Association of Hard Ticks (Ixodidae) Infestation with Milk Production and Udder Health of Extensively Reared Dairy Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Herds and Animals
2.2. Data Collection
2.3. Data Handling
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Effect of Tick Infestation on Goat Productivity and Udder Health
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eskezia, B.G.; Desta, A.H. Review on the impact of ticks on livestock health and productivity. J. Biol. Agric. Healthc. 2016, 6, 1–7. [Google Scholar]
- Gelasakis, A.I.; Valergakis, G.E.; Arsenos, G. Health and welfare of indigenous goat breeds from dairy farms in Greece. In Sustainable Goat Production in Adverse Environments: Volume I; Simões, J., Gutiérrez, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 223–246. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Venzal, J.M. Climate niches of tick species in the Mediterranean region: Modelling of occurrence data, distributional constraints, and impact of climate change. J. Med. Entomol. 2006, 44, 1130–1138. [Google Scholar] [CrossRef]
- Estrada-Peña, A. Tick-borne pathogens, transmission rates and climate change. Front. Biosci. 2009, 14, 2674–2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organisation. Module 1. Ticks: Acaricide Resistance: Diagnosis, Management and Prevention. In Guidelines Resistance Management and Integrated Parasite Control in Ruminants; FAO: Rome, Italy, 2004; pp. 25–77. Available online: http://www.fao.org/tempref/docrep/fao/010/ag014e/ag014e05.pdf (accessed on 30 April 2020).
- Hurtado, O.J.B.; Giraldo-Ríos, C. Economic and health impact of the ticks in production animals. In Ticks and Tick-Borne Pathogens; Abubakar, M., Perera, P.K., Eds.; IntechOpen: London, UK, 2018; pp. 1–19. [Google Scholar] [CrossRef] [Green Version]
- Inokuma, H.; Kerlin, R.L.; Kemp, D.H.; Willadsen, P. Effects of cattle tick (Boophilus microplus) infestation on the bovine immune system. Vet. Parasitol. 1993, 47, 107–118. [Google Scholar] [CrossRef]
- Norval, R.A.I.; Sutherst, R.W.; Kurki, J.; Kerr, J.D.; Gibson, J.D. The effects of the brown-ear tick, Rhipicephalus appendiculatus, on milk production of Sanga cattle. Med. Vet. Entomol. 1997, 11, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Norval, R.A.I.; Sutherst, R.W.; Gibson, J.D.; Kerr, J.D.; Thorne, L.M.; Ellenhauge, A. The effects of the brown-ear tick, Rhipicephalus appendiculatus, on milk production in dairy cattle. Med. Vet. Entomol. 1997, 11, 155–158. [Google Scholar] [CrossRef]
- Jonsson, N.N.; Mayer, D.G.; Matschoss, A.L.; Green, P.E.; Ansell, J. Production effects of cattle tick (Boophilus microplus) infestation on high yielding dairy cows. Vet. Parasitol. 1998, 78, 65–77. [Google Scholar] [CrossRef]
- Jonsson, N.N. The productivity effects of cattle tick (Boophilus microplus) infestation on cattle, with particular reference to Bos indicus cattle and their crosses. Vet. Parasitol. 2006, 137, 1–10. [Google Scholar] [CrossRef]
- Moges, N.; Hailemariam, T.; Fentahun, T.; Chanie, M.; Melaku, A. Bovine mastitis and associated risk factors in small holder lactating dairy farms in Hawassa, Southern Ethiopia. Glob. Vet. 2012, 9, 441–446. [Google Scholar] [CrossRef]
- Rodrigues, D.S.; Leite, R.C. Economic impact of Rhipicephalus (Boophilus) microplus: Estimate of decreased milk production on a dairy farm. Arq. Bras. Med. Vet. Zootec. 2013, 65, 1570–1572. [Google Scholar] [CrossRef] [Green Version]
- Heath, A.C.G.; Pearce, D.M.; Tenquist, J.D.; Cole, D.J.W. Some effects of a tick infestation (Haemaphysalis longicornis) on sheep. J. Agric. Res. 1977, 20, 19–22. [Google Scholar] [CrossRef]
- Al-Hosary, A.A.T.; Ellah, M.R.A.; Ahmed, L.S.E.D. Evaluation of Oxidative Stress in Sheep Infested with Ticks and Concurrent Diagnosis of Theileriosis. Asian J. Anim. Vet. Adv. 2018, 13, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Ghafar, A.; Abbas, T.; Rehman, A.; Sandhu, Z.U.D.; Cabezas-Cruz, A.; Jabbar, A. Systematic review of ticks and tick-borne pathogens of small ruminants in Pakistan. Pathogens 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Perveen, N.; Muzafar, S.B.; Al-Deeb, M.A. Ticks and tick-borne diseases of livestock in the Middle East and North Africa: A review. Insects 2021, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Sanhokwe, M.; Mupangwa, J.; Masika, P.J.; Maphosa, V.; Muchenje, V. Medicinal plants used to control internal and external parasites in goats. Onderstepoort J. Vet. Res. 2016, 83, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Soundararajan, C.; Nagarajan, K.; Muthukrishnan, S.; Prakash, M.A. Tick infestation on sheep, goat, horse and wild hare in Tamil Nadu. J. Parasit Dis. 2018, 42, 127–129. [Google Scholar] [CrossRef]
- Soundararajan, C.; Latha, B.R.; Pandian, A. Prevalence of tick infestation in goats under different system of management. Int. J. Agric. Sci. Vet. Med. 2014, 2, 4–9. [Google Scholar]
- Dimanopoulou, A.P.; Starras, A.G.; Diakou, A.; Lefkaditis, M.; Giadinis, N.D. Prevalence of tick species in sheep and goat flocks in areas of southern Greece. J. Hell. Vet. Med. Soc. 2017, 68, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Matei, I.A.; Ionicӑ, A.M.; Corduneanu, A.; Domşa, C.; Sándor, A.D. The presence of ticks collected from ungulates in continental Eastern Europe. J. Vet. Res. 2021, 65, 71–275. [Google Scholar] [CrossRef]
- Alessandra, T.; Santo, C. Tick-borne diseases in sheep and goats: Clinical and diagnostic aspects. Small Rumin. Res. 2012, 106, 6–11. [Google Scholar] [CrossRef]
- Yin, H.; Luo, J. Ticks of small ruminants in China. Parasitol. Res. 2007, 101, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Goats Population—Annual Data. Available online: http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=apro_mt_lsgoat&lang=en (accessed on 12 April 2020).
- Gelasakis, A.I.; Rose, G.; Giannakou, R.; Valergakis, G.E.; Theodoridis, A.; Fortomaris, P.; Arsenos, G. Typology and characteristics of dairy goat production systems in Greece. Livest. Sci. 2017, 197, 22–29. [Google Scholar] [CrossRef]
- Papazahariadou, M.G.; Papadopoulos, E.G.; Himonas, C.A. Seasonal activity of ixodid ticks on goats in northern Greece. Vet. Rec. 1995, 136, 586–588. [Google Scholar]
- Pavlidou, V.; Gerou, S.; Kahrimanidou, M.; Papa, A. Ticks infesting domestic animals in Northern Greece. Exp. Appl. Acarol. 2008, 45, 195–198. [Google Scholar] [CrossRef]
- Chaligiannis, I.; Papa, A.; Sotiraki, S. Ticks feeding on ruminants and humans in Greece. Parasites Vectors 2014, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Chaligiannis, I.; Musella, V.; Rinaldi, L.; Cringoli, G.; de la Fuente, J.; Papa, A.; Sotiraki, S. Species diversity and spatial distribution of ixodid ticks on small ruminants in Greece. Parasitol. Res. 2016, 115, 4673–4680. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation 2016/885 of 3 June 2016. Amending Regulation (EU) No 37/2010 as Regards the Substance “Eprinomectin” (Text with EEA Relevance). OJ L 148. 2016, pp. 1–3. Available online: http://data.europa.eu/eli/reg_impl/2016/885/oj (accessed on 1 March 2021).
- Rostang, A.; Devos, J.; Chartier, C. Review of the Eprinomectin effective doses required for dairy goats: Where do we go from here? Vet. Parasitol. 2020, 277, 108992. [Google Scholar] [CrossRef]
- Papadopoulos, B.; Morel, P.C.; Aeschlimann, A. Ticks of domestic animals in the Macedonia region of Greece. Vet. Parasitol. 1996, 63, 25–40. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Angelidis, A.S.; Giannakou, R.; Filioussis, G.; Kalamaki, M.S.; Arsenos, G. Bacterial subclinical mastitis and its effect on milk yield in low-input dairy goat herds. J. Dairy Sci. 2016, 99, 3698–3708. [Google Scholar] [CrossRef] [Green Version]
- Gelasakis, A.I.; Angelidis, A.; Giannakou, R.; Arsenos, G. Bacterial subclinical mastitis and its effect on milk quality traits in low-input dairy goat herds. Vet. Rec. 2018, 183, 449. [Google Scholar] [CrossRef]
- International Committee for Animal Recording. ICAR Recording Guidelines. International Agreement of Recording Practices. Available online: http://www.icar.org/wp-content/uploads/2016/Guidelines-Edition-2016.pdf/ (accessed on 7 June 2018).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 30 December 2021).
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification; Springer: New York, NY, USA, 2017; pp. 75–404. [Google Scholar]
- Norval, R.A.I.; Sutherst, R.W.; Jorgensen, O.G.; Kerr, J.D. The effects of the bont tick Amblyomma hebraeum, on milk production of Sanga and Sanga × Brahman cattle. Med. Vet. Entomol. 1997, 11, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Siddique, F.; Mahmood, M.S.; Shamim, A.; Zafar, T.; Rasheed, I.; Saleem, I.; Ahmad, W. Prevalence and impact of ectoparasitic fauna infesting goats (Capra hircus) of district Toba Tek Singh, Punjab, Pakistan. Glob. Vet. 2014, 12, 158–164. [Google Scholar] [CrossRef]
- White, E.C.; Hinckley, L.S. Prevalence of mastitis pathogens in goat milk. Small Rumin. Res. 1999, 33, 117–121. [Google Scholar] [CrossRef]
- Al-Rubaie, M.A. Isolation and Characterization of Klebsiella spp. and Staphylococcus aureus from Engorged Adult Females of Rhipicephalus spp. J. Pure Appl. Microbiol. 2019, 13, 1763–1767. [Google Scholar] [CrossRef] [Green Version]
- Andreotti, R.; de León, A.A.P.; Dowd, S.E.; Guerrero, F.D.; Bendele, K.G.; Scoles, G.A. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirecci, E.; Wasan, M.S.; Metin, T.U.; Bawar, A.M.; Omer, A.N.; Rızgar, M.K. Isolation and identification of tick-borne bacterial pathogens in Turkey and Iraq. Afr. J. Microbiol. Res. 2015, 9, 1608–1612. [Google Scholar] [CrossRef] [Green Version]
- Bergonier, D.; de Crémoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef] [Green Version]
- Koop, G.; van Werven, T.; Schuiling, H.J.; Nielen, M. The effect of subclinical mastitis on milk yield in dairy goats. J. Dairy Sci. 2010, 93, 5809–5817. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]


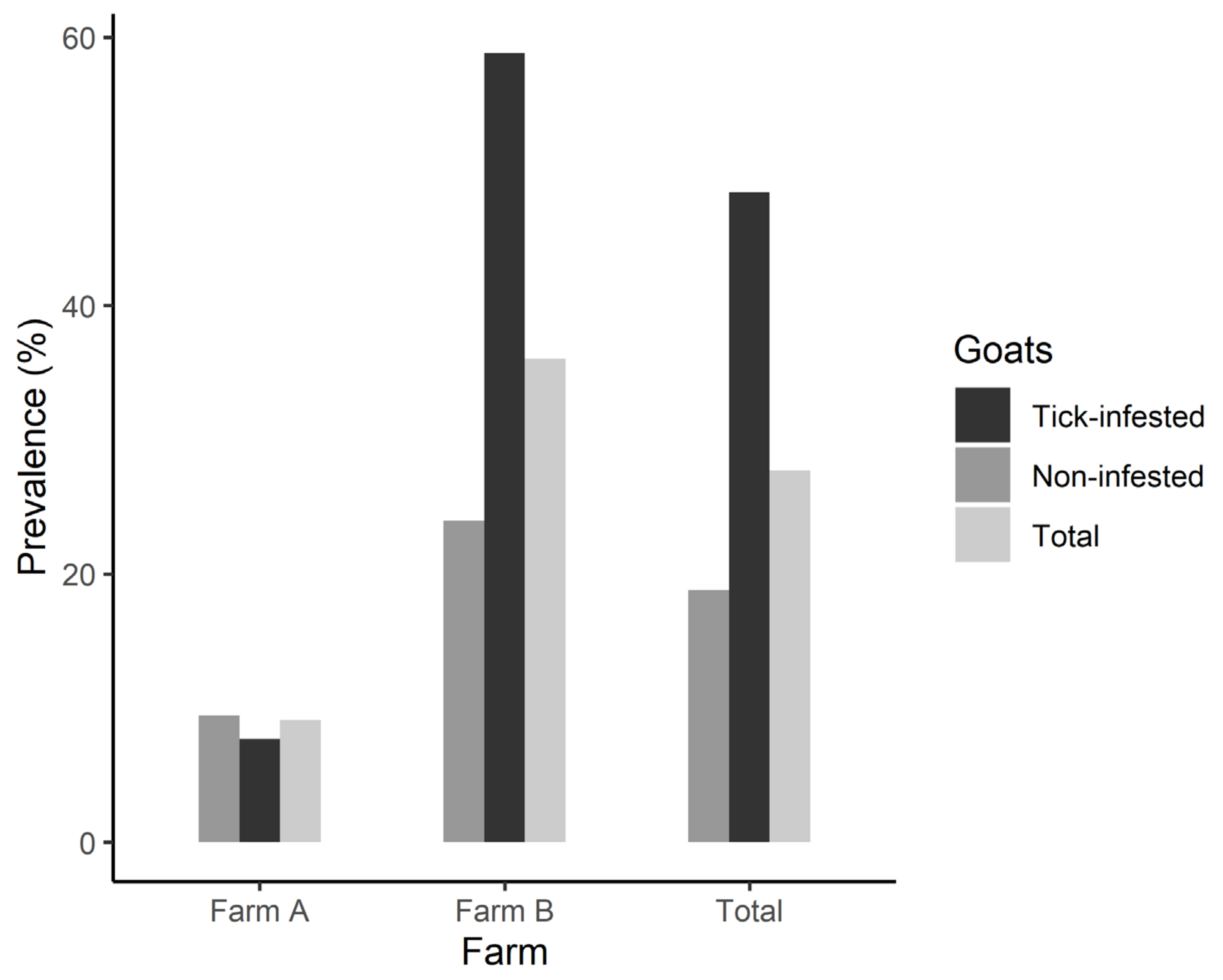
| Characteristic | Farm A | Farm B |
|---|---|---|
| Breed | Skopelos | Eghoria |
| Number of adult goats | 250 | 1200 |
| Number of bucks | 20 | 85 |
| Number of yearlings | 70 | 215 |
| Goat replacement rate (%) | 15 | 15 |
| Buck replacement rate (%) | 25 | 30 |
| Age of yearlings at first mating (months) | 9 | 7 |
| Milk production (kg/goat/lactation period) | 280 | 180 |
| Kidding season | December | November |
| Ectoparasitic treatment | ivermectin | ivermectin |
| Last ectoparasitic treatment (months ago) | 7 | 9 |
| Sampling month | May | June |
| Grazing duration (hours/day) | 10 | 6 |
| Type of pastureland | grassland/shrubland/woodland | grassland/shrubland/woodland |
| Farm A | Farm B | Total | |||||
|---|---|---|---|---|---|---|---|
| Trait | Goats | N | Mean (±SD 1) | N | Mean (±SD 1) | N | Mean (±SD 1) |
| Daily milk yield (g) | Tick-infested | 32 | 1156.7 (419.82) | 55 | 659.0 (173.61) | 87 | 842.1 (375.14) |
| Non-infested | 120 | 1063.2 (348.81) | 97 | 742.0 (186.53) | 217 | 919.6 (328.81) | |
| Total | 152 | 1082.9 (365.42) | 152 | 712.0 (185.74) | 304 | 897.4 (343.87) | |
| Daily fat yield (g) | Tick-infested | 26 | 57.1 (17.95) | 52 | 31.6 (8.54) | 78 | 40.1 (17.30) |
| Non-infested | 99 | 55.2 (15.53) | 97 | 35.0 (8.75) | 196 | 45.2 (16.18) | |
| Total | 125 | 55.6 (16.01) | 149 | 33.8 (8.80) | 274 | 43.8 (16.63) | |
| Daily protein yield (g) | Tick-infested | 26 | 44.6 (14.82) | 52 | 24.4 (5.84) | 78 | 31.2 (13.62) |
| Non-infested | 99 | 39.7 (10.89) | 97 | 26.1 (6.46) | 196 | 33.0 (11.26) | |
| Total | 125 | 40.7 (11.92) | 149 | 25.5 (6.28) | 274 | 32.5 (11.98) | |
| Daily lactose yield (g) | Tick-infested | 26 | 49.4 (20.52) | 52 | 28.2 (7.18) | 78 | 35.3 (16.48) |
| Non-infested | 99 | 43.9 (13.89) | 97 | 31.4 (7.96) | 196 | 37.7 (12.93) | |
| Total | 125 | 45.0 (15.57) | 149 | 30.3 (7.82) | 274 | 37.0 (14.04) | |
| Daily SNF 2 yield (g) | Tick-infested | 26 | 104.7 (39.22) | 52 | 58.7 (14.01) | 78 | 74.0 (33.23) |
| Non-infested | 99 | 93.2 (27.47) | 97 | 64.2 (15.83) | 196 | 78.8 (26.72) | |
| Total | 125 | 95.6 (30.47) | 149 | 62.3 (15.40) | 274 | 77.5 (28.74) | |
| Milk SCC 3 (×103 cells/mL) | Tick-infested | 13 | 1455.1 (2052.42) | 52 | 3238.8 (3736.53) | 65 | 2882.0 (3525.98) |
| Non-infested | 53 | 1723.4 (3940.92) | 96 | 1377.8 (1693.78) | 149 | 1500.7 (2706.63) | |
| Total | 66 | 1670.6 (3635.10) | 148 | 2031.7 (2737.25) | 214 | 1920.3 (3038.30) | |
| Milk TVC 4 (×103 cfu/mL) | Tick-infested | 32 | 10.2 (8.17) | 54 | 323.9 (581.77) | 86 | 207.2 (484.09) |
| Non-infested | 120 | 53.1 (265.77) | 97 | 87.7 (251.11) | 217 | 68.6 (259.30) | |
| Total | 152 | 44.1 (236.62) | 151 | 172.2 (415.75) | 303 | 107.9 (343.46) | |
| Trait | β-Coefficient | SE 1 | p-Value |
|---|---|---|---|
| Daily milk yield (g, ln) | −0.04 | 0.040 | 0.333 |
| Daily fat yield (g, ln) | −0.06 | 0.039 | 0.136 |
| Daily protein yield (g, ln) | 0.00 | 0.037 | 0.984 |
| Daily lactose yield (g, ln) | −0.03 | 0.042 | 0.433 |
| Daily SNF 2 yield (g, ln) | −0.02 | 0.039 | 0.659 |
| Milk SCC 3 (cells/mL, ln) | 0.61 | 0.176 | <0.001 |
| Milk TVC 4 (cfu/mL, ln) | 0.58 | 0.162 | <0.001 |
| Udder health status (odds ratio) | 3.65 | 1.24 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vouraki, S.; Gelasakis, A.I.; Papanikolopoulou, V.; Papadopoulos, E.; Arsenos, G. Association of Hard Ticks (Ixodidae) Infestation with Milk Production and Udder Health of Extensively Reared Dairy Goats. Animals 2022, 12, 354. https://doi.org/10.3390/ani12030354
Vouraki S, Gelasakis AI, Papanikolopoulou V, Papadopoulos E, Arsenos G. Association of Hard Ticks (Ixodidae) Infestation with Milk Production and Udder Health of Extensively Reared Dairy Goats. Animals. 2022; 12(3):354. https://doi.org/10.3390/ani12030354
Chicago/Turabian StyleVouraki, Sotiria, Athanasios I. Gelasakis, Vasiliki Papanikolopoulou, Elias Papadopoulos, and Georgios Arsenos. 2022. "Association of Hard Ticks (Ixodidae) Infestation with Milk Production and Udder Health of Extensively Reared Dairy Goats" Animals 12, no. 3: 354. https://doi.org/10.3390/ani12030354
APA StyleVouraki, S., Gelasakis, A. I., Papanikolopoulou, V., Papadopoulos, E., & Arsenos, G. (2022). Association of Hard Ticks (Ixodidae) Infestation with Milk Production and Udder Health of Extensively Reared Dairy Goats. Animals, 12(3), 354. https://doi.org/10.3390/ani12030354









