Multidrug-Resistant and Genetic Characterization of Extended-Spectrum Beta-Lactamase-Producing E. coli Recovered from Chickens and Humans in Egypt
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Identification
2.3. Antimicrobial Susceptibility Pattern and ESBL Screening of the Isolated E. coli
2.3.1. Antimicrobial Sensitivity Test (AST)
2.3.2. Double Disc Synergy Test (DDST)
2.4. Genotypic Characterizations of ESBL
3. Results
3.1. E. coli Isolation, Identification, and Serotyping
3.2. Antimicrobial Susceptibility Pattern of the Isolated E. coli
3.2.1. Antimicrobial Sensitivity Test (AST)
3.2.2. ESBL Screening Test
3.2.3. Double Disc Synergy Test (DDST)
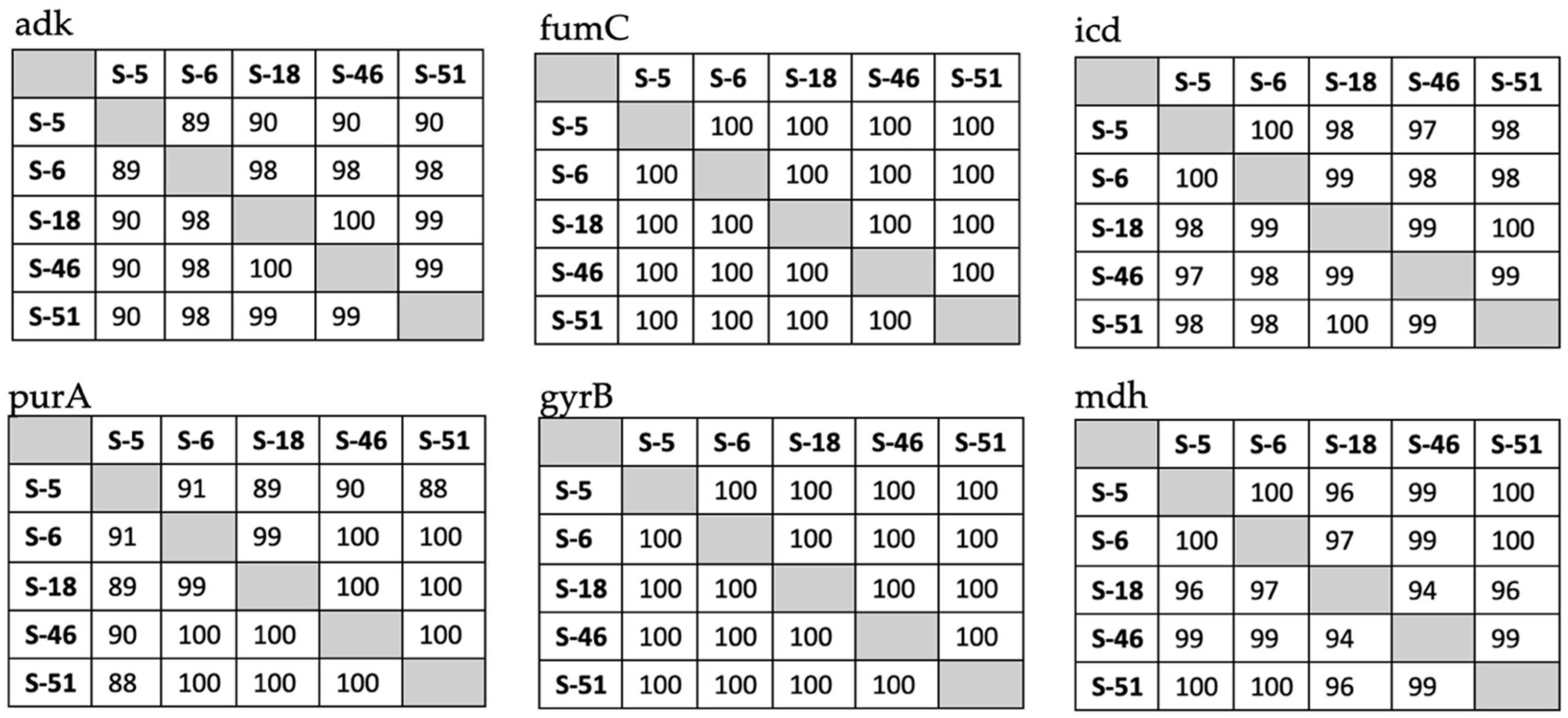
3.3. Molecular Detection and Identity Matrices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Percival, S.L.; Williams, D.W. Chapter Six—Escherichia coli. In Microbiology of Waterborne Diseases, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: London, UK, 2014; pp. 89–117. [Google Scholar]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Giufrè, M.; Mazzolini, E.; Cerquetti, M.; Brusaferro, S. Extended-spectrum β-lactamase-producing Escherichia coli from extraintestinal infections in humans and from food-producing animals in Italy: A ‘One Health’ study. Int. J. Antimicrob. Agents 2021, 58, 106433. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, M.V.; Liakopoulos, A.; Brouwer, M.; Kant, A.; Pizauro, L.J.L.; Borzi, M.M.; Mevius, D.; de Ávila, F.A. Occurrence and Molecular Characteristics of Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Recovered From Chicken, Chicken Meat, and Human Infections in Sao Paulo State, Brazil. Front. Microbiol. 2021, 12, 628738. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- White, A.; Hughes, J.M. Critical Importance of a One Health Approach to Antimicrobial Resistance. EcoHealth 2019, 16, 404–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and One Health approaches. Proc. Biol. Sci. 2018, 285. [Google Scholar] [CrossRef]
- Coppola, N.; Freire, B.; Umpiérrez, A.; Cordeiro, N.F.; Ávila, P.; Trenchi, G.; Castro, G.; Casaux, M.L.; Fraga, M.; Zunino, P.; et al. Transferable Resistance to Highest Priority Critically Important Antibiotics for Human Health in Escherichia coli Strains Obtained From Livestock Feces in Uruguay. Front. Vet. Sci. 2020, 7, 588919. [Google Scholar] [CrossRef] [PubMed]
- Bubpamala, J.; Khuntayaporn, P.; Thirapanmethee, K.; Montakantikul, P.; Santanirand, P.; Chomnawang, M.T. Phenotypic and genotypic characterizations of extended-spectrum beta-lactamase-producing Escherichia coli in Thailand. Infect. Drug Resist. 2018, 11, 2151–2157. [Google Scholar] [CrossRef] [Green Version]
- Chishimba, K.; Hang’Ombe, B.; Muzandu, K.; Mshana, S.; Matee, M.; Nakajima, C.; Suzuki, Y. Detection of extended-spectrum beta-lactamase-producing Escherichia coli in market-ready chickens in Zambia. Int. J. Microbiol. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Falgenhauer, L.; Imirzalioglu, C.; Oppong, K.; Akenten, C.W.; Hogan, B.; Krumkamp, R.; Poppert, S.; Levermann, V.; Schwengers, O.; Sarpong, N. Detection and characterization of ESBL-producing Escherichia coli from humans and poultry in Ghana. Front. Microbiol. 2019, 9, 3358. [Google Scholar] [CrossRef] [Green Version]
- Eibach, D.; Dekker, D.; Boahen, K.G.; Akenten, C.W.; Sarpong, N.; Campos, C.B.; Berneking, L.; Aepfelbacher, M.; Krumkamp, R.; Owusu-Dabo, E. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in local and imported poultry meat in Ghana. Vet. Microbiol. 2018, 217, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Aschenbrenner, K.; Stamm, I.; Bethe, A.; Semmler, T.; Stubbe, A.; Stubbe, M.; Batsajkhan, N.; Glupczynski, Y.; Wieler, L.H.; et al. Comparable high rates of extended-spectrum-beta-lactamase-producing Escherichia coli in birds of prey from Germany and Mongolia. PLoS ONE 2012, 7, e53039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluytmans, J.A.; Overdevest, I.T.; Willemsen, I.; Kluytmans-van den Bergh, M.F.; van der Zwaluw, K.; Heck, M.; Rijnsburger, M.; Vandenbroucke-Grauls, C.M.; Savelkoul, P.H.; Johnston, B.D.; et al. Extended-spectrum β-lactamase-producing Escherichia coli from retail chicken meat and humans: Comparison of strains, plasmids, resistance genes, and virulence factors. Clin. Infect. Dis. 2013, 56, 478–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kola, A.; Kohler, C.; Pfeifer, Y.; Schwab, F.; Kühn, K.; Schulz, K.; Balau, V.; Breitbach, K.; Bast, A.; Witte, W.; et al. High prevalence of extended-spectrum-β-lactamase-producing Enterobacteriaceae in organic and conventional retail chicken meat, Germany. J. Antimicrob. Chemother. 2012, 67, 2631–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leverstein-van Hall, M.A.; Dierikx, C.M.; Cohen Stuart, J.; Voets, G.M.; van den Munckhof, M.P.; van Essen-Zandbergen, A.; Platteel, T.; Fluit, A.C.; van de Sande-Bruinsma, N.; Scharinga, J.; et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin. Microbiol. Infect. 2011, 17, 873–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moawad, A.A.; Hotzel, H.; Neubauer, H.; Ehricht, R.; Monecke, S.; Tomaso, H.; Hafez, H.M.; Roesler, U.; El-Adawy, H. Antimicrobial resistance in Enterobacteriaceae from healthy broilers in Egypt: Emergence of colistin-resistant and extended-spectrum β-lactamase-producing Escherichia coli. Gut. Pathog. 2018, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Husna, A.; Elshabrawy, H.A.; Alam, J.; Runa, N.Y.; Badruzzaman, A.T.M.; Banu, N.A.; Al Mamun, M.; Paul, B.; Das, S.; et al. Isolation and molecular characterization of multidrug-resistant Escherichia coli from chicken meat. Sci. Rep. 2020, 10, 21999. [Google Scholar] [CrossRef]
- Nolan, L.; Barnes, H.; Vaillancourt, J.; Abdul-Aziz, T.; Logue, C. Diseases of Poultry, 13th ed.; Swayne, D.E., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- World Health Organization. Manual for the Laboratory Identification and Antimicrobial Susceptibility Testing of Bacterial Pathogens of Public Health Importance in the Developing World: Haemophilus influenzae, Neisseria meningitidis, Streptococcus pneumoniae, Neisseria gonorrhoea, Salmonella serotype Typhi, Shigella, and Vibrio cholerae / Principal authors: Mindy J. Perilla … [et al.]; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2021. [Google Scholar]
- Anago, E.; Ayi-Fanou, L.; Akpovi, C.D.; Hounkpe, W.B.; Agassounon-Djikpo Tchibozo, M.; Bankole, H.S.; Sanni, A. Antibiotic resistance and genotype of beta-lactamase producing Escherichia coli in nosocomial infections in Cotonou, Benin. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Ryoo, N.H.; Kim, E.C.; Hong, S.G.; Park, Y.J.; Lee, K.; Bae, I.K.; Song, E.H.; Jeong, S.H. Dissemination of SHV-12 and CTX-M-type extended-spectrum beta-lactamases among clinical isolates of Escherichia coli and Klebsiella pneumoniae and emergence of GES-3 in Korea. J. Antimicrob. Chemother. 2005, 56, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.; Ochman, H.; et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [Green Version]
- Hosuru Subramanya, S.; Bairy, I.; Nayak, N.; Padukone, S.; Sathian, B.; Gokhale, S. Low rate of gut colonization by extended-spectrum β-lactamase producing Enterobacteriaceae in HIV infected persons as compared to healthy individuals in Nepal. PLoS ONE 2019, 14, e0212042. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ashworth, A.J.; Willett, C.; Cook, K.; Upadhyay, A.; Owens, P.R.; Ricke, S.C.; DeBruyn, J.M.; Moore, P.A., Jr. Review of Antibiotic Resistance, Ecology, Dissemination, and Mitigation in U.S. Broiler Poultry Systems. Front. Microbiol. 2019, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.D.; Ahmed, M.F.; El-Adawy, H.; Hotzel, H.; Engelmann, I.; Weiß, D.; Monecke, S.; Ehricht, R. Surveillance of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli in Dairy Cattle Farms in the Nile Delta, Egypt. Front. Microbiol. 2016, 7, 1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanin, F.S.; Hassan, M.A.; Shaltout, F.A.; Shawqy, N.A.; Abd-Elhameed, G.A. Bacteriological criteria of chicken giblets. Benha Vet. Med. J. 2017, 33, 447–456. [Google Scholar] [CrossRef]
- Al-Agamy, M.H. Phenotypic and molecular characterization of extended-spectrum β-lactamases and AmpC β-lactamases in Klebsiella pneumoniae. Pak. J. Pharm. Sci. 2013, 26. [Google Scholar]
- Benklaouz, M.B.; Aggad, H.; Benameur, Q. Resistance to multiple first-line antibiotics among Escherichia coli from poultry in Western Algeria. Vet. World 2020, 13, 290. [Google Scholar] [CrossRef]
- Aworh, M.K.; Kwaga, J.; Okolocha, E.; Harden, L.; Hull, D.; Hendriksen, R.S.; Thakur, S. Extended-spectrum ß-lactamase-producing Escherichia coli among humans, chickens and poultry environments in Abuja, Nigeria. One Health Outlook 2020, 2, 8. [Google Scholar] [CrossRef]
- Parvin, M.S.; Talukder, S.; Ali, M.Y.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Antimicrobial Resistance Pattern of Escherichia coli Isolated from Frozen Chicken Meat in Bangladesh. Pathogens 2020, 9, 420. [Google Scholar] [CrossRef]
- Gundran, R.S.; Cardenio, P.A.; Villanueva, M.A.; Sison, F.B.; Benigno, C.C.; Kreausukon, K.; Pichpol, D.; Punyapornwithaya, V. Prevalence and distribution of bla(CTX-M), bla(SHV), bla(TEM) genes in extended- spectrum β- lactamase- producing E. coli isolates from broiler farms in the Philippines. BMC Vet. Res. 2019, 15, 227. [Google Scholar] [CrossRef]
- Seo, K.W.; Kim, Y.B.; Jeon, H.Y.; Lim, S.K.; Lee, Y.J. Comparative genetic characterization of third-generation cephalosporin-resistant Escherichia coli from chicken meat produced by integrated broiler operations in South Korea. Poult. Sci. 2018, 97, 2871–2879. [Google Scholar] [CrossRef]
- Kawamura, K.; Goto, K.; Nakane, K.; Arakawa, Y. Molecular epidemiology of extended-spectrum β-lactamases and Escherichia coli isolated from retail foods including chicken meat in Japan. Foodborne Pathog. Dis. 2014, 11, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Younas, S.; Abosalif, K.O.A.; Junaid, K.; Alzahrani, B.; Alsrhani, A.; Abdalla, A.E.; Ullah, M.I.; Qamar, M.U.; Hamam, S.S.M. Molecular analysis of blaSHV, blaTEM, and blaCTX-M in extended-spectrum β-lactamase producing Enterobacteriaceae recovered from fecal specimens of animals. PLoS ONE 2021, 16, e0245126. [Google Scholar] [CrossRef] [PubMed]
- Valentin, L.; Sharp, H.; Hille, K.; Seibt, U.; Fischer, J.; Pfeifer, Y.; Michael, G.B.; Nickel, S.; Schmiedel, J.; Falgenhauer, L.; et al. Subgrouping of ESBL-producing Escherichia coli from animal and human sources: An approach to quantify the distribution of ESBL types between different reservoirs. Int. J. Med. Microbiol. 2014, 304, 805–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.M.; Shimamoto, T.; Shimamoto, T. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int. J. Med. Microbiol. 2013, 303, 475–483. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, D.; Nasef, S.; Mahmoud, F.; Jonas, D. Expanded spectrum β–lactamase producing Escherichia coli isolated from chickens with colibacillosis in Egypt. Poult. Sci. 2017, 96, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Locatelli, A.; Amoureux, L.; Depret, G.; Jolivet, C.; Gueneau, E.; Neuwirth, C. Occurrence of CTX-M Producing Escherichia coli in Soils, Cattle, and Farm Environment in France (Burgundy Region). Front. Microbiol. 2012, 3, 83. [Google Scholar] [CrossRef] [Green Version]
- Dahms, C.; Hübner, N.O.; Kossow, A.; Mellmann, A.; Dittmann, K.; Kramer, A. Occurrence of ESBL-Producing Escherichia coli in Livestock and Farm Workers in Mecklenburg-Western Pomerania, Germany. PLoS ONE 2015, 10, e0143326. [Google Scholar] [CrossRef] [PubMed]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K.; et al. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216–1222. [Google Scholar] [CrossRef]
- Doi, Y.; Paterson, D.L.; Egea, P.; Pascual, A.; López-Cerero, L.; Navarro, M.D.; Adams-Haduch, J.M.; Qureshi, Z.A.; Sidjabat, H.E.; Rodríguez-Baño, J. Extended-spectrum and CMY-type beta-lactamase-producing Escherichia coli in clinical samples and retail meat from Pittsburgh, USA and Seville, Spain. Clin. Microbiol. Infect. 2010, 16, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 1181–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Nagar, A.; Ibrahim, A. Case study of the Egyptian poultry sector. In Proceedings of the International Poultry Conference, Bangkok, Thailand, 5–7 November 2007. [Google Scholar]

| Gene | Sequence (5′–3′) | Temperatures of Annealing Step (°C) | Product Size | References |
|---|---|---|---|---|
| blaTeM-F blaTeM-R | ATG AGT ATT CAA CAT TTC CGT TTA CCA ATG CTT AAT CAG TGA | 58 | 861 bp | [23] |
| blaVeB-F blaVeB-R | GCC AGA ATA GGA GTA GCA AT TGG ACT CTG CAA CAA ATA CG | 58 | 703 bp | [9] |
| blaOXa2-F blaOXa2-R | ATG GCA ATC CGA ATC TTC GC GCA CGA TTG CCT CCC TCT T | 60 | 670 bp | [9] |
| blaOXa10-F blaOXa10-R | ATG AAA ACA TTT GCC GCA TAT G TTA GCC ACC AAT GAT GCC CT | 60 | 801 bp | [9] |
| blages-F blages-R | TAC TGG CAG SGA TCG CTC AC TTG TCC GTG CTC AGG ATG AG | 62 | 838 bp | [9] |
| blaPeR-F blaPeR-R | CTC AGC GCA ATC CCC ACT GT TTG GGC TTA GGG CAG AAA GCT | 62 | 851 bp | [9] |
| blashV-F blashV-R | CGC CTG TGT ATT ATC TCC CTG TTA GCG TTG CCA GTG CTC GAT | 64 | 849 bp | [9] |
| blaCTX-M 1-F blaCTX-M 1-R | AGT TCA CGC TGA TGG CGA CG GAC GAT TTT AGC CGC CGA CG | 67 | 839 bp | [9] |
| blaCTX-M 9-F blaCTX-M 9-R | GCG TGC ATT CCG CTG CTG C ACA GCC CTT CGG CGA TGA TTC | 67 | 832 bp | [9] |
| Antimicrobial Agent | Resistant No. (%) 1 | Intermediate No. (%) 1 | Sensitive No. (%) 1 | |||
|---|---|---|---|---|---|---|
| Poultry (n = 56) | Human (n = 9) | Poultry (n = 56) | Human (n = 9) | Poultry (n = 56) | Human (n = 9) | |
| Amoxicillin-clavulanic acid (AMC30) | 54 (96.4%) | 3 (33.3%) | 2 (3.6%) | 1 (11.1%) | 0 | 5 (55.6%) |
| Ampicillin (AMP10) | 52 (92.9%) | 9 (100%) | 4 (7.1%) | 0 (0%) | 0 | 0 (0%) |
| Aztreonam (ATM30) | 18 (32.1%) | 2 (22.2%) | 6 (10.7%) | 2 (22.2%) | 32 (57.2%) | 5 (55.6%) |
| Cefepime (FEP30) | 20 (35.7%) | 3 (33.3%) | 24 (42.9%) | 3 (33.3%) | 12 (21.4%) | 3 (33.3%) |
| Cefotaxime (CTX30) | 43 (76.8%) | 5 (55.6%) | 9 (16.1%) | 2 (22.2%) | 4 (7.1%) | 2 (22.2%) |
| Ceftazidime (CAZ30) | 30 (53.6%) | 4 (44.4%) | 16 (28.6%) | 4 (44.4%) | 10 (17.8%) | 1 (11.1%) |
| Ceftriaxone (CRO30) | 25 (44.7%) | 3 (33.3%) | 11 (19.6%) | 0 (0%) | 20 (35.7%) | 6 (66.7%) |
| Cephalexin (CL30) | 56 (100%) | 9 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Cephalothin (KF30) | 56 (100%) | 9 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ciprofloxacin (CIP5) | 37 (66.1%) | 1 (11.1%) | 7 (12.5%) | 0 (0%) | 12 (21.4%) | 8 (88.9%) |
| Colistin sulphate (CT10) | 23 (41.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 33 (58.9%) | 9 (100%) |
| Imipenem (IPM10) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 56 (100%) | 9 (100%) |
| Norfloxacin (NOR10) | 37 (66.1%) | 1 (11.1%) | 4 (7.1%) | 0 (0%) | 15 (26.8%) | 8 (88.9%) |
| Sulfamethoxazole-trimethoprim (SXT25) | 46 (82.1%) | 5 (55.6%) | 1 (1.8%) | 0 (0%) | 9 (16.1%) | 4 (44.4%) |
| Antibiotic Disc for ESBL Screening Test | Interpretation of Conduct ESBL-Testing | ESBL Production Screening | |
|---|---|---|---|
| Poultry (%) 1 | Human (%) 1 | ||
| Aztreonam (ATM30) | ≤27 mm | 48 (85.7%) | 9 (100%) |
| Cefotaxime (CTX30) | ≤27 mm | 53 (94.6%) | 9 (100%) |
| Ceftazidime (CAZ30) | ≤22 mm | 48 (85.7%) | 8 (88.9%) |
| Ceftriaxone (CRO30) | ≤25 mm | 44 (78.6%) | 5 (55.6%) |
| Antibiotic Disc for ESBL Screening Test | ESBL Production Confirmation | |
|---|---|---|
| Poultry (%) 1 | Human (%) 1 | |
| Cefepime (CPM30) | 22 (39.3%) | 8 (88.9%) |
| Cefotaxime (CTX30) | 22 (39.3%) | 7 (77.8%) |
| Ceftazidime (CAZ30) | 20 (35.7%) | 6 (66.7%) |
| Ceftriaxone (CRO30) | 28 (50%) | 8 (88.9%) |
| Antibiotic Resistance Genes | PCR Result: Positive Result/Total Examined Isolates (%) | |
|---|---|---|
| Poultry | Human | |
| blaTEM | 33/56 (58.9%) | 0/9 (0%) |
| blaVEB | 7/56 (12.5%) | 0/9 (0%) |
| blaOXA group 2 | 1/56 (1.8%) | 0/9 (0%) |
| blaOXA group 10 | 9/56 (16.1%) | 0/9 (0%) |
| blaGES | 10/56 (17.9%) | 2/9 (22.2%) |
| blaPER | 27/56 (48.2%) | 0/9 (0%) |
| blaSHV | 14/56 (25%) | 0/9 (0%) |
| blaCTX-M group 1 | 19/56 (33.9%) | 9/9 (100%) |
| blaCTX-M group 9 | 36/56 (65.3%) | 9/9 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, H.; Reda, R.M.; Hagag, N.M.; Kamel, E.; Elnomrosy, S.M.; Mansour, A.I.; Shahein, M.A.; Ali, S.F.; Ali, H.R. Multidrug-Resistant and Genetic Characterization of Extended-Spectrum Beta-Lactamase-Producing E. coli Recovered from Chickens and Humans in Egypt. Animals 2022, 12, 346. https://doi.org/10.3390/ani12030346
Badr H, Reda RM, Hagag NM, Kamel E, Elnomrosy SM, Mansour AI, Shahein MA, Ali SF, Ali HR. Multidrug-Resistant and Genetic Characterization of Extended-Spectrum Beta-Lactamase-Producing E. coli Recovered from Chickens and Humans in Egypt. Animals. 2022; 12(3):346. https://doi.org/10.3390/ani12030346
Chicago/Turabian StyleBadr, Heba, Reem M. Reda, Naglaa M. Hagag, Essam Kamel, Sara M. Elnomrosy, Amal I. Mansour, Momtaz A. Shahein, Samah F. Ali, and Hala R. Ali. 2022. "Multidrug-Resistant and Genetic Characterization of Extended-Spectrum Beta-Lactamase-Producing E. coli Recovered from Chickens and Humans in Egypt" Animals 12, no. 3: 346. https://doi.org/10.3390/ani12030346
APA StyleBadr, H., Reda, R. M., Hagag, N. M., Kamel, E., Elnomrosy, S. M., Mansour, A. I., Shahein, M. A., Ali, S. F., & Ali, H. R. (2022). Multidrug-Resistant and Genetic Characterization of Extended-Spectrum Beta-Lactamase-Producing E. coli Recovered from Chickens and Humans in Egypt. Animals, 12(3), 346. https://doi.org/10.3390/ani12030346








