Eye Region Surface Temperature and Corticosterone Response to Acute Stress in a High-Arctic Seabird, the Little Auk
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Fieldwork
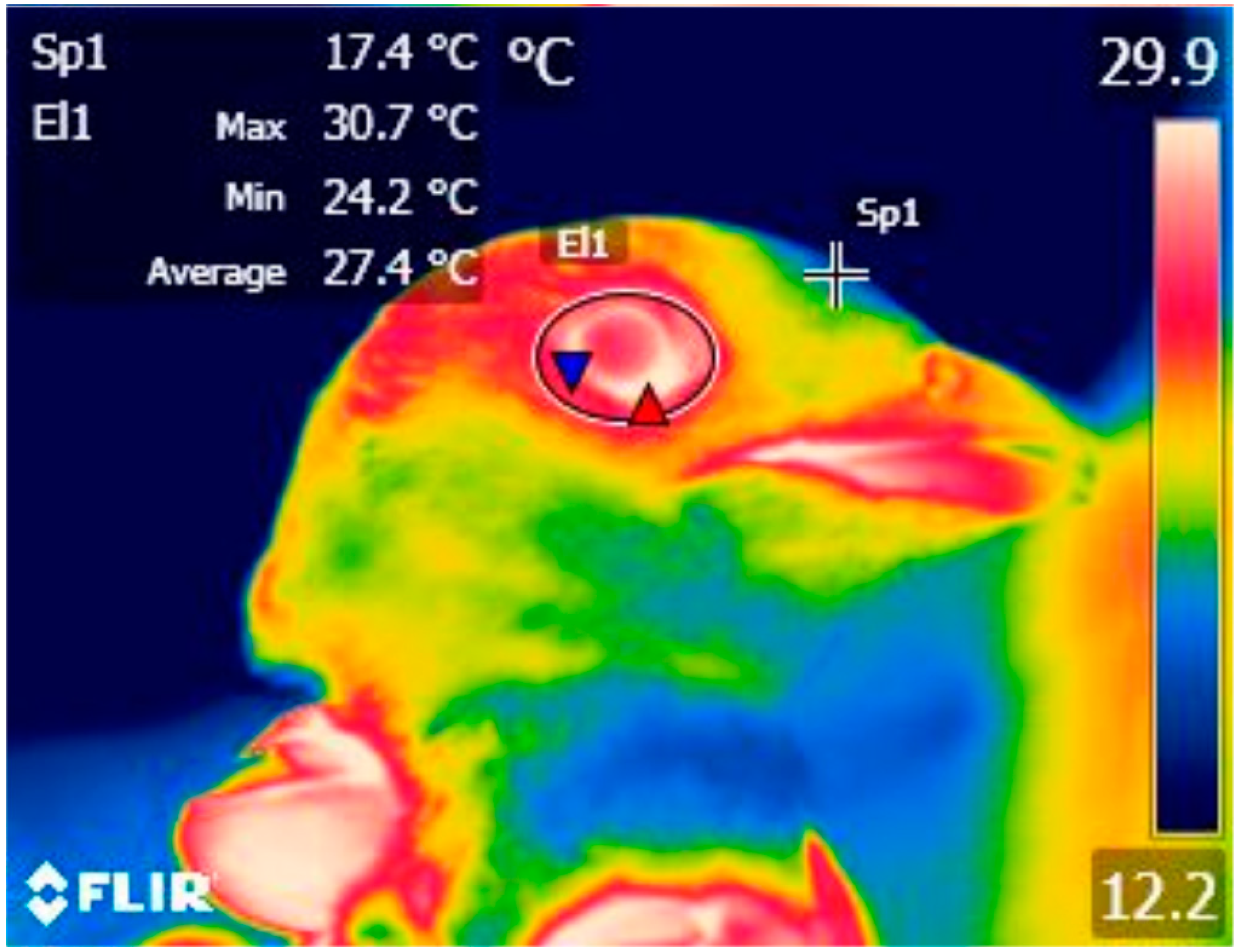
2.3. Processing of Thermal Images
2.4. Hormone Assay
2.5. Molecular Sexing
2.6. Data Analyses
2.7. Statistical Analyses
3. Results
3.1. Factors Affecting Maximal Eye-Region Temperatures (TEYE)
3.2. Factors Affecting Corticosterone Level (CORT)
3.3. Factors Affecting Thermal Stress Response (TEYE SR)
4. Discussion
4.1. Factors Affecting Maximal Eye-Region Temperatures
4.2. Magnitude and Direction of Thermal Response to Acute Stress
4.3. Thermal vs. Hormonal Response to Acute Stress
4.4. Implementation of Infrared Thermography in the Field Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero, M.; Wingfield, J. Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Romero, L.M. Adrenocortical Responses to Stress and Their Modulation in Free-Living Vertebrates. In Comprehensive Physiology; American Cancer Society: Atlanta, GA, USA, 2001; pp. 211–234. [Google Scholar]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingfield, J.C. Control of behavioural strategies for capricious environments. Anim. Behav. 2003, 66, 807–816. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Oka, K.; Hori, T. Mechanisms and Mediators of Psychological Stress-Induced Rise in Core Temperature. Psychosom. Med. 2001, 63, 476–486. [Google Scholar] [CrossRef]
- Bouwknecht, A.J.; Olivier, B.; Paylor, R.E. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: A review of pharmacological and genetic studies in the mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef]
- Herborn, K.A.; Graves, J.L.; Jerem, P.; Evans, N.P.; Nager, R.; McCafferty, D.J.; McKeegan, D.E.F. Skin temperature reveals the intensity of acute stress. Physiol. Behav. 2015, 152, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Cabanac, A.J.; Guillemette, M. Temperature and heart rate as stress indicators of handled common eider. Physiol. Behav. 2001, 74, 475–479. [Google Scholar] [CrossRef]
- Gray, D.A.; Maloney, S.K.; Kamerman, P.R. Restraint increases afebrile body temperature but attenuates fever in Pekin ducks (Anas platyrhynchos). Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R1666–R1671. [Google Scholar] [CrossRef] [Green Version]
- de Aguiar Bittencourt, M.; Melleu, F.F.; Marino-Neto, J. Stress-induced core temperature changes in pigeons (Columba livia). Physiol. Behav. 2015, 139, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Foster, S.; Ijichi, C. The association between infrared thermal imagery of core eye temperature, personality, age and housing in cats. Appl. Anim. Behav. Sci. 2017, 189, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Vinkers, C.H.; Penning, R.; Hellhammer, J.; Verster, J.C.; Klaessens, J.H.G.M.; Olivier, B.; Kalkman, C.J. The effect of stress on core and peripheral body temperature in humans. Stress 2013, 16, 520–530. [Google Scholar] [CrossRef]
- Gormally, B.M.G.; Romero, L.M. What are you actually measuring? A review of techniques that integrate the stress response on distinct time-scales. Funct. Ecol. 2020, 34, 2030–2044. [Google Scholar] [CrossRef]
- Jerem, P.; Herborn, K.; McCafferty, D.; McKeegan, D.; Nager, R. Thermal imaging to study stress non-invasively in unrestrained birds. J. Vis. Exp. 2015, 2015, 53184. [Google Scholar] [CrossRef] [Green Version]
- Jerem, P.; Jenni-Eiermann, S.; McKeegan, D.; McCafferty, D.J.; Nager, R.G. Eye region surface temperature dynamics during acute stress relate to baseline glucocorticoids independently of environmental conditions. Physiol. Behav. 2019, 210, 112627. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.K.; Mastromonaco, G.; Burness, G. Evidence that stress-induced changes in surface temperature serve a thermoregulatory function. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef]
- Winder, L.A.; White, S.A.; Nord, A.; Helm, B.; McCafferty, D.J. Body surface temperature responses to food restriction in wild and captive great tits. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef] [PubMed]
- Jerem, P.; Jenni-Eiermann, S.; Herborn, K.; McKeegan, D.; McCafferty, D.J.; Nager, R.G. Eye region surface temperature reflects both energy reserves and circulating glucocorticoids in a wild bird. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Herborn, K.A.; Jerem, P.; Nager, R.G.; McKeegan, D.E.F.; McCafferty, D.J. Surface temperature elevated by chronic and intermittent stress. Physiol. Behav. 2018, 191, 47–55. [Google Scholar] [CrossRef]
- Tabh, J.K.R.; Burness, G.; Wearing, O.H.; Tattersall, G.J.; Mastromonaco, G.F. Infrared thermography as a technique to measure physiological stress in birds: Body region and image angle matter. Physiol. Rep. 2021, 9, e14865. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Edgar, J.L.; Nicol, C.J.; Pugh, C.A.; Paul, E.S. Surface temperature changes in response to handling in domestic chickens. Physiol. Behav. 2013, 119, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Stempniewicz, L. BWP update. Little Auk (Alle alle). J. Birds West. Palearct. 2001, 3, 175–201. [Google Scholar]
- Wojczulanis-Jakubas, K.; Jakubas, D.; Stempniewicz, L. The Little Auk Alle alle: An ecological indicator of a changing Arctic and a model organism. Polar Biol. 2022, 45, 163–176. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Deviche, P.; Sharbaugh, S.; Astheimer, L.B.; Holberton, R.; Suydam, R.; Hunt, K. Seasonal changes of the adrenocortical responses to stress in redpolls, Acanthis flammea, in Alaska. J. Exp. Zool. 1994, 270, 372–380. [Google Scholar] [CrossRef]
- Johnstone, C.P.; Reina, R.D.; Lill, A. Interpreting indices of physiological stress in free-living vertebrates. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Wojczulanis-Jakubas, K.; Jakubas, D.; Chastel, O. Behavioural and hormonal stress responses during chick rearing do not predict brood desertion by female in a small Arctic seabird. Horm. Behav. 2013, 64, 448–453. [Google Scholar] [CrossRef]
- Wojczulanis-Jakubas, K.; Jakubas, D.; Stempniewicz, L. Sex-specific parental care by incubating little auks (Alle alle). Ornis Fenn. 2009, 86, 140–148. [Google Scholar]
- Jakubas, D.; Wojczulanis, K. Predicting the Sex of Dovekies by Discriminant Analysis. Waterbirds 2007, 30, 92–96. [Google Scholar] [CrossRef]
- Wojczulanis-Jakubas, K.; Jakubas, D.; Chastel, O.; Kulaszewicz, I. A big storm in a small body: Seasonal changes in body mass, hormone concentrations and leukocyte profile in the little auk (Alle alle). Polar Biol. 2015, 38, 1203–1212. [Google Scholar] [CrossRef]
- Wojczulanis-Jakubas, K.; Jakubas, D.; Kulpińska-Chamera, M.; Chastel, O. Sex- and breeding stage-specific hormonal stress response of seabird parents. Horm. Behav. 2018, 103, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Keslinka, L.K.; Wojczulanis-Jakubas, K.; Jakubas, D.; Neubauer, G. Determinants of the little auk (Alle alle) breeding colony location and size in W and NW coast of Spitsbergen. PLoS ONE 2019, 14, e0212668. [Google Scholar] [CrossRef]
- Nord, A.; Folkow, L.P. Ambient temperature effects on stress-induced hyperthermia in Svalbard ptarmigan. Biol. Open 2019, 8, bio043497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Zhang, H.F.; Du, R.; Gu, X.H.; Zhang, Z.Y.; Buyse, J.; Decuypere, E. Thermoregulation responses of broiler chickens to humidity at different ambient temperatures. II. Four weeks of age. Poult. Sci. 2005, 84, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiris, P.T. Thermophysical and transport properties of humid air at temperature range between 0 and 100 °C. Energy Convers. Manag. 2008, 49, 1098–1110. [Google Scholar] [CrossRef]
- Playà-Montmany, N.; Tattersall, G.J. Spot size, distance and emissivity errors in field applications of infrared thermography. Methods Ecol. Evol. 2021, 12, 828–840. [Google Scholar] [CrossRef]
- Griffiths, R.; Double, M.C.; Orr, K.; Dawson, R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998, 7, 1071–1075. [Google Scholar] [CrossRef]
- Pakkala, J.J.; Ryan Norris, D.; Newman, A.E.M. An experimental test of the capture-restraint protocol for estimating the acute stress response. Physiol. Biochem. Zool. 2013, 86, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, T.M.; Gaston, K.J.; Loder, N. Geographic gradients in body size: A clarification of Bergmann’s rule. Divers. Distrib. 1999, 5, 165–174. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach, 2nd ed.; Spinger: Berlin, Germany, 2002; ISBN 0387953647. [Google Scholar]
- Hegyi, G.; Garamszegi, L.Z. Using information theory as a substitute for stepwise regression in ecology and behavior. Behav. Ecol. Sociobiol. 2011, 65, 69–76. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.10.0. 2017. Available online: https://r-forge.r-project.org/projects/mumin/ (accessed on 1 February 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef]
- Halekoh, U.; Højsgaard, S. A kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models-the R package pbkrtest. J. Stat. Softw. 2014, 59, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R News 2002, 2, 7–10. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Romero, L.M.; Soma, K.K.; Wingfield, J.C. Hypothalamic-pituitary-adrenal axis changes allow seasonal modulation of corticosterone in a bird. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 274, R1338–R1344. [Google Scholar] [CrossRef]
- Romero, L.M. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 2002, 128, 1–24. [Google Scholar] [CrossRef]
- Goymann, W.; Trappschuh, M.; Urasa, F. Corticosterone concentrations reflect parental expenditure in contrasting mating systems of two coucal species. Front. Ecol. Evol. 2017, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Jenni-Eiermann, S.; Glaus, E.; Grüebler, M.; Schwabl, H.; Jenni, L. Glucocorticoid response to food availability in breeding barn swallows (Hirundo rustica). Gen. Comp. Endocrinol. 2008, 155, 558–565. [Google Scholar] [CrossRef]
- Hill, R.W.; Beaver, D.L.; Veghte, J.H. Body Surface Temperatures and Thermoregulation in the Black-Capped Chickadee (Parus atricapillus). Physiol. Zool. 1980, 53, 305–321. [Google Scholar] [CrossRef]
- Carere, C.; Van Oers, K. Shy and bold great tits (Parus major): Body temperature and breath rate in response to handling stress. Physiol. Behav. 2004, 82, 905–912. [Google Scholar] [CrossRef]
- Sharma, H.S.; Hoopes, P.J. Hyperthermia induced pathophysiology of the central nervous system. Int. J. Hyperth. 2009, 19, 325–354. [Google Scholar] [CrossRef] [PubMed]
- Sundgren-Andersson, A.K.; Östlund, P.; Bartfai, T. Simultaneous Measurement of Brain and Core Temperature in the Rat during Fever, Hyperthermia, Hypothermia and Sleep. Neuroimmunomodulation 1998, 5, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P. Body temperature and fever in a free-living bird. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2010, 156, 68–74. [Google Scholar] [CrossRef]
- Lewden, A.; Nord, A.; Petit, M.; Vézina, F. Body temperature responses to handling stress in wintering Black-capped Chickadees (Poecile atricapillus L.). Physiol. Behav. 2017, 179, 49–54. [Google Scholar] [CrossRef]
- Humphries, M.M.; Careau, V. Heat for nothing or activity for free? Evidence and implications of activity-thermoregulatory heat substitution. In Proceedings of the Integrative and Comparative Biology; Oxford Academic: Oxford, UK, 2011; Volume 51, pp. 419–431. [Google Scholar]
- O’Connor, R.S.; Le Pogam, A.; Young, K.G.; Robitaille, F.; Choy, E.S.; Love, O.P.; Elliott, K.H.; Hargreaves, A.L.; Berteaux, D.; Tam, A.; et al. Limited heat tolerance in an Arctic passerine: Thermoregulatory implications for cold-specialized birds in a rapidly warming world. Ecol. Evol. 2021, 11, 1609–1619. [Google Scholar] [CrossRef]
- Box, J.E.; Colgan, W.T.; Christensen, T.R.; Schmidt, N.M.; Lund, M.; Parmentier, F.J.W.; Brown, R.; Bhatt, U.S.; Euskirchen, E.S.; Romanovsky, V.E.; et al. Key indicators of Arctic climate change: 1971–2017. Environ. Res. Lett. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Cohen, J.; Zhang, X.; Francis, J.; Jung, T.; Kwok, R.; Overland, J.; Ballinger, T.J.; Bhatt, U.S.; Chen, H.W.; Coumou, D.; et al. Divergent Consensuses on Arctic Amplification Influence on Midlatitude Severe Winter Weather; Nature Research: Berlin, Germany, 2020; Volume 10. [Google Scholar]
- Gabrielsen, G.W.; Taylor, J.R.E.; Konarzewski, M.; Mehlum, F. Field and Laboratory Metabolism and Thermoregulation in Dovekies (Alle alle). Auk 1991, 108, 71–78. [Google Scholar]
- Gaston, A.J.; Elliott, K.H. Effects of climate-induced changes in parasitism, predation and Predator-Predator interactions on reproduction and survival of an Arctic Marine Bird. Arctic 2013, 66, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Jakubas, D.; Wojczulanis-Jakubas, K.; Iliszko, L.M.; Strøm, H.; Stempniewicz, L. Habitat foraging niche of a High Arctic zooplanktivorous seabird in a changing environment. Sci. Rep. 2017, 7, 16203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, G.A.; Macdonald, R.W.; Outridge, P.M.; Wilson, S.; Chételat, J.; Cole, A.; Hintelmann, H.; Loseto, L.L.; Steffen, A.; Wang, F.; et al. How does climate change influence arctic mercury? Sci. Total Environ. 2012, 414, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Fort, J.; Robertson, G.J.; Grémillet, D.; Traisnel, G.; Bustamante, P. Spatial Ecotoxicology: Migratory Arctic Seabirds Are Exposed to Mercury Contamination While Overwintering in the Northwest Atlantic. Environ. Sci. Technol. 2014, 48, 11560–11567. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Q.; Macaballug, P.; Chen, H.; Hodach, K.; Tang, S.; Francis, J.S. Infrared thermography is an effective, noninvasive measure of HPA activation. Stress 2020, 24, 584–589. [Google Scholar] [CrossRef]
- Buchanan, K.L.; Burt de Perera, T.; Carere, C.; Carter, T.; Hailey, A.; Hubreacht, R.; Jenning, D.J.; Metcalfe, N.B.; Pitcher, T.E.; Péron, F.; et al. Guidelines for the Use of Animals Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2012, 83, 301–309. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLOS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]


| Model Parameters | df | logLik | AICc | ΔAICc | Akaike’s Weights |
|---|---|---|---|---|---|
| global model #1: TEYE ~ Session ∗ Sex ∗ AMBT ∗ Humidity + (1|Bird ID), N = 41 | |||||
| Int + AMBT + Session + (1|Bird ID) | 5 | −182.189 | 375.2 | 0.00 | 0.530 |
| Int + AMBT + Session + Sex + (1|Bird ID) | 6 | −181.684 | 376.5 | 1.32 | 0.274 |
| Int + AMBT + (1|Bird ID) | 4 | −184.323 | 377.2 | 2.00 | 0.195 |
| global model #2: TEYE ~ Session ∗ BodySize ∗ AMBT ∗ Humidity + (1|Bird ID), N = 41 | |||||
| Int + AMBT + Session + (1|Bird ID) | 5 | −182.189 | 375.2 | 0.00 | 0.564 |
| Int + AMBT + Session + BodySize + (1|Bird ID) | 6 | −181.932 | 377.0 | 1.81 | 0.228 |
| Int + AMBT + (1|Bird ID) | 4 | −184.323 | 377.2 | 2.00 | 0.208 |
| global model #3: TEYE ~ Session ∗ BodyCond ∗ AMBT ∗ Humidity + (1|Bird ID), N = 38 | |||||
| Int + AMBT + Session + (1|Bird ID) | 5 | −169.920 | 350.7 | 0.0 | 0.701 |
| Int + AMBT + (1|Bird ID) | 4 | −171.919 | 352.4 | 1.7 | 0.299 |
| global model #4: log(CORT) ~ Session ∗ Sex ∗ TEYE ∗ AMBT + (1|Bird ID), N = 35 | |||||
| Int + Session + (1|Bird ID) | 4 | −73.448 | 155.5 | 0.00 | 1.000 |
| global model #5: log(CORT) ~ Session ∗ BodySize ∗ TEYE ∗ AMBT + (1|Bird ID), N = 35 | |||||
| Int + Session + (1|Bird ID) | 4 | −73.448 | 155.5 | 0.00 | 1.000 |
| global model #6: log(CORT) ~ Session ∗ BodyCond ∗ TEYE ∗ AMBT + (1|Bird ID), N = 33 | |||||
| Int + Session + (1|Bird ID) | 4 | −69.197 | 147.0 | 0.00 | 1.000 |
| Response Variable | Predictor | Estimate | SE | df | t | p |
|---|---|---|---|---|---|---|
| The highest-ranked model within the global LMM #1, R2c = 0.616 | ||||||
| TEYE | Intercept | 22.895 | 1.298 | 60.268 | 17.636 | <0.001 |
| Session | 0.887 | 0.423 | 47.841 | 2.100 | 0.0411 | |
| AMBT | 0.600 | 0.125 | 59.838 | 4.782 | <0.001 | |
| Bird ID r.e. | 19.851 | - | 2 | - | <0.001 | |
| The highest-ranked model within the global LMM #4, R2c = 0.712 | ||||||
| log(CORT) | Intercept | 1.620 | 0.114 | 68 | 14.17 | <0.001 |
| Session | 2.111 | 0.162 | 68 | 13.06 | <0.001 | |
| Bird ID r.e. | 170.6 | - | 1 | <0.001 | ||
| Model Parameters | df | logLik | AICc | ΔAICc | Akaike’s Weights |
|---|---|---|---|---|---|
| global model #1: TEYE SR ~ Sex ∗ CORT SR ∗ CORT BL ∗ AMBT BL, N = 35 | |||||
| Int | 2 | −78.810 | 162.0 | 0.00 | 0.527 |
| Int + CORT SR | 3 | −78.308 | 163.4 | 1.39 | 0.262 |
| Int + AMBT BL | 3 | −78.526 | 163.8 | 1.83 | 0.211 |
| global model #2: TEYE SR ~ BodySize ∗ CORT SR ∗ CORT BL ∗ AMBT BL, N = 35 | |||||
| Int | 2 | −78.810 | 162.0 | 0.00 | 0.410 |
| Int + BodySize | 3 | −78.228 | 163.2 | 1.23 | 0.221 |
| Int + CORT SR | 3 | −78.308 | 163.4 | 1.39 | 0.204 |
| Int + CORT BL | 3 | −78.526 | 163.8 | 1.83 | 0.164 |
| global model #3: TEYE SR ~ log(BodyCond) ∗ CORT SR ∗ CORT BL ∗ AMBT BL, N = 32 | |||||
| Int | 2 | −72.115 | 148.6 | 0.00 | 0.552 |
| Int + CORT SR | 3 | −71.727 | 150.3 | 1.67 | 0.240 |
| Int + CORT BL | 3 | −71.865 | 150.6 | 1.94 | 0.209 |
| Response Variable | Predictor | Estimate | SE | df | F | p |
|---|---|---|---|---|---|---|
| The second-highest ranked model within the global LM#1, R2 = 0.028 | ||||||
| TEYE SR | CORT SR | −0.012 | 0.012 | 1 | 0.961 | 0.334 |
| The second-highest ranked model within the global LM#2, R2 = 0.033 | ||||||
| TEYE SR | BodySize | −15.980 | 16.770 | 1 | 1.117 | 0.298 |
| The second-highest ranked model within the global LM#3, R2 = 0.024 | ||||||
| TEYE SR | CORT SR | −0.010 | 0.012 | 1 | 0.738 | 0.397 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubas, D.; Wojczulanis-Jakubas, K.; Grissot, A.; Devogel, M.; Cendrowska, M.; Chastel, O. Eye Region Surface Temperature and Corticosterone Response to Acute Stress in a High-Arctic Seabird, the Little Auk. Animals 2022, 12, 499. https://doi.org/10.3390/ani12040499
Jakubas D, Wojczulanis-Jakubas K, Grissot A, Devogel M, Cendrowska M, Chastel O. Eye Region Surface Temperature and Corticosterone Response to Acute Stress in a High-Arctic Seabird, the Little Auk. Animals. 2022; 12(4):499. https://doi.org/10.3390/ani12040499
Chicago/Turabian StyleJakubas, Dariusz, Katarzyna Wojczulanis-Jakubas, Antoine Grissot, Marion Devogel, Martyna Cendrowska, and Olivier Chastel. 2022. "Eye Region Surface Temperature and Corticosterone Response to Acute Stress in a High-Arctic Seabird, the Little Auk" Animals 12, no. 4: 499. https://doi.org/10.3390/ani12040499






