The Indicators of Clinical and Subclinical Mastitis in Equine Milk
Abstract
:Simple Summary
Abstract
1. Introduction
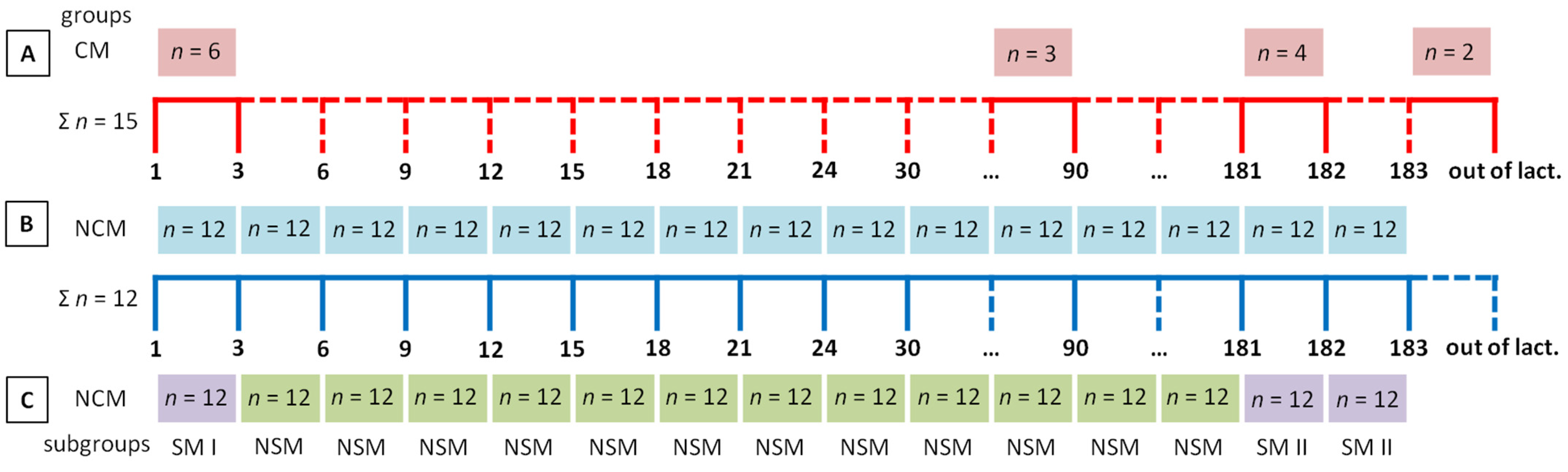
2. Materials and Methods
2.1. Animals
2.2. Sample Collection
2.3. Examination of Milk and Inflammatory Secretions from the Mammary Gland
2.4. Statistical Analysis
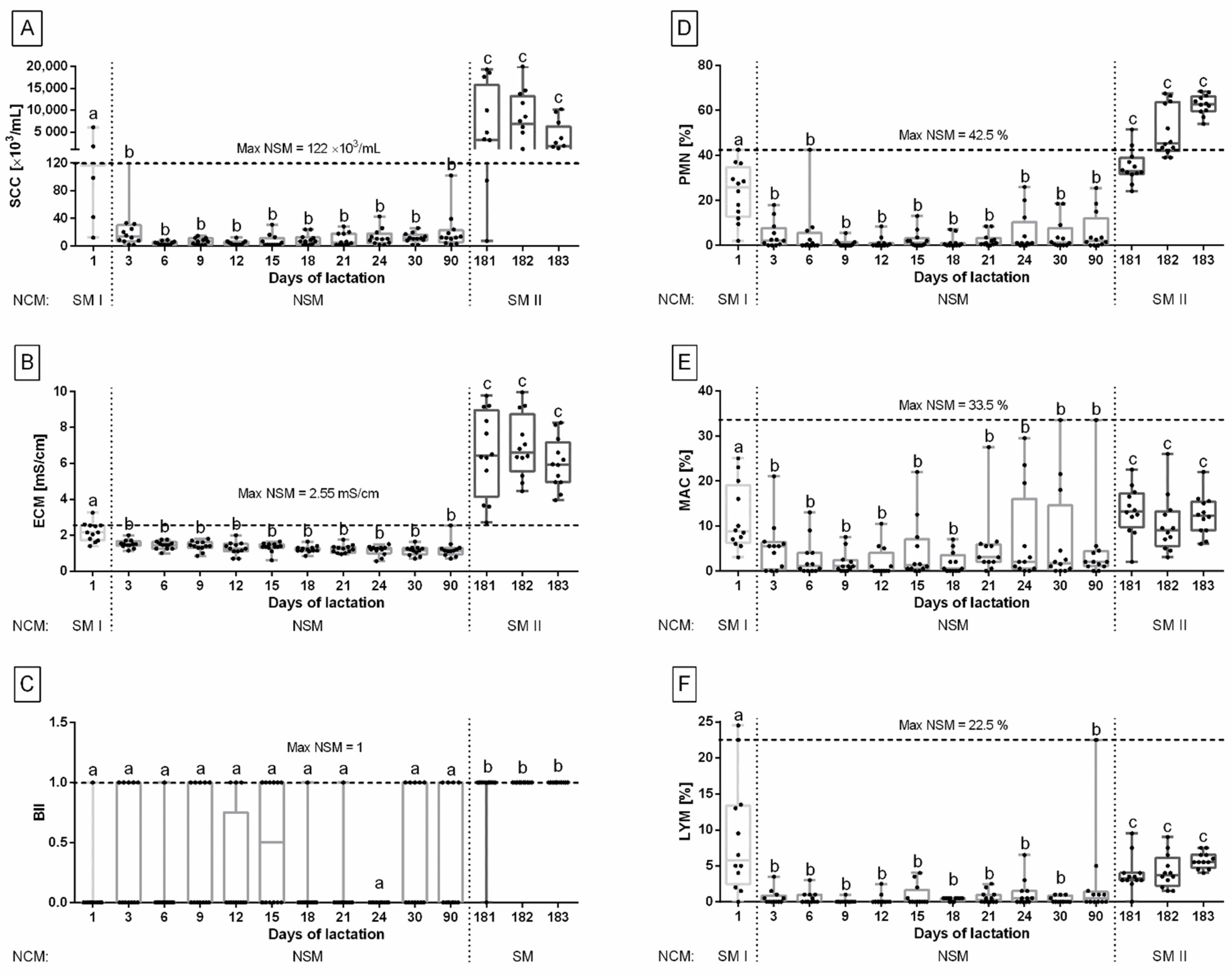
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brumini, D.; Criscione, A.; Bordonaro, S.; Vegarud, G.E.; Marletta, D. Whey proteins and their antimicrobial properties in donkey milk: A brief review. Dairy Sci. Technol. 2016, 96, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fotschki, J.; Szyc, A.M.; Laparra, J.M.; Markiewicz, L.H.; Wróblewska, B. Immune-modulating properties of horse milk administered to mice sensitized to cow milk. J. Dairy Sci. 2016, 99, 9395–9404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushugulova, A.; Kozhakhmetov, S.; Sattybayeva, R.; Nurgozhina, A.; Ziyat, A.; Yadav, H.; Marotta, F. Mare’s milk as a prospective functional product. Funct. Food Health Dis. 2018, 8, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Aspri, M.; Leni, G.; Galaverna, G.; Papademas, P. Bioactive properties of fermented donkey milk, before and after in vitro simulated gastrointestinal digestion. Food Chem. 2018, 268, 476–484. [Google Scholar] [CrossRef]
- Miraglia, N.; Salimei, E.; Fantuz, F. Equine milk production and valorization of marginal areas—A review. Animals 2020, 10, 353. [Google Scholar] [CrossRef] [Green Version]
- Drogoul, C.; Prevost, H.; Maubois, J.L. Le lait de juments un produit. Une filiere a developer? In Quoi de Neuf en Matiere d’Etudes de Recherches sur le Cheval. 18eme Journee d’Etude. 4 Mars; CEREOPA: Paris, France, 1992; pp. 37–51. [Google Scholar]
- Doreau, M.; Martin-Rosset, W. Animals that produce dairy foods—horse. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Elsevier Academy Press: London, UK, 2011; Volume 1, pp. 358–364. [Google Scholar]
- Salimei, E.; Fantuz, F. Horse and donkey milk. In Milk and Dairy Products in Human Nutrition: Production. Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2013; pp. 594–613. [Google Scholar]
- McLean, A.K.; Navas Gonzalez, F.J. Can scientists influence donkey welfare? Historical perspective and a contemporary view. J. Equine Vet. Sci. 2018, 65, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Businco, L.; Giampietro, P.G.; Lucenti, P.; Lucaroni, F.; Pini, C.; Di Felice, G.; Iacovacci, P.; Curadi, C.; Orlandi, M. Allergenicity of mare’s milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2000, 105, 1031–1034. [Google Scholar] [CrossRef]
- Sarti, L.; Martini, M.; Brajon, G.; Barni, S.; Salari, F.; Altomonte, I.; Ragona, G.; Mori, F.; Pucci, N.; Muscas, G.; et al. Donkey’s milk in the management of children with cow’s milk protein allergy: Nutritional and hygienic aspects. Ital. J. Pediatrics 2019, 45, 102–110. [Google Scholar] [CrossRef]
- Stuparu, A.A.; Strugariu, C.E.; Oroian, T. Pharmaceuticals and Dietary Supplements Extracted from Mare’s Milk. Bul. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2016, 73, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Uniacke-Lowe, T.; Huppertz, T.; Fox, P.F. Equine milk proteins: Chemistry, structure and nutritional significance. Int. Dairy J. 2010, 20, 609–629. [Google Scholar] [CrossRef]
- Pastuszka, R.; Barłowska, J.; Litwińczuk, Z. Allergenicity of milk of different animal species in relation to human milk. Postepy Hig. I Med. Dosw. 2016, 70, 1451–1459. [Google Scholar] [CrossRef]
- Fantuz, F.; Salimei, E.; Papademas, P. Macro- and micronutrients in non-cow milk and products and their impact on human health. In Non-Bovine Milk and Milk Products, 1st ed.; Tsakalidou, E., Papadimitriou, K., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 209–261. [Google Scholar]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, M.; Delavaud, C.; Laud, K.; Gourdou, I.; Leroux, C.; Djiane, J.; Chilliard, Y. Mammary leptin synthesis, milk leptin and their putative physiological roles. Reprod. Nutr. Dev. 2002, 42, 399–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inglingstad, R.A.; Devold, T.G.; Eriksen, E.K.; Holm, H.; Jacobsen, M.; Liland, K.H.; Rukke, E.O.; Vegarud, G.E. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milk by human gastrointestinal enzymes. Dairy Sci. Technol. 2010, 90, 549–563. [Google Scholar] [CrossRef] [Green Version]
- Morand-Fehr, P.; Fedele, V.; Decandia, M.; Le Frileux, Y. Influence of farming and feeding systems on composition and quality of goat and sheep milk. Small Rumin. Res. 2007, 68, 20–34. [Google Scholar] [CrossRef]
- Mostafa, T.H.; El-Malky, O.M.; Abd El-Salaam, A.M.; Nabih, A.M. Some Studies on Milk Production and its Composition In Maghrebi She-Camel Farming And Traditional Pastoral Systems In Egypt. Int. J. Hortic. Agric. 2017, 2, 1–9. [Google Scholar]
- Vicini, J.; Etherton, T.; Kris-Etherton, P.; Ballam, J.; Denham, S.; Staub, R.; Goldstein, D.; Cady, R.; McGrath, M.; Lucy, M. Survey of retail milk composition as affected by label claims regarding farm-management practices. J. Am. Diet. Assoc. 2008, 108, 1198–1203. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Guo, H. The nutritional ingredients and antioxidant activity of donkey milk and donkey milk powder. Food Sci. Biotechnol. 2018, 27, 393–400. [Google Scholar] [CrossRef]
- Kaic, A.; Luštrek, B.; Simcic, M.; Potocnik, K. Milk quantity. composition and hygiene traits of routinely machine milked Lipizzan mares. Slov. Vet. Res. 2019, 56, 115–123. [Google Scholar]
- Colavita, G.; Amadoro, C.; Rossi, F.; Fantuz, F.; Salimei, E. Hygienic characteristics and microbiological hazard identification in horse and donkey raw milk. Vet Ital 2016, 52, 21–29. [Google Scholar]
- Cieslak, J.; Mackowski, M.; Czyzak-Runowska, G.; Wojtowski, J.; Puppel, K.; Kuczynska, B.; Pawlak, P. Screening for the most suitable reference genes for gene expression studies in equine milk somatic cells. PLoS ONE 2015, 10, e0139688. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Podico, G.; Ellerbrock, R.E. Diagnosis and treatment of mastitis in mares. Equine Vet. Educ. 2021, 33, 320–326. [Google Scholar] [CrossRef]
- Hughes, K. Development and Pathology of the Equine Mammary Gland. J. Mammary Gland Biol. Neoplasia 2021, 26, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, M.; Leta, S.; Mamo, G.; Tesfaye, S. Prevalence of bovine subclinical mastitis and isolation of its major causes in Bishoftu Town. Ethiopia. BMC Res. Notes 2017, 10, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Dhama, K.; Tiwari, R.; Iqbal Yatoo, M.; Khurana, S.K.; Khandia, R.; Munja, A.; Munuswamy, P.; Kumar, M.A.; Singh, M.; et al. Technological interventions and advances in the diagnosis of intramammary infections in animals with emphasis on bovine population—A review. Vet. Q. 2019, 39, 76–94. [Google Scholar] [CrossRef] [Green Version]
- Perkins, N.R.; Threlfall, W.R. Mastitis in the mare. Equine Vet. Educ. 2002, 5, 99–102. [Google Scholar] [CrossRef]
- Murata, H.; Shimada, N.; Yoshioka, M. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef]
- McCue, P.M.; Wilson, W.D. Equine mastitis—A review of 28 cases. Equine Vet. J. 1989, 21, 351–353. [Google Scholar] [CrossRef]
- Rhaymah, M.; Al-Obaidi, Q.T.; Hamad, M.A.; Altalby, M.A. Mastitis in mare: Case report. Iraqi J. Vet. Sci. 2018, 32, 109–111. [Google Scholar]
- Böhm, K.H.; Klug, E.; Jacobs, B.J. Mastitis in the mare-a long-term study on the incidence, clinical symptoms, diagnostics, microbiology, therapy and economic importance. as well as recommendations for veterinary practice. Prakt. Tierarzt. 2009, 90, 842–849. [Google Scholar]
- Motta, R.G.; Listoni, F.J.P.; Ribeiro, M.G.; Bussalaro, V.A.; Link, A.; Almeida, R.P.; Cappellozza, B.I. Microbiologic characterization of equine mastitis. J. Bacteriol. Parasitol. 2014, 5, 186–188. [Google Scholar]
- Kitchen, B.J. Review of the progress of dairy science: Bovine mastitis: Milk compositional changes and related diagnostic tests. J. Dairy Sci. 1981, 64, 167–188. [Google Scholar]
- Kamphuis, C.; Pietersma, D.; Van der Tol, R.; Wiedemann, M.; Hogeveen, H. Using sensor data patterns from an automatic milking system to develop predictive variables for classifying clinical mastitis and abnormal milk. Comput. Electron. Agric. 2008, 62, 169–181. [Google Scholar] [CrossRef]
- Prescott, S.C.; Breed, R.S. The determination of the number of body cells in milk by a direct method. J. Infect. Dis. 1910, 7, 632–640. [Google Scholar] [CrossRef]
- Kulisa, M.; Makieła, K.; Hofman, J. Zawartość komórek somatycznych w mleku klaczy a rasa, wiek, kolejność i miesiąc laktacji. Rocz. Nauk. Zootech. 2010, 37, 131–135. [Google Scholar]
- Schröder, A. Untersuchung zum Zelldifferentialbild in Milch und Blut unter Berücksichtigung des Gesundheitsstatus der bovinen Milchdrüse. Ph.D. Thesis, University of Veterinary Medicine, Hannover, Germany, 2003. [Google Scholar]
- Danków, R.; Wójtowski, J.; Pikul, J.; Niżnikowski, R.; Cais-Sokolińska, D. Effect of lactation on the hygiene quality and some milk physicochemical traits of the Wielkopolska mares. Arch. Tierzucht. Dummerstorf 2006, 49, 201–206. [Google Scholar]
- Danków, R.; Pikul, J.; Osten-Sacken, N.; Teichert, J. Charakterystyka i właściwości prozdrowotne mleka klaczy. Nauka Przyr. Technol. 2012, 6, 2–16. [Google Scholar]
- Dohoo, I.; Martin, W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; VER Inc.: Charlottetown, PE, Canada, 2009. [Google Scholar]
- Oftedal, O.T.; Dhouailly, D. Evo-devo of the mammary gland. J. Mammary Gland Biol. Neoplasia 2013, 18, 105–120. [Google Scholar] [CrossRef]
- Oftedal, O.T. The evolution of lactation in mammalian species. Milk. Mucosal Immun. Microbiome Impact Neonate 2020, 94, 1–10. [Google Scholar]
- Oviedo-Boyso, J.; Valdez-Alarcon, J.J.; Cajero-Juarez, M.; Ochoa-Zarzosa, A.; Lopez-Meza, J.E.; Bravo-Patino, A.; Baizabal-Aguirre, M. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J. Infect. 2007, 54, 399–409. [Google Scholar] [CrossRef]
- Rieland, E. Untersuchungen zu den Aktivitäten der Enzyme Lysosym, LDH, Gamma—GT, GOT, GTP. Laktoperoxydase und Bestimmung der Zellzahl in Stutenmilch im Laktationsverlauf. Ph.D. Thesis, Justus Liebig-Universität, Giessen, Germany, 1997; pp. 1–98. [Google Scholar]
- Markiewicz-Kęszycka, M. Wartość Odżywcza Oraz Prozdrowotna Siary i Mleka Klaczy w Diecie Człowieka. Ph.D. Thesis, Uniwersytet Przyrodniczy w Poznaniu, Poznań, Poland, 2012; pp. 3–106. [Google Scholar]
- Kuy, G. Untersuchungen zur Qualität von Frischer und Tiefgefriergetrockneter Stutenmilch. Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 1998; pp. 1–103. [Google Scholar]
- Pecka, E.; Dobrzański, Z.; Zachwieja, A.; Szulc, T.; Czyż, K. Studies of composition and major protein level in milk and colostrum of mares. Anim. Sci. J. 2012, 83, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kehrli, M.E., Jr.; Shuster, D.E. Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J. Dairy Sci. 1994, 77, 619–627. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, N.K.; Bhadwal, M.S. Relationship of somatic cell count and mastitis: An overview. Asian-Australas J. Anim. Sci. 2011, 24, 429–438. [Google Scholar] [CrossRef]
- Litwińczuk, Z.; Król, J.; Brodziak, A.; Barłowska, J. Changes of protein content and its fractions in bovine milk from different breeds subject to somatic cell count. J. Dairy Sci. 2011, 94, 684–691. [Google Scholar] [CrossRef] [Green Version]
- Prestes, N.C.; Langoni, H.; Cordeiro, L.A.V. Study of the milk in healthy mares or mares with subclinics mastitis using Whiteside test. microbiological culture and somatic cell count. Braz. J. Vet. Res. Anm. Sci. 1999, 36, 144–148. [Google Scholar]
- Wendt, K.; Bostedt, H.; Mielke, H.; Fuchs, W. Euter- und Gesäugekrankheiten; Fischer Verlag: Stuttgart, Germany, 1994. [Google Scholar]
- Jackson, P.G.G. Equine mastitis: Comparative lessons. Equine Vet. J. 1986, 18, 88–89. [Google Scholar] [CrossRef]
- Bostedt, H.; Lehmann, B.; Peip, D. Zur Problematik der Mastitis bei Stuten. Tierärztl. Prax. 1988, 16, 367–371. [Google Scholar]
- Oshima, M. Detection of abnormal quarter milk by quarter difference of electrical conductivity and its theoretical basis. Jpn. Agric. Res. Q. 1977, 11, 239–245. [Google Scholar]
- Sordillo, L.M.; Streicher, K.L. Mammary gland immunity and mastitis susceptibility. J. Mammary Gland Biol. Neopla. 2002, 7, 135–146. [Google Scholar] [CrossRef]
- Salimei, E.; Park, Y.W. Mare milk. In Handbook of Milk of Non-Bovine Mammals, 2nd ed.; Park, Y.W., Haenlein, G.F.W., Wendor, W.L., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2017; pp. 369–408. [Google Scholar]
- Ying, G.S.; Maguire, M.G.; Glynn, R.J.; Rosner, B. Calculating sensitivity, specificity, and predictive values for correlated eye data. IOVS 2020, 61, 29. [Google Scholar] [CrossRef]
- Sumon, S.M.R.; Parvin, M.S.; Ehsan, M.A.; Islam, M.T. Relationship between somatic cell counts and subclinical mastitis in lactating dairy cows. Vet. World 2020, 13, 1709. [Google Scholar] [CrossRef] [PubMed]
- Norberg, E.; Hogeveen, H.; Korsgaard, I.R.; Friggens, N.C.; Sloth, K.H.M.N.; Løvendahl, P. Electrical Conductivity of Milk: Ability to Predict Mastitis Status. J. Dairy Sci. 2004, 87, 1099–1107. [Google Scholar] [CrossRef] [Green Version]

| Groups | SM I | SM II | CM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Threshold | NSM Mean | m + SD | m + 2SD | Max NSM | NSM Mean | m + SD | m + 2SD | Max NSM | NSM Mean | m + SD | m + 2SD | Max NSM |
| SCC in the milk | ||||||||||||
| Se | 1.00 | 0.92 | 0.92 | 0.77 | 0.97 | 0.97 | 0.97 | 0.95 | 1.00 | 1.00 | 1.00 | 1.00 |
| Sp | 0.63 | 0.88 | 0.95 | 1.00 | 0.63 | 0.88 | 0.95 | 1.00 | 0.63 | 0.88 | 0.95 | 1.00 |
| PPV | 0.21 | 0.43 | 0.63 | 1.00 | 0.44 | 0.70 | 0.84 | 1.00 | 0.14 | 0.33 | 0.53 | 1.00 |
| NPV | 1.00 | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 | 0.99 | 0.98 | 1.00 | 1.00 | 1.00 | 1.00 |
| ECM in the milk | ||||||||||||
| Se | 1.00 | 0.92 | 0.62 | 0.15 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Sp | 0.41 | 0.82 | 0.98 | 1.00 | 0.41 | 0.82 | 0.98 | 1.00 | 0.41 | 0.82 | 0.98 | 1.00 |
| PPV | 0.14 | 0.34 | 0.73 | 1.00 | 0.34 | 0.63 | 0.93 | 1.00 | 0.09 | 0.26 | 0.73 | 1.00 |
| NPV | 1.00 | 0.99 | 0.96 | 0.92 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domańska, D.; Trela, M.; Pawliński, B.; Podeszewski, B.; Domino, M. The Indicators of Clinical and Subclinical Mastitis in Equine Milk. Animals 2022, 12, 440. https://doi.org/10.3390/ani12040440
Domańska D, Trela M, Pawliński B, Podeszewski B, Domino M. The Indicators of Clinical and Subclinical Mastitis in Equine Milk. Animals. 2022; 12(4):440. https://doi.org/10.3390/ani12040440
Chicago/Turabian StyleDomańska, Dominika, Michał Trela, Bartosz Pawliński, Bartłomiej Podeszewski, and Małgorzata Domino. 2022. "The Indicators of Clinical and Subclinical Mastitis in Equine Milk" Animals 12, no. 4: 440. https://doi.org/10.3390/ani12040440






