1. Introduction
There has been a growing trend towards the use of natural feed additives to improve performance and maintain the health of birds. Many researchers are currently focusing their efforts on discovering viable alternatives for achieving better production and profitability. In the poultry industry, “friendly additives” must be sought to replace the frequent use of vaccinations, drugs, and antibiotics [
1]. Therefore, the use of probiotics, prebiotics, herbal powders, and algal products in poultry diets, to improve growth and reproductive attributes, as well as innate immunity that provides adequate protection to birds, has become popular as an alternative to antibiotics worldwide in recent years [
2,
3,
4,
5]. The genus Sargassum is a tropical and sub-tropical brown seaweed, comprising 150 species [
6]. When compared to red and green seaweeds, brown seaweeds exhibited good antioxidant activities, according to Seenivasan [
7]. Phenolic compounds, which range from 20 to 30% of the dry weight of brown seaweeds [
8], were one of the most potent antioxidants [
9]. Brown seaweeds contain sulphated polysaccharides, proteins, omega-3 polyunsaturated fatty acids, pigments, minerals, and vitamins, in addition to their prebiotic properties [
10,
11,
12]. Seaweeds have been used in poultry to improve the immunological status, reduce the microbial burden in their digestive tract, and increase their growth performance [
13]. Seaweeds contain a variety of active compounds, including carotenoids, vitamin B
12, vitamin C, thiamine, riboflavin, and pyridoxine, which can be used to improve the performance of layers and broilers [
13,
14]. These seaweed components have been evaluated as feed additives to help broilers perform better by preventing pathogenic bacteria from colonising the small intestine, stimulating beneficial bacteria, improving intestinal architecture, enhancing the antioxidant status, and increasing the immunological response [
15,
16,
17]. Previously, Wiseman [
18] reported that feeding broiler chickens 3% sun-dried brown seaweed improved their body weight but did not affect their cecum weight. Incorporating 0.5 brown seaweed into a broiler diet resulted in an increased body weight gain, enhanced immunological response, and a lower mortality rate, as compared to the control diet [
19]. Adding 5% brown seaweed to the diet of pigs increased their propionic and butyric acid levels, according to Hoebler [
20]. Laminarin, a beneficial component found in brown seaweed, improved mucosal absorption and butyrate synthesis, according to Deville et al. [
21]. The dietary inclusion of seaweed has been shown to improve bird health and feed efficiency by increasing the abundance of beneficial gut bacteria and by strengthening the host’s innate immune system [
22]. A basal diet supplementation with 100 and 200 mg/kg of a fucoxanthin extract (a brown seaweed derivate) increased catalase (CAT) and superoxide dismutase (SOD) activities, as well as glutathione (GSH) levels. Fucoxanthins can be used to regulate antioxidant metabolism and improve the immune system of broilers [
16]. Therefore, this study aimed to evaluate growth performance, cecal fermentation, microbial populations, duodenal histomorphology, antioxidant status, and the immunological response in growing Japanese quails fed a
S. siliquastrum-supplemented diet.
2. Materials and Methods
The research protocol was permitted by the Animal Care and Use Committees of the Scientific Research and Technological Applications (Protocol No. 52-3U-12021), Alexandria, Egypt.
2.1. Collection and Preparation of Brown Seaweed (Sargassum siliquastrum)
Brown seaweed (
Sargassum siliquastrum) was hand-picked from the Red Sea near Hurgada with the help of the National Institute of Oceanography and Fisheries—Hurghada Branch, Egypt. Before being sundried for 2 to 3 days, the collected marine
S. siliquastrum was adequately washed and rinsed three times in freshwater to remove sand, debris, and other extraneous matter attached to the thalli. The dried samples were ground into a fine powder and were stored in airtight bags for further chemical analyses.
S. siliquastrum was evaluated as a feed supplement in a powder form, and the chemical composition of the
S. siliquastrum sample is illustrated in
Table 1.
2.2. Experimental Design, Birds, and Diets
A total of 450 Japanese quails, at seven days of age, with an average body weight of 27 ± 0.23 g, were randomly allocated to one of three dietary treatments for a five-week experiment. The three dietary treatments were a basal diet, supplemented with 0%, 1%, or 2%
Sargassum siliquastrum. Each treatment consisted of 150 unsexed quails, with five replications, with 30 unsexed quail chicks each. Quails were reared in wire battery cages (W × H × L cm: 50 × 35 × 95) in a well-ventilated room and maintained under the same conditions, with 23 h of light to 1 h of darkness. The quails had free access to water and feed ad libitum throughout the experiment. The experimental diet was formulated according to the NRC [
23]. The composition of the experimental diet is shown in
Table 2.
2.3. Growth Performance
The weights of individual quails were recorded every week to determine the final body weight (FBW). The amount of feed consumption and residual in each cage was recorded daily to evaluate the average daily feed intake (ADFI). The average daily gain (ADG) and the feed conversion ratio (FCR; g feed/g gain) were calculated. The mortality rate was recorded daily and at the end of the experiment the percentage was recorded for each group.
2.4. Nutrient Digestibility
On the last week of the experiment, six quails (three males and three females) were selected from each replicate per treatment and were placed in individual battery cages for the digestibility study. The quails were allowed to acclimate for 2 days, then the feed consumption was recorded, and feces were collected every day before feeding in the morning for 5 consecutive days. Once collected, all quails were removed from the excreta, and the cleaned samples were weighed and oven-dried at 70 °C for 48 h, before being ground and stored for chemical analysis. Diet and feces samples were analysed for dry matter (DM), organic matter (OM), crude protein (PC), crude fibre (CF), neutral detergent fiber (NDF), and acid detergent fiber (ADF). AOAC [
24] procedures were used to determine the CP (Method No. 954.01) and ash (Method No. 942.05) contents. The ether extract (EE) was determined using petroleum ether as an extracting agent (40–60 °C) according to the Soxhlet extract method (Method No. 930.09) [
24]. The NDF and ADF contents were determined according to the method described by van Soest [
25]. The calculation for the nitrogen-free extract is: %NFE = 100% − (%EE + %CP + %Ash + %CF).
2.5. Some Digestive Tract Characteristics
At the end of the feeding trial, 30 quails (15 males and 15 females) from each treatment were slaughtered, to determine the weights and lengths of the intestines and cecum of the Japanese quails. The gut intestinal tract (GIT), from the esophagus to the cloaca, was carefully excised. Any digesta remaining in the whole GIT and the cecum were emptied by gentle pressure. The length (cm) of the whole GIT was measured, as well as the cecum. The GIT’s full and empty weights, and the cecum (g/g body weight), were expressed as a percentage of the body weight.
2.6. Cecal Microbes and Fermentation Traits
At the end of the experiment, four quails were euthanized from each replicate and the cecum was removed to determine the cecal microbial counts (two quails/replicate) and cecal fermentation (two quails/replicate). One gram of cecum content was transferred to 9 mL of 0.1% peptone water (Oxoid, Basingstoke, UK), and was homogenised to enumerate the cecal bacteria. Ten-fold dilutions of each sample were performed with buffered peptone water and were directly inoculated on de Man–Rogosa–Sharpe (MRS) agar for the total aerobic bacterial, anaerobic bacterial, and Lactobacillus sp. Counts, and were incubated anaerobically at 37 °C using gas generating kits (Oxoid) for 48 h. The E. coli were subcultured on a MacConkey agar and were incubated aerobically at 37 °C for 24 h. Clostridium perfringens were subcultured on a Perfringens agar base (Oxoid) mixed with 400 mg of D-cycloserine/liter and were incubated anaerobically using gas generating kits (Oxoid) at 37 °C for 48 h. Depending on the growth characteristics of the bacterial species, bacterial colonies were counted on dishes using a range of 30–300 cfu/g. The overall population was expressed as the log of cfu/g.
The cecal contents of the two quails used to determine cecal fermentation were squeezed out into clean beakers. Immediately, cecal contents were strained through two layers of sterile gauze and the resultant strained liquors were used to measure pH values using an electronic digital pH meter (GLP 21 model; CRISON, Barcelona, Spain). Thereafter, the contents were centrifuged at 7000×
g for 10 min at 20 °C. The supernatant fluid was divided into two parts. One part was treated with a solution of 5% orthophosphoric acid (
v/
v) plus 1% mercuric chloride (
w/
v) (0.1 mL-mL
−1 sample) for the determination of the total VFA concentration and the individual VFA proportions, while the other was acidified with 0.2 M hydrochloric acid solution (one mL-mL
−1 sample) to be used for the determination of ammonia nitrogen (NH
3-N) concentration. The total VFA concentration was measured via steam distillation, according to Eadie et al. [
26]. The percentage concentration of VFA was analysed using high performance liquid chromatography (HPLC; Model Water 600; UV detector, Millipore Crop.) according to the method of Mathew et al. [
27]. After the results of the percentage concentrations of the particular VFA had been received, the concentrations (mmol·L
−1) of acetic, propionic, and butyric acids were calculated. cecal NH
3-N concentrations were measured using spectrophotometry according to Chaney and Marbach [
28].
2.7. Blood Antioxidant Activity and Immunoglobulin Concentration
At the end of the experiment, blood samples were collected from the slaughtered quails into clean tubes and were immediately centrifuged at 1000× g for 20 min at 20 °C. The serum was separated and kept at −20 °C until determination of total antioxidant capacity (TAC), superoxide dismutase activity (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and thiobarbituric acid reactive substances (TBARS) were calorimetrically determined using commercial kits from Biodiagnostic Company (Giza, Egypt) and a spectrophotometer (Optizen Pop, Mecasys, Korea). Serum concentrations of immunoglobulins (Ig) A, G, and M were determined using kits (Bethyl Laboratories, Montgomery, TX, USA). The ELISA procedure was performed according to the manufacturer’s protocol, and the absorbance was measured at 450 nm.
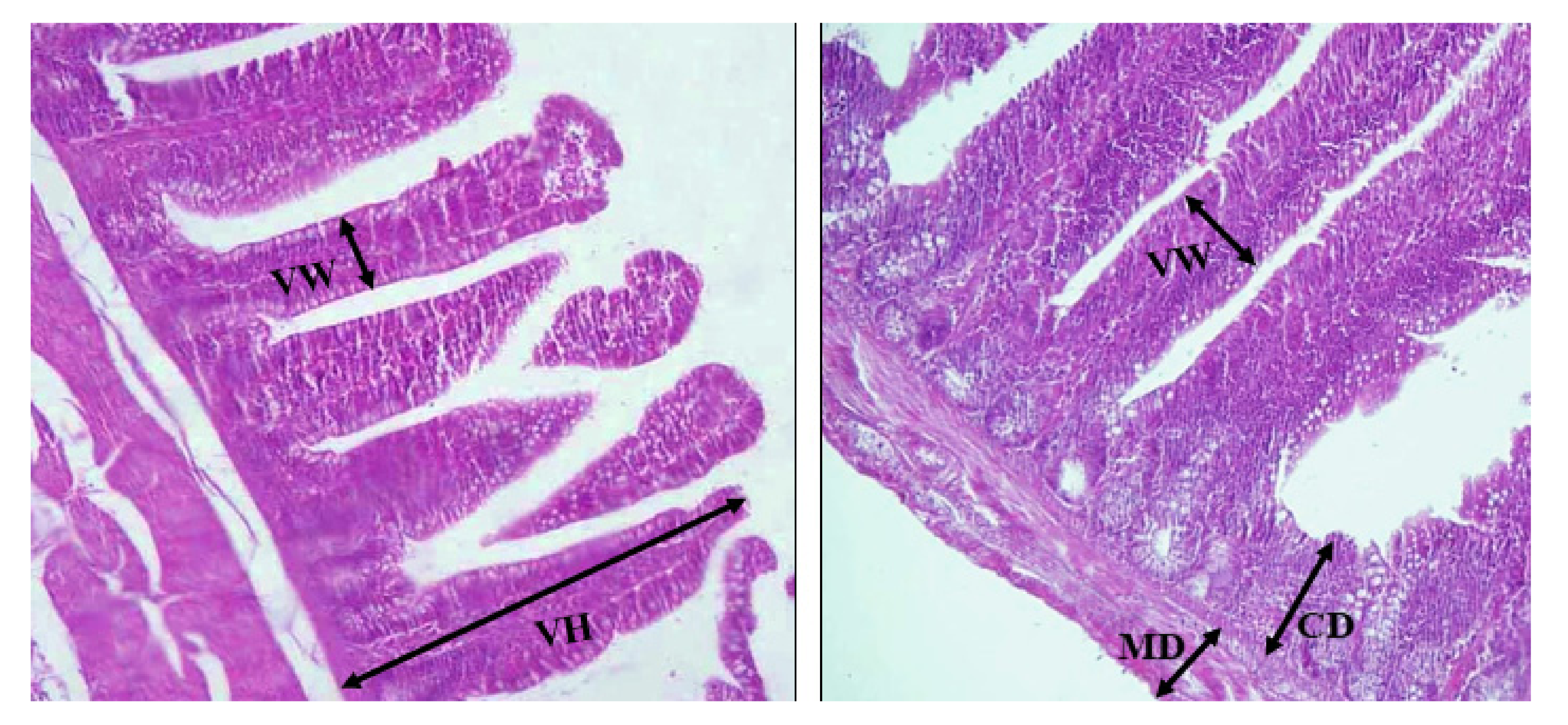
2.8. Histopathology Measurements
At the end of the experiment, five additional quails from each treatment were euthanized to determine the histopathology analysis. A 2-cm long segment of the duodenum was transected from each quail, and any digesta remaining in these segments was emptied by gentle washing with normal saline before being fixed in a 10% neutral buffered formalin solution. The fixed samples were processed through a normal alcohol dehydration-xylene procedure before being embedded in paraffin. Histological sections were prepared from 5-µm paraffin blocks of samples and were stained using the haematoxylin and eosin (H&E) technique. The measurements of the villi lengths and crypt depths in the samples were performed using an image analysis program Image J software. A number of well-oriented intact crypt–villus units were selected in triplicate for each cross-section in the duodenum. The appearance of the entire lamina propria served as the villus classification criteria. According to Wilson et al. [
29], the length of the villus was measured from the tip of the villus to the villus–crypt junction.
2.9. Statistical Analyses
Data were subjected to statistical analyses in a randomized complete block design using general linear model procedures of SAS/STAT (Statistical Analysis System, version 9.3, SAS Institute Inc., Cary, NC, USA) [
30]. The obtained data were tested by an analysis of variance with a one-way design to test the treatment at each sampling, according to the following model:
where y
ij is the measured value, μ is the overall mean effect, T
i is the ith treatment effect, and Ɛ
ij is the random error associated with the jth quails assigned to the ith treatment. Significant differences among the treatments were determined at
p < 0.05. All results are presented as least-squares means.
4. Discussion
In this study,
S. siliquastrum supplementation improved the FCR of Japanese quails, which is consistent with the findings of [
14]. Choi et al. [
19] found that supplementing broilers with seaweed improved their BWG significantly; however, the feed efficiency was not affected. Abu Hafsa et al. [
5], on laying quails, and Rizk et al. [
31] on laying hens found that the dietary inclusion of marine seaweeds improved their performance. According to Abu Hafsa et al. [
32], the growth performance improved significantly in rabbits fed 4% marine seaweeds. Adding seaweed to bird diets improved growth and health, as well as improving intestinal microflora [
33]. This can be attributed to the improved immune response, providing adequate protection for the birds.
Brown seaweeds are rich in a variety of polysaccharides, such as fucans and alginic acids that function as prebiotics, stimulating growth and improving health, according to Wijesinghe et al. [
34]. Our results also showed that Japanese quails fed
S. siliquastrum-supplemented diets exhibited improved nutrient digestibility. Wong and Cheung [
35] reported that the presence of phenolic compounds in seaweed, especially phlorotanin in brown seaweed, might affect protein digestion. Similarly, Balasubramanian et al. [
36] showed that broilers fed red seaweed-supplemented diets exhibited improved nutrient digestibility. The total digestible nutrients increased significantly in rabbits fed 4% marine seaweed, according to Abu Hafsa et al. [
32]. The current study revealed that growing Japanese quails fed
S. siliquastrum performed as good as, or better than, quails fed a control diet. Seaweed, which is a natural source of many compounds, such as proteins, minerals, fatty acids, polysaccharides, and essential vitamins, can stimulate body metabolism and improve nutrient digestion and absorption, which could explain why the quails in the
S. siliquastrum groups gained more weight than those in the control group.
S. siliquastrum improved dietary palatability while simultaneously enhancing digestibility, FCR and intestinal absorption, resulting in increased quail performance and cost efficiency. The beneficial effects in quails can also be ascribed to the rich content of crude proteins and minerals in
S. siliquastrum. Kumar [
14] found that the dietary supplementation of dried
S. wightii powder at 1%, 2%, 3%, and 4% increased broiler BW, FI, and FCR. Adding 0.25% and 0.5% of sun-dried
A. nodosum improved broilers’ growth performance [
18].
The weight of the full and empty intestines increased significantly when quails were fed
S. siliquastrum-supplemented diets. In addition, the length of the intestine increased by 9.07% and 9.45%, respectively. The villi’s function is to provide a vastly enlarged surface area for more efficient nutrition absorption. The surface area that is accessible for nutrients to move through affects the absorption efficiency; the more villi there are, the better the absorption is. Despite this, feed intake was unanimously equal, on the whole, in the experiments. Thus, it was easy to demonstrate that the improved BW in
S. siliquastrum-supplemented birds was attributable to the improved nutrient digestion and absorption, as a result of an increased intestine weight and length. It was observed that the villus height in the small intestine of
S. siliquastrum-supplemented quails was significantly taller, which is correlated with more efficient nutrient absorption and improved growth performance. Overall, we believe that dietary supplementation with
S. siliquastrum can improve the growth performance of Japanese quails, as well as their nutrient digestibility, by strengthening their intestinal integrity and immune system. In quails fed the
S. siliquastrum-supplemented diet, their cecum length increased by 8.13% and 7.70%, respectively, above the control value. These findings could be attributable to the fact that seaweeds have been shown to increase immune function and lower the microbial load in the gastrointestinal system [
37].
The supplementation of
S. siliquastrum into the quail diet increased the abundance of
Lactobacillus in the cecum, while decreasing
E. coli and
C. perfringens concentrations. Macroalgae polysaccharides could be prebiotic ingredients for animal health applications, promoting the growth and/or activity of beneficial gut microbiota, such as
Lactobacillus sp., which, in turn, confers health benefits to the host by reducing pathogen invasions and disease [
38,
39].
Lactobacillus bacteria inhibit the growth of harmful microorganisms in the gut by producing lactic and acetic acids, which lower the pH of the gut and make it unfavourable for pathogen growth.
Lactobacillus bacteria also enhance immunity by up-regulating intestinal mucins synthesis, which disrupts pathogen adhesion to the intestinal epithelium, consequently preventing pathogen translocation. These results are consistent with those of [
40,
41]. The increased count of
Lactobacillus bacteria that contribute to the development of resistance to
C. perfringens and
E. coli colonization through a competitive exclusion mechanism may be responsible for the observed improvements in the microbial community. Seaweed contains a variety of active compounds, including polysaccharides, such as laminarin and fucoidan [
42]; proteins such as lectins [
43]; phlorotannins [
44]; and pigments, such as carotenoids [
45] which act as prebiotics in promoting the growth of beneficial bacteria while inhibiting harmful microorganisms, hence, improving overall health [
46].
The weight of the cecum is an indicator of the fermentation in the cecum. An increase in cecum weight is associated with a rise in the count of beneficial bacteria. [
47]. The total VFA, which is a primary end product of microbial fermentation and improves gastrointestinal fluid absorption, is affected by changes in the bacterial community structure. [
48]. The concentration of VFA in the intestine is determined primarily by the fermentative substrate and the microbial diversity in the intestine [
49]. Supplementing Japanese quails with
S. siliquastrum increased VFA and propionic acid significantly; however, both acetic and butyric acids were not affected. According to Gomez-Ordonez et al. [
50], the propionate concentration in the cecum was increased dramatically when seaweed was included in feed. The greater propionate acid concentrations found in this investigation was consistent with previous findings in broilers [
51]. Deville et al. [
21] reported that laminarin, a brown seaweed polysaccharide, improved mucosal absorption and butyrate synthesis. Kulshreshtha et al. [
47] found that the concentration of VFA, including acetic, propionic, and butyric acids, was substantially greater in red seaweed treatments than in a control. The VFA is an important source of energy for enterocytes, and is also key in signaling molecules for gut health maintenance. In addition, the VFA can indirectly stimulate cell proliferation, resulting in an increase in the weight of the intestine and cecum. [
52]. It is, thus, probable that the elevated VFA concentration in this study contributed to the larger intestine and cecum weights. As a result, it’s likely that the higher VFA content in our study led to the increased intestine and cecum weight.
The antioxidant status of birds is extensively documented as being significant for their resistance to infections, health maintenance, and their productivity [
53]. Increases in TAS, SOD, GRx, and GPx, but a decrease in TBARS, were indicative of lower lipid peroxidation levels, according to Abdel-Daim et al. [
54]. The
S. siliquastrum-treated group had significantly lower TBARS levels than in the control group. This supports the findings of Droge [
55] who reported that
Spirulina platensis had a high antioxidant capacity in broilers, which was linked to the presence of β-carotene zeaxanthin, phycocyanin, and allophycocyanin [
56]. Superoxide dismutase (SOD), the most significant antioxidant enzyme, is essential for the removal of superoxide anions in animals [
57]. The considerable increase in SOD values in the groups supplemented with
S. siliquastrum could imply a strong association between a microalgae addition and the improved antioxidant capacity, as documented by Droge [
55]. It is known that the immunological status of the host has a significant impact on various infection resistance. In addition to protecting the apical surface of the brush border, IgA plays a vital function in gastrointestinal defence and secretion in the gut lumen [
58]. Therefore, IgA has the potential to alter the microbial population by targeting those species that the immune system considers harmful [
58]. IgG is the main serum antibody in the mucosal immune response [
59]. According to our findings, the elevated concentrations of IgA, IgG, and IgM antibodies in the supplemented groups imply that
S. siliquastrum in the diet can strengthen the immune system for antibody production in Japanese quails. Alginates, the active components of brown seaweed, consist of two types of uronic acids: mannuronic and guluronic [
60], which function as prebiotics, as well as immunomodulators that enhance innate immunological resistance in birds and animals [
61].