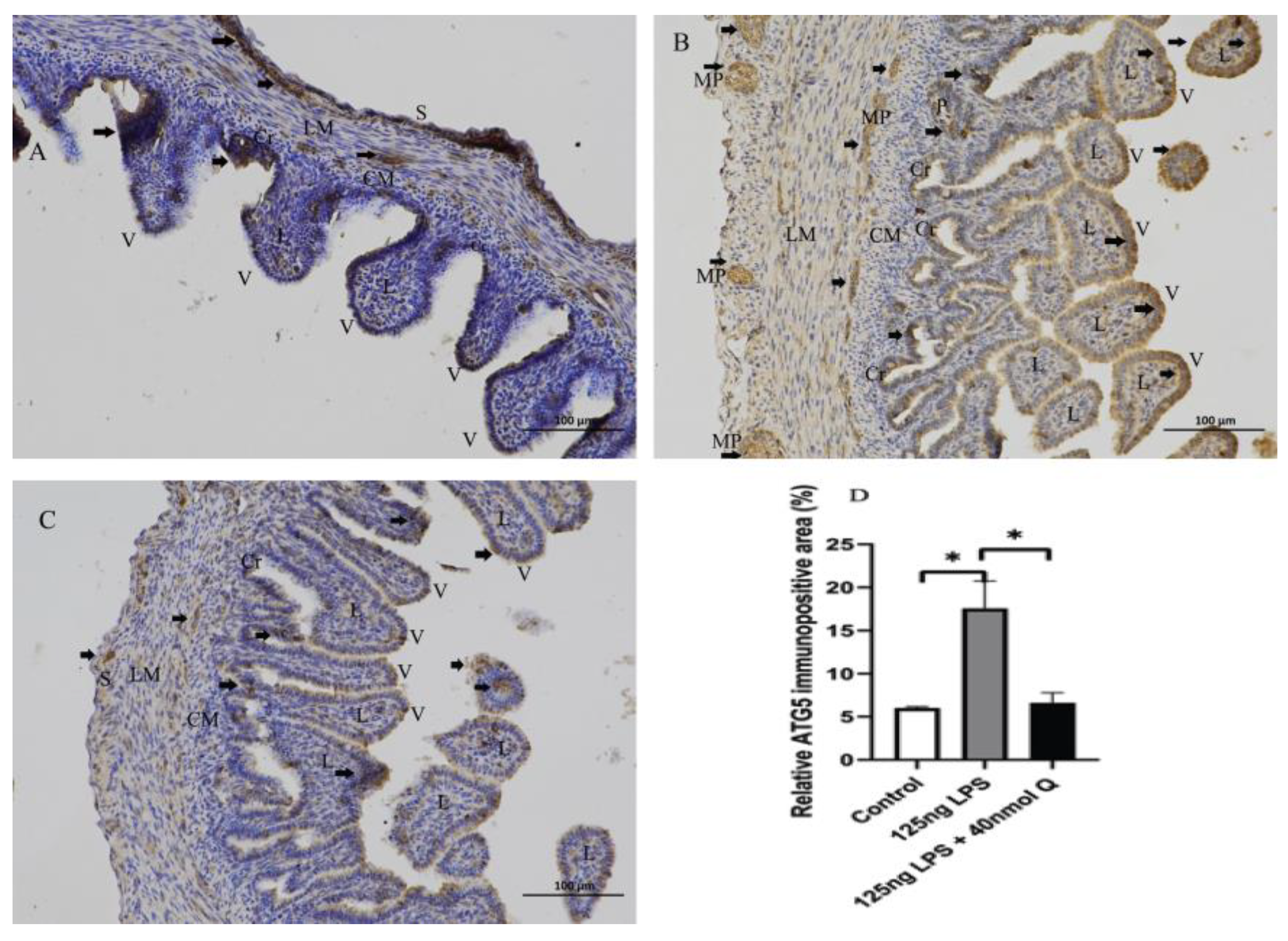
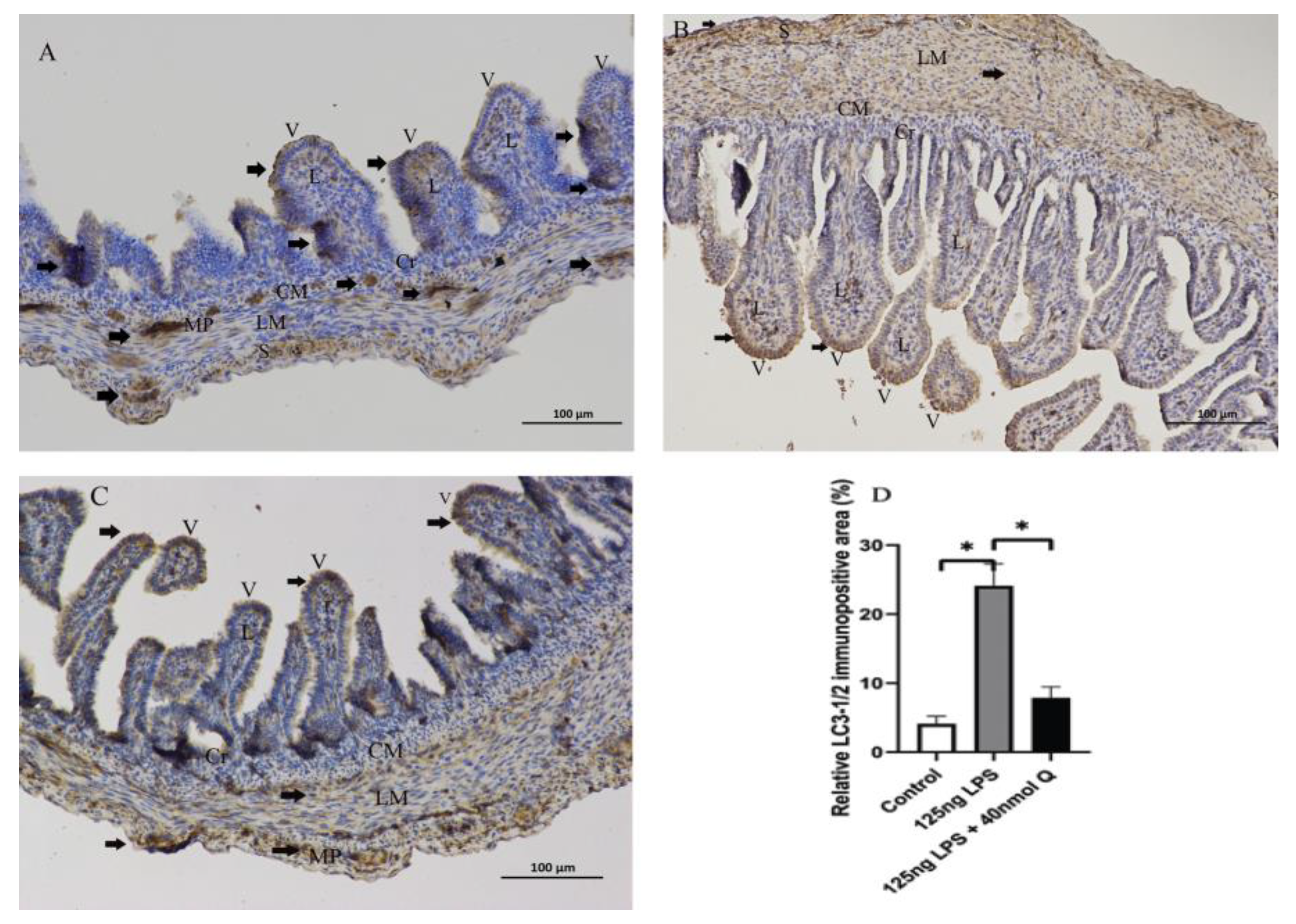
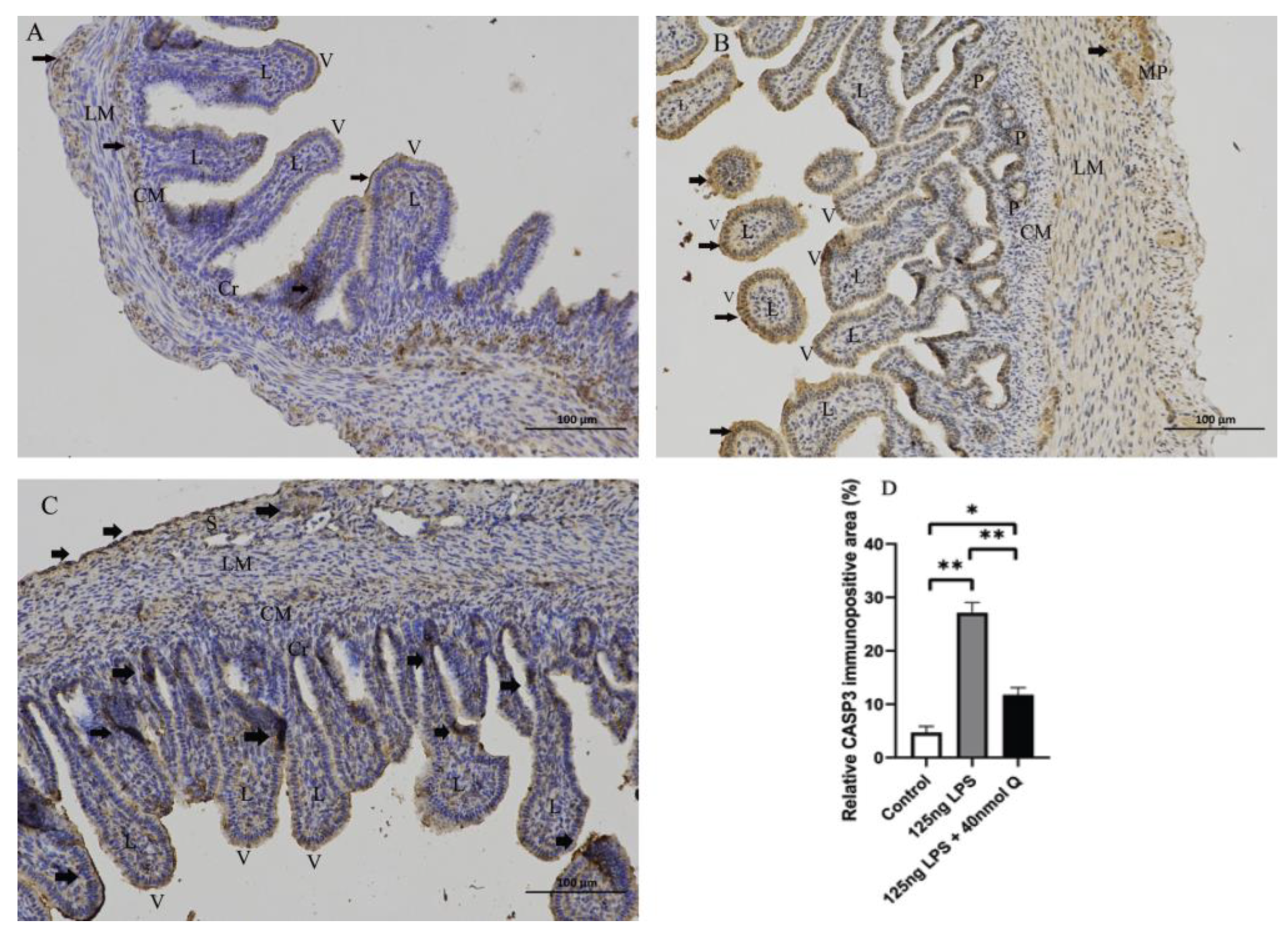
3.3. Quercetin Ameliorates Gut Inflammation through Modulating Autophagy, Programmed Cell Depth, Barrier Function in Chicken Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.J.; Zimsen, S.R.M.; Albertson, S.; Stanaway, J.D.; Deshpande, A.; Brown, A.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Mountzouris, K.C.; Balaskas, C.; Xanthakos, I.; Tzivinikou, A.; Fegeros, K. Effects of a multi-species probiotic on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella enteritidis. Br. Poult. Sci. 2009, 50, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Elbediwi, M.; Tang, Y.; Shi, D.; Ramadan, H.; Xu, Y.; Xu, S.; Li, Y.; Yue, M. Genomic Investigation of Antimicrobial-Resistant Salmonella enterica Isolates From Dead Chick Embryos in China. Front. Microbiol. 2021, 12, 684400. [Google Scholar] [CrossRef] [PubMed]
- Al-Ogaili, A.S.; Hameed, S.S.; Noori, N. LPS-induced NLRP3 gene expression in chicken. Open Vet. J. 2022, 12, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Im, E.; Riegler, F.M.; Pothoulakis, C.; Rhee, S.H. Elevated lipopolysaccharide in the colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G490–G497. [Google Scholar] [CrossRef] [PubMed]
- Manders, T.T.M.; Matthijs, M.G.R.; Veraa, S.; van Eck, J.H.H.; Landman, W.J.M. Success rates of inoculation of the various compartments of embryonated chicken eggs at different incubation days. Avian Pathol. 2021, 50, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Li, Y.; Luo, X.; He, J. Excessive Selenium Supplementation Induced Oxidative Stress and Endoplasmic Reticulum Stress in Chicken Spleen. Biol. Trace. Elem. Res. 2016, 172, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2019, 60, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goliomytis, M.; Tsoureki, D.; Simitzis, P.E.; Charismiadou, M.A.; Hager-Theodorides, A.L.; Deligeorgis, S.G. The effects of quercetin dietary supplementation on broiler growth performance, meat quality, and oxidative stability. Poult. Sci. 2014, 93, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Pappas, A.C.; Goliomytis, M.; Simitzis, P.E.; Sotirakoglou, K.; Tavrizelou, S.; Danezis, G.; Georgiou, C.A. Quercetin and Egg Metallome. Antioxidants 2021, 10, 80. [Google Scholar] [CrossRef]
- Zeng, Y.; Nikitkova, A.; Abdelsalam, H.; Li, J.; Xiao, J. Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch. Oral. Biol. 2019, 98, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhang, L.F.; Xu, J.G. Chemical composition, antibacterial activity and action mechanism of different extracts from hawthorn (Crataegus pinnatifida Bge.). Sci. Rep. 2020, 10, 8876. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.H.; Alghamdi, Y.S.; Mostafa Abdel-Hafez, S.; Soliman, M.M.; Alotaibi, S.H.; Hassan, M.Y.; Hany, N.A.; Amer, H.H. Susceptibility Assessment of Multidrug Resistant Bacteria to Natural Products. Dose Response. 2020, 18, 1559325820936189. [Google Scholar] [CrossRef]
- Givisiez, P.E.N.; Moreira Filho, A.L.B.; Santos, M.R.B.; Oliveira, H.B.; Ferket, P.R.; Oliveira, C.J.B.; Malheiros, R.D. Chicken embryo development: Metabolic and morphological basis for in ovo feeding technology. Poult. Sci. 2020, 99, 6774–6782. [Google Scholar] [CrossRef]
- Ramasamy, K.T.; Verma, P.; Reddy, M.R. Toll-like receptors gene expression in the gastrointestinal tract of Salmonella serovar Pullorum-infected broiler chicken. Appl. Biochem. Biotechnol. 2014, 173, 356–364. [Google Scholar] [CrossRef]
- Li, D.; Lv, B.; Wang, D.; Xu, D.; Qin, S.; Zhang, Y.; Chen, J.; Zhang, W.; Zhang, Z.; Xu, F. Network Pharmacology and Bioactive Equivalence Assessment Integrated Strategy Driven Q-markers Discovery for Da-Cheng-Qi Decoction to Attenuate Intestinal Obstruction. Phytomedicine 2020, 72, 153236. [Google Scholar] [CrossRef]
- Bamias, G.; Cominelli, F. Cytokines and intestinal inflammation. Curr. Opin. Gastroenterol. 2016, 32, 437–442. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, J.M.; Li, X.S.; Li, J. Quercetin ameliorates LPS-induced inflammation in human peripheral blood mononuclear cells by inhibition of the TLR2-NF-kappaB pathway. Genet. Mol. Res. 2016, 15, gmr8297. [Google Scholar]
- Raqib, R.; Wretlind, B.; Andersson, J.; Lindberg, A.A. Cytokine secretion in acute shigellosis is correlated to disease activity and directed more to stool than to plasma. J. Infect. Dis. 1995, 171, 376–384. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Rennick, D.M.; Fort, M.M. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(-/-) mice and intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G829–G833. [Google Scholar] [CrossRef] [PubMed]
- Uribe, J.H.; Collado-Romero, M.; Zaldivar-Lopez, S.; Arce, C.; Bautista, R.; Carvajal, A.; Cirera, S.; Claros, M.G.; Garrido, J.J. Transcriptional analysis of porcine intestinal mucosa infected with Salmonella Typhimurium revealed a massive inflammatory response and disruption of bile acid absorption in ileum. Vet. Res. 2016, 47, 11. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Freeman, S.L.; Hayes, M.P. Inhibition of IL-10 expression by IFN-gamma up-regulates transcription of TNF-alpha in human monocytes. J. Immunol. 1995, 155, 1420–1427. [Google Scholar] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef] [PubMed]
- Keestra, A.M.; van Putten, J.P. Unique properties of the chicken TLR4/MD-2 complex: Selective lipopolysaccharide activation of the MyD88-dependent pathway. J. Immunol. 2008, 181, 4354–4362. [Google Scholar] [CrossRef]
- Karnati, H.K.; Pasupuleti, S.R.; Kandi, R.; Undi, R.B.; Sahu, I.; Kannaki, T.R.; Subbiah, M.; Gutti, R.K. TLR-4 signalling pathway: MyD88 independent pathway up-regulation in chicken breeds upon LPS treatment. Vet. Res. Commun. 2015, 39, 73–78. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Ozkan, E.; Bakar-Ates, F. The Trinity of Matrix Metalloproteinases, Inflammation, and Cancer: A Literature Review of Recent Updates. Antiinflamm. Antiallergy Agents Med. Chem. 2020, 19, 206–221. [Google Scholar] [CrossRef]
- Handley, S.A.; Miller, V.L. General and specific host responses to bacterial infection in Peyer’s patches: A role for stromelysin-1 (matrix metalloproteinase-3) during Salmonella enterica infection. Mol. Microbiol. 2007, 64, 94–110. [Google Scholar] [CrossRef]
- Bai, X.; Bai, G.; Tang, L.; Liu, L.; Li, Y.; Jiang, W. Changes in MMP-2, MMP-9, inflammation, blood coagulation and intestinal mucosal permeability in patients with active ulcerative colitis. Exp. Ther. Med. 2020, 20, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kofla-Dlubacz, A.; Matusiewicz, M.; Krzesiek, E.; Noga, L.; Iwanczak, B. Metalloproteinase-3 and -9 as novel markers in the evaluation of ulcerative colitis activity in children. Adv. Clin. Exp. Med. 2014, 23, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Engers, J.; Haque, M.; King, S.; Al-Omari, D.; Ma, T.Y. Matrix Metalloproteinase-9 (MMP-9) induced disruption of intestinal epithelial tight junction barrier is mediated by NF-kappaB activation. PLoS ONE 2021, 16, e0249544. [Google Scholar] [CrossRef] [PubMed]
- Nighot, P.; Al-Sadi, R.; Rawat, M.; Guo, S.; Watterson, D.M.; Ma, T. Matrix metalloproteinase 9-induced increase in intestinal epithelial tight junction permeability contributes to the severity of experimental DSS colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G988–G997. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, K.; Wen, L.; Yang, Y.; Yin, X.; Liu, K.; Chen, Y.; He, Y.; Yang, M.; Wei, Y.; et al. Autophagy and Gastrointestinal Diseases. Adv. Exp. Med. Biol. 2020, 1207, 529–556. [Google Scholar]
- Wu, Y.; Tang, L.; Wang, B.; Sun, Q.; Zhao, P.; Li, W. The role of autophagy in maintaining intestinal mucosal barrier. J. Cell Physiol. 2019, 234, 19406–19419. [Google Scholar] [CrossRef]
- Haq, S.; Grondin, J.; Banskota, S.; Khan, W.I. Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 2019, 26, 19. [Google Scholar] [CrossRef]
- Cadwell, K.; Patel, K.K.; Komatsu, M.; Virgin, H.W.t.; Stappenbeck, T.S. A common role for Atg16L1, Atg5 and Atg7 in small intestinal Paneth cells and Crohn disease. Autophagy 2009, 5, 250–252. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inatomi, O.; Imai, T.; Kume, S.; Kawahara, M.; Maegawa, H.; Andoh, A. Targeted deletion of Atg5 in intestinal epithelial cells promotes dextran sodium sulfate-induced colitis. J. Clin. Biochem. Nutr. 2021, 68, 156–163. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Saikia, R.; Joseph, J. AMPK: A key regulator of energy stress and calcium-induced autophagy. J. Mol. Med. 2021, 99, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.B.; Xu, J.W.; Xing, T.; Li, J.L.; Zhang, L.; Gao, F. Creatine nitrate supplementation strengthens energy status and delays glycolysis of broiler muscle via inhibition of LKB1/AMPK pathway. Poult. Sci. 2021, 101, 101653. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhou, Y.; Wu, X.; Sun, Y.; Xiao, T.; Gao, Y.; Wang, J. TREM1 Blockade Ameliorates Lipopolysaccharide-Induced Acute Intestinal Dysfunction through Inhibiting Intestinal Apoptosis and Inflammation Response. Biomed. Res. Int. 2021, 2021, 6635452. [Google Scholar] [CrossRef] [PubMed]
- Dupaul-Chicoine, J.; Yeretssian, G.; Doiron, K.; Bergstrom, K.S.; McIntire, C.R.; LeBlanc, P.M.; Meunier, C.; Turbide, C.; Gros, P.; Beauchemin, N.; et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 2010, 32, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Liu, G.; Liu, Q.; Zhou, Y. Swine Influenza Virus Induces RIPK1/DRP1-Mediated Interleukin-1 Beta Production. Viruses 2018, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Buchrieser, J.; Oliva-Martin, M.J.; Moore, M.D.; Long, J.C.D.; Cowley, S.A.; Perez-Simon, J.A.; James, W.; Venero, J.L. RIPK1 is a critical modulator of both tonic and TLR-responsive inflammatory and cell death pathways in human macrophage differentiation. Cell Death Dis. 2018, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Vereecke, L.; Bertrand, M.J.; Duprez, L.; Berger, S.B.; Divert, T.; Goncalves, A.; Sze, M.; Gilbert, B.; Kourula, S.; et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 2014, 513, 95–99. [Google Scholar] [CrossRef]
- Karlowitz, R.; van Wijk, S.J.L. Surviving death: Emerging concepts of RIPK3 and MLKL ubiquitination in the regulation of necroptosis. FEBS J. 2021, 1–18. [Google Scholar] [CrossRef]
- Dannappel, M.; Vlantis, K.; Kumari, S.; Polykratis, A.; Kim, C.; Wachsmuth, L.; Eftychi, C.; Lin, J.; Corona, T.; Hermance, N.; et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014, 513, 90–94. [Google Scholar] [CrossRef]
- Alizadeh, A.; Akbari, P.; Garssen, J.; Fink-Gremmels, J.; Braber, S. Epithelial integrity, junctional complexes, and biomarkers associated with intestinal functions. Tissue Barriers 2022, 10, 1996830. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Kim, J.O.; Kim, K.K. Autophagy-mediated upregulation of cytoplasmic claudin 1 stimulates the degradation of SQSTM1/p62 under starvation. Biochem. Biophys. Res. Commun. 2018, 496, 159–166. [Google Scholar] [CrossRef] [PubMed]





| Botanic Name | English Common Name | Name in Chinese Pinyin | Abbreviation |
|---|---|---|---|
| Folium Artemisiae argyi | Moxa | Aiye | AIYE |
| Paeoniae Radix Alba | White peony root | Baishao | BS |
| Pulsatilliae Radix | Chinese Pulsatilla root | Baitouweng | BTW |
| Platycladi cacumen | Platycladus orientalis leaves | Cebaiye | CBY |
| Radix Bupleuri | Chinese thorowax root | Chaihu | CH |
| Ailanthi Cortex | Bark of ailanthus | Chunpi | CP |
| Radix Sanguisorbae officinalis L. | Garden burnet root | Diyu | DIYU |
| Caryophylli flos, Syzygium aromaticum | Clove | Dingxiang | DX |
| Glycyrrhizae radix et Rhizoma | Licorice | Gancao | GC |
| Alpiniae officinarum Rhizome | Galangal | Gaoliangjiang | GLJ |
| Zanthoxylum bungeanum Maxim. Pericarpium | Pepper pericarp | Huajiao | HJ |
| Coptidis Rhizoma | Coptis root | Huanglian | HL |
| Hedysarum Multijugum Maxim., Astragalus membranaceus | Astragali radix | Huangqi | HQ |
| Scutellariae Radix | Baical skullcap root | Huangqin | HQIN |
| Lonicerae Japonicae Flos | Honeysuckle flower | Jinyinhua | JYH |
| Rosae Laevigatae Fructus | Cherokee rose fruit | Jinyingzi | JYZ |
| Portulacae Herba | Purslane | Machixian | MCX |
| Chaenomeles sinensis (Thouin) Koehne | Pawpaw | Mugua | MG |
| Aucklandiae Radix | Costustoot | Muxiang | MX |
| Equiseti Hiemalis Herba | Scouring rush herb | Muzei | MZ |
| Pollen Typhae | Cattail pollen | Puhuang | PH |
| Panax ginseng C. A. Mey. | Ginseng | Renshen | RS |
| Herba Taxilli | Chinese taxillus twig | Sangjisheng | SJS |
| Granati Pericarpium | Pomegranate bark | Shiliupi | SLP |
| Crataegi folium | Hawthorn leaf | Shanzhaye | SZY |
| Mume Fructus | Dark plum fruit | Wumei | WM |
| Evodiae fructus | Evodia fruit | Wuzhuyu | WZY |
| Leonuri Herba | Motherwort | Yimucao | YMC |
| Genes Name | Primer Sequence (5′-3′) | Gene Bank ID |
|---|---|---|
| ATG5 | F:CCCATCCCTGGTCCGTAAC; R:CGGCGGCGTATACGAAGTA | NM_001006409 |
| Bcl-2 | F: TGGCTGCTTTACTCTTGGGG; R:TATCTCGCGGTTGTCGTAGC | NM_205339 |
| CASP1 | F:CACTTCCACTTCGGATGGCT; R:CCACGAGACAGTATCAGGCG | XM_015295935 |
| CASP3 | F:ACCGAGATACCGGACTGTCA; R:GCCATGGCTTAGCAACACAC | NM_204725 |
| CASP12 | F:AATAGTGGGCATCTGGGTCA; R:CGGTGTGATTTAGACCCGTAAGAC | [7] |
| Claudin 1 | F:CTGGGTCTGGTTGGTGTGTT; R:CGAGCCACTCTGTTGCCATA | NM_001013611 |
| Drp1 | F: GGCAGTCACAGCAGCTAACA; R:GCATCCATGAGATCCAGCTT | NM_001079722 |
| Fas | F:GTCAGTGCTGCACGAAATGT; R:AACCTCCAAACCGAGTGCTT | NM_001199487 |
| GAPDH | F: GAGAAACCAGCCAAGTATGATG; R: CACAGGAGACAACCTGGTCC | NM_204305 |
| IFNγ | F:CTGACAAGTCAAAGCCGCAC; R:CTTCACGCCATCAGGAAGGT | NM_205149 |
| IL-10 | F:TGCGAGAAGAGGAGCAAAGC; R:AACTCCCCCATGGCTTTGTAG | AJ621254 |
| IL-1β | F:GCTCAACATTGCGCTGTACC; R:AGGCGGTAGAAGATGAAGCG | FJ537850 |
| IL-6 | F:ACGAGGAGAAATGCCTGACG; R:CTTCAGATTGGCGAGGAGGG | NM_204628 |
| IL-8 | F:TGCCAGTGCATTAGCACTCA; R:TTGGCGTCAGCTTCACATCT | HM179639 |
| LC3-1 | F:GCATCCAAACAAAATCCCAGTC; R:AAGCCATCCTCATCCTTCTCCT | XM_040688401 |
| LC3-2 | F:CTTCTTCCTCCTGGTGAACG; R:GCACTCCGAAAGTCTCCTGA | NM_001031461 |
| LKB1 | F:AGCAGAGGCATTGCATCCAT; R:CCTGCGGACCAGATGTCTAC | NM_001045833 |
| MMP9 | F:ACACAGACTCTATGCTGCCTG; R:GAGAGTAGGGCGGGGAAAAT | NM_204667 |
| MMP3 | F:ATCAGGCTCTACAGTGGTG; R:ATGGGATACATCAAGGCAC | XM_025152201 |
| Mucin 2 | F:TCAGATCAGATGGCAGTGTGTC; R:AATCTGCAGCTGAAGCCCAAA | JX284122 |
| MYD88 | F:TTAGTCTTTCCCCAGGGGCT; R:GCCAGTCTTGTCCAGAACCA | NM_001030962 |
| NFKB1 | F:TCAACGCAGGACCTAAAGACAT; R:GCAGATAGCCAAGTTCAGGATG | NM_001396396 |
| Occludin | F:TACATCATGGGCGTCAACCC; R:CCAGATCTTACTGCGCGTCT | NM_205128 |
| p38 | F:GGTCGGTGAGCTGGTAAAGG; R:CGCTTTCAGCTTCTGTCGGA | XM_015296032 |
| RIPK1 | F:GATCCATTTGCGAAGCTGCC; R:CTTAGGCTAATGGCGCTGGT | NM_204402 |
| TLR4 | F:GGCTCAACCTCACGTTGGTA; R:AGTCCGTTCTGAAATGCCGT | KP410249 |
| ZO-1 | F:TATGAAGATCGTGCGCCTCC; R:GAGGTCTGCCATCGTAGCTC | XM_015278977 |
| TNFα | F:CCCATCCCTGGTCCGTAAC; R:CGGCGGCGTATACGAAGTA | MF000729 |
| Gene ID | Gene Name Abbreviation | Gene Name |
|---|---|---|
| 5243 | ABCB1 | ATP Binding Cassette Subfamily B Member 1) |
| 135 | ADORA2A | Adenosine A2a Receptor |
| 196 | AHR | Aryl Hydrocarbon Receptor |
| 207 | AKT1 | AKT Serine/Threonine Kinase 1 |
| 240 | ALOX5 | Arachidonate 5-Lipoxygenase |
| 3577 | CXCR1 | C-X-C Motif Chemokine Receptor 1 |
| 1956 | EGFR | Epidermal Growth Factor Receptor |
| 2100 | ESR2 | Estrogen Receptor 2 |
| 4314 | MMP3 | Matrix Metallopeptidase 3 |
| 4318 | MMP9 | Matrix Metallopeptidase 9 |
| 4350 | MPG | N-Methylpurine DNA Glycosylase |
| 6850 | SYK | Spleen Associated Tyrosine Kinase |
| 7015 | TERT | Telomerase Reverse Transcriptase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Hu, G.; Cao, H.; Guo, X. Quercetin Ameliorates Lipopolysaccharide-Induced Duodenal Inflammation through Modulating Autophagy, Programmed Cell Death and Intestinal Mucosal Barrier Function in Chicken Embryos. Animals 2022, 12, 3524. https://doi.org/10.3390/ani12243524
Yu J, Hu G, Cao H, Guo X. Quercetin Ameliorates Lipopolysaccharide-Induced Duodenal Inflammation through Modulating Autophagy, Programmed Cell Death and Intestinal Mucosal Barrier Function in Chicken Embryos. Animals. 2022; 12(24):3524. https://doi.org/10.3390/ani12243524
Chicago/Turabian StyleYu, Jinhai, Guoliang Hu, Huabin Cao, and Xiaoquan Guo. 2022. "Quercetin Ameliorates Lipopolysaccharide-Induced Duodenal Inflammation through Modulating Autophagy, Programmed Cell Death and Intestinal Mucosal Barrier Function in Chicken Embryos" Animals 12, no. 24: 3524. https://doi.org/10.3390/ani12243524
APA StyleYu, J., Hu, G., Cao, H., & Guo, X. (2022). Quercetin Ameliorates Lipopolysaccharide-Induced Duodenal Inflammation through Modulating Autophagy, Programmed Cell Death and Intestinal Mucosal Barrier Function in Chicken Embryos. Animals, 12(24), 3524. https://doi.org/10.3390/ani12243524






