The Shape of the Nasal Cavity and Adaptations to Sniffing in the Dog (Canis familiaris) Compared to Other Domesticated Mammals: A Review Article
Abstract
Simple Summary
Abstract
1. Introduction
2. Functional Anatomy of Smell
3. The Nose Structure and Airflow Routes in Animals
4. Comparison of the Nasal Structure in Dogs with Those of Other Domestic Animal Species in Terms of Olfactory Ability
4.1. Dogs
4.2. Cats
4.3. Horses
4.4. Pigs
4.5. Ruminants
5. Brachycephalia, the Brachycephalic Syndrome and Anatomical Changes in the Nose Balances in Dogs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lord, K.; Schneider, R.A.; Coppinger, R. In The Origins and Evolution. In The Domestic Dog: Its Evolution, Behaviour and Interactions with People; Cambridge University Press: Cambridge, UK, 2016; Volume 4, pp. 21–50. [Google Scholar]
- Schoenebeck, J.J.; Ostrander, E.A. The genetics of canine skull shape variation. Genetics 2013, 193, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sablin, M.; Khlopachev, G. The earliest ice age dogs: Evidence from Eliseevichi. Curr. Anthropol. 2002, 43, 795–799. [Google Scholar] [CrossRef]
- Coppinger, R.; Coppinger, L. Dogs: A Startling New Understanding of Canine Origin, Behaviour, and Evolution; Crosskeys: London, UK, 2004. [Google Scholar]
- Plogmann, D.; Kruska, D. Volumetric comparison of auditory structures in the brains of european wild boars (sus scrofa) and domestic pigs (sus scrofa f. dom.). Brain Behav. Evol. 1990, 35, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, P.; Röhrs, M. Volumetric analysis of brain structures, especially of the visual system in wild and domestic turkeys (Meleagris gallopavo). J. Hirnforsch. 1995, 36, 219–228. [Google Scholar]
- Kruska, D. The effect of domestication on brain size and composition in the mink (mustela vison). J. Zool. 1996, 239, 645–661. [Google Scholar] [CrossRef]
- Kruska, D. Mammalian domestication and its effect on brain structure and behaviour. In Intelligence and Evolutionary Biology; Springer: Berlin/Heidelberg, Germany, 1988; pp. 211–250. [Google Scholar] [CrossRef]
- Lord, K.; Schneider, R.A.; Coppinger, R. Evolution of working dogs. In The Domestic Dog: Its Evolution, Behaviour and Interactions with People; Cambridge University Press: Cambridge, UK, 2016; pp. 42–66. [Google Scholar] [CrossRef]
- Zeder, M.A. Pathways to animal domestication. In Biodiversity in Agriculture; Cambridge University Press: Cambridge, UK, 2012; pp. 227–259. [Google Scholar] [CrossRef]
- Trut, L. Early canid domestication: The farm-fox experiment. Am. Sci. 1999, 87, 160–169. [Google Scholar] [CrossRef]
- Schoenebeck, J.J.; Hutchinson, S.A.; Byers, A.; Beale, H.C.; Carrington, B.; Faden, D.L.; Rimbault, M.; Decker, B.; Kidd, J.M.; Sood, R.; et al. Variation of BMP3 contributes to dog breed skull diversity. PLoS Genet. 2012, 8, e1002849. [Google Scholar] [CrossRef]
- Ghosh, S.; Jana, A.; Mahanti, B. An updated review on medical detection of dog. Asian J. Pharm. Anal. 2016, 6, 47–52. [Google Scholar] [CrossRef]
- Kokocińska-Kusiak, A.; Woszczyło, M.; Zybala, M.; Maciocha, J.; Barłowska, K.; Dzięcioł, M. Canine olfaction: Physiology, behaviour, and possibilities for practical applications. Animals 2021, 11, 2463. [Google Scholar] [CrossRef]
- Marchal, S.; Bregeras, O.; Puaux, D.; Gervais, R.; Ferry, B. Rigorous training of dogs leads to high accuracy in human scent matching-to-sample performance. PLoS ONE 2016, 11, e0146963. [Google Scholar] [CrossRef]
- Cambau, E.; Poljak, M. Sniffing animals as a diagnostic tool in infectious diseases. Clin. Microbiol. Infec. 2020, 26, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.B.; Walker, J.C.; Cavnar, P.J.; Taylor, J.L.; Pickel, D.H.; Hall, S.B.; Suarez, J.C. Naturalistic quantification of canine olfactory sensitivity. Appl. Anim. Behav. Sci. 2006, 97, 241–254. [Google Scholar] [CrossRef]
- Jezierski, T.; Ensminger, J.; Papet, L.E. Canine Olfaction Science and Law: Advances in Forensic Science, Medicine, Conservation, and Environmental Remediation; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Taherali, F.; Varum, F.; Basit, A.W. A slippery slope: On the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 2018, 123, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Galibert, F.; Azzouzi, N.; Quignon, P.; Chaudieu, G. The genetics of canine olfaction. J. Vet. Behav. 2016, 16, 86–93. [Google Scholar] [CrossRef]
- Evans, H.E.; Delahunta, A.; Miller, M.E. Miller’s Anatomy of the Dog; Elsevier Pte. Ltd.: Singapore, 2016; pp. 122–218. [Google Scholar]
- Jenkins, E.K.; DeChant, M.T.; Perry, E.B. When the nose doesn’t know: Canine olfactory function associated with health, management, and potential links to microbiota. Front Vet. Sci. 2018, 5, 56. [Google Scholar] [CrossRef]
- Harper, R.J.; Furton, K.G. Biological detection of explosives. In Counterterrorist Detection Techniques of Explosives; Yinon, J., Ed.; Elsevier: Amsterdam, The Netherland, 2007; pp. 395–431. [Google Scholar]
- Trotier, D. Vomeronasal organ and human pheromones. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 184–190. [Google Scholar] [CrossRef]
- Dennis, J.C.; Allgier, J.G.; Desouza, L.S.; Eward, W.C.; Morrison, E.E. Immunohistochemistry of the canine vomeronasal organ. J. Anat. 2003, 203, 329–338. [Google Scholar] [CrossRef]
- Salazar, I.; Cifuentes, J.M.; Sánchez-Quinteiro, P. Morphological and immunohistochemical features of the vomeronasal system in dogs. Anat. Rec. 2013, 296, 146–155. [Google Scholar] [CrossRef]
- Adams, D.R.; Wiekamp, M.D. The canine vomeronasal organ. J. Anat. 1984, 138, 771–787. [Google Scholar]
- Lee, K.H.; Park, C.; Kim, J.; Moon, C.; Ahn, M.; Shin, T. Histological and lectin histochemical studies of the vomeronasal organ of horses. Tissue Cell 2016, 48, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Dzięcioł, M.; Podgórski, P.; Stańczyk, E.; Szumny, A.; Woszczyło, M.; Pieczewska, B.; Niżański, B.; Nicpoń, W.; Wrzosek, M.A. MRI Features of the Vomeronasal Organ in Dogs (Canis Familiaris). Front. Vet. Sci. 2020, 7, 159. [Google Scholar] [CrossRef]
- Asproni, P.; Cozzi, A.; Verin, R.; Lafont-Lecuelle, C.; Bienboire-Frosini, C.; Poli, A.; Pageat, P. Pathology and behaviour in feline medicine: Investigating the link between vomeronasalitis and aggression. J. Feline Med. Surg. 2016, 18, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Crowell-Davis, S.; Houpt, K.A. The ontogeny of flehmen in horses. Anim. Behav. 1985, 33, 739–745. [Google Scholar] [CrossRef]
- Pageat, P.; Gaultier, E. Current research in canine and feline pheromones. Vet. Clin. Small. Anim. 2003, 33, 187–211. [Google Scholar] [CrossRef]
- DePorter, T.L. Use of pheromones in feline practice. In Feline Behavioural Health and Welfare; Rodan, I., Heath, S., Eds.; Saunders: London, UK, 2015; pp. 235–244. [Google Scholar]
- Barrios, A.W.; Sánchez-Quinteiro, P.; Salazar, I. Dog and mouse: Toward a balanced view of the mammalian olfactory system. Front. Neuroanat. 2014, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Craven, B.A.; Paterson, E.G.; Settles, G.S. The fluid dynamics of canine olfaction: Unique nasal airflow patterns as an explanation of macrosmia. J. R. Soc. Interface 2009, 7, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Jendrny, P.; Schulz, C.; Twele, F.; Meller, S.; von Köckritz-Blickwede, M.; Osterhaus, A.D.M.E.; Ebbers, J.; Pilchová, V.; Pink, I.; Welte, T.; et al. Scent dog identification of samples from COVID-19 patients—A pilot study. BMC Infec. Dis. 2020, 20, 536. [Google Scholar] [CrossRef]
- Sakr, S.; Ghaddar, A.; Sheet, I.; Eid, A.H.; Hamam, B. Knowledge, attitude and practices related to COVID-19 among young lebanese population. BMC Public Health 2021, 21, 653. [Google Scholar] [CrossRef]
- Mason, M.J.; Wenger, L.M.D.; Hammer, Ø.; Blix, A.S. Structure and function of respiratory turbinates in phocid seals. Polar. Biol. 2020, 43, 157–173. [Google Scholar] [CrossRef]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy, 5th ed.; Saunders: St. Louis, MO, USA; Elsevier: St. Louis, MO, USA, 2010. [Google Scholar]
- König, H.E.; Liebich, H. Anatomia zwierząt domowych. Galaktyka 2002, 1–66. (In Polsh) [Google Scholar]
- D’Arce, R.D.; Flechtmann, C.H.W. Introdução à Anatomia e Fisiologia Animal; Nobel: São Paulo, Brazil, 1989; pp. 37–39. [Google Scholar]
- Sisson, S.; Grossman, J.D.; Getty, R. Sisson and Grossman’s Anatomy of the Domestic Animal; Saunders: Philadelphia, PA, USA, 1975; pp. 1377–1411. [Google Scholar]
- Gracis, M. Radiographic study of the maxillary canine tooth of four mesaticephalic cats. J. Vet. Dent. 1999, 16, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.E.; Miller, M.E. Miller’s Anatomy of the Dog; Saunders: Philadelphia, PA, USA, 1993; pp. 128–168. [Google Scholar]
- Ginn, J.A.; Kumar, M.S.A.; McKiernan, B.C.; Powers, B.E. Nasopharyngeal turbinates in brachycephalic dogs and cats. J. Am. Anim. Hosp. Assoc. 2008, 44, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, E.A. Both ends of the leash—The human links to good dogs with bad genes. N. Engl. J. Med. 2012, 367, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.J.; Smith, D.W.; Wynne, C.D.L. Effect of odour preexposure on acquisition of an odour discrimination in dogs. Learn Behav. 2014, 42, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Polgár, Z.; Kinnunen, M.; Újváry, D.; Miklósi, Á.; Gácsi, M. A Test of canine olfactory capacity: Comparing various dog breeds and wolves in a natural detection task. PLoS ONE 2016, 11, e0154087. [Google Scholar] [CrossRef]
- Jezierski, T.; Adamkiewicz, E.; Walczak, M.; Sobczyńska, M.; Górecka-Bruzda, A.; Ensminger, J.; Papet, E. Efficacy of drug detection by fully-trained police dogs varies by breed, training level, type of drug and search environment. Forensic Sci. Int. 2014, 237, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; McGreevy, P.; Valenzuela, M. Human induced rotation and reorganization of the brain of domestic dogs. PLoS ONE 2010, 5, e11946. [Google Scholar] [CrossRef]
- Adamkiewicz, E.; Jezierski, T.; Walczak, M.; Górecka-Bruzda, A.; Sobczyńska, M.; Prokopczyk, M.; Ensminger, J. Traits of drug and explosive detection in dogs of two breeds as evaluated by their handlers and trainers. Anim. Sci. Rep. 2013, 31, 205–217. [Google Scholar]
- Lesniak, A.; Walczak, M.; Jezierski, T.; Sacharczuk, M.; Gawkowski, M.; Jaszczak, K. Canine olfactory receptor gene polymorphism and its relation to odour detection performance by sniffer dogs. J. Hered. 2008, 99, 518–527. [Google Scholar] [CrossRef]
- Svartberg, K. Shyness-boldness predicts performance in working dogs. Appl. Anim. Behav. Sci. 2002, 79, 157–174. [Google Scholar] [CrossRef]
- Bird, D.J.; Jacquemetton, C.; Buelow, S.A.; Evans, A.W.; Van Valkenburgh, B. Domesticating olfaction: Dog breeds, including scent hounds, have reduced cribriform plate morphology relative to wolves. Anat. Rec. 2021, 304, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Nickel, R.; Schummer, A.; Seiferle, E.; Sack, W.O. Respiratory system. In The Viscera of the Domestic Mammals; Springer: Cham, Germany, 1979; pp. 211–281. [Google Scholar] [CrossRef]
- Asher, L.; Diesel, G.; Summers, J.F.; McGreevy, P.D.; Collins, L.M. Inherited defects in pedigree dogs. Part 1: Disorders related to breed standards. Vet. J. 2009, 182, 402–411. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Hendricks, A.; Tivers, M.S.; Burn, C.C. Impact of facial conformation on canine health: Brachycephalic obstructive airway syndrome. PLoS ONE 2015, 10, e0137496. [Google Scholar] [CrossRef] [PubMed]
- Stockard, C.R.; Anderson, O.D.; James, W.T. The Genetic and Endocrinic Basis for Differences in Form and Behaviour: As Elucidated by Studies of Contrasted Pure-Line Dog Breeds and Their Hybrids; The Wistar Institute of Anatomy and Biology: Philadelphia, PA, USA, 1941. [Google Scholar]
- Losonsky, J.M.; Abbott, L.C.; Kuriashkin, I.V. Computed tomography of the normal feline nasal cavity and paranasal sinuses. Vet. Radiol. Ultrasoun. 1997, 38, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, C.; Budras, K.D.; Ludewig, E.; Mayrhofer, E.; Koenig, H.E.; Walter, A.; Oechtering, G.U. Brachycephalic feline noses: CT and anatomical study of the relationship between head conformation and the nasolacrimal drainage system. J. Feline Med. Surg. 2009, 11, 891–900. [Google Scholar] [CrossRef]
- Hayden, S.; Bekaert, M.; Crider, T.A.; Mariani, S.; Murphy, W.J.; Teeling, E.C. Ecological adaptation determines functional mammalian olfactory subgenomes. Genome Res. 2009, 20, 1–9. [Google Scholar] [CrossRef]
- Pang, B.; Yee, K.K.; Lischka, F.W.; Rawson, N.E.; Haskins, M.E.; Wysocki, C.J.; Craven, B.A.; Van Valkenburgh, B. The influence of nasal airflow on respiratory and olfactory epithelial distribution in felids. J. Exp. Biol. 2016, 219, 1866–1874. [Google Scholar] [CrossRef]
- Lawson, M.J.; Craven, B.A.; Paterson, E.G.; Settles, G.S.A. Computational study of odourant transport and deposition in the canine nasal cavity: Implications for olfaction. Chem. Senses 2012, 37, 553–566. [Google Scholar] [CrossRef]
- Zhao, K.; Dalton, P.; Yang, G.C.; Scherer, P.W. Numerical modeling of turbulent and laminar airflow and odourant transport during sniffing in the human and rat nose. Chem. Senses 2005, 31, 107–118. [Google Scholar] [CrossRef]
- Yang, G.C.; Scherer, P.W.; Mozell, M.M. Modeling inspiratory and expiratory steady-state velocity fields in the sprague-dawley rat nasal cavity. Chem. Senses 2007, 32, 215–223. [Google Scholar] [CrossRef][Green Version]
- Yang, G.C.; Scherer, P.W.; Zhao, K.; Mozell, M.M. Numerical modeling of odourant uptake in the rat nasal cavity. Chem. Senses 2007, 32, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Craven, B.A.; Paterson, E.G.; Settles, G.S.; Lawson, M.J. Development and verification of a high-fidelity computational fluid dynamics model of canine nasal airflow. J. Biomech. Eng. 2009, 131, 091002. [Google Scholar] [CrossRef] [PubMed]
- Vlaminck, L.; Crijns, C.; Gielen, I. Nasal cavity and sinuses in equines. In Proceedings of the 3rd International CT-User Meeting, Ghent, Belgium, 13–14 November 2020; pp. 148–149. [Google Scholar]
- Krüger, K.; Flauger, B. Olfactory recognition of individual competitors by means of faeces in horse (Equus caballus). Anim. Cogn. 2011, 14, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Merkies, K.; Paraschou, G.; McGreevy, P.D. Morphometric characteristics of the skull in horses and donkeys—A pilot study. Animals 2020, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Saslow, C.A. Understanding the perceptual world of horses. Appl. Anim. Behav. Sci. 2002, 78, 209–224. [Google Scholar] [CrossRef]
- Salazar, I.; Quinteiro, P.S.; Cifuentes, J.M. The soft-tissue components of the vomeronasal organ in pigs, cows and horses. Anat. Histol. Embryol. J. Vet. Med. Ser. C 1997, 26, 179–186. [Google Scholar] [CrossRef]
- Rørvang, M.V.; Nielsen, B.L.; McLean, A.N. Sensory abilities of horses and their importance for equitation science. Front. Vet. Sci. 2020, 633. [Google Scholar] [CrossRef]
- Holcombe, S.J.; Ducharme, N.G. Upper airway function of normal horses during exercise. In Equine Exercise Physiology: The Science of Exercise in the Athletic Horse, 1st ed.; Hinchcliff, K.W., Geor, R.J., Kaneps, A.J., Eds.; Elsevier: Saunder, UK; St. Louis, MO, USA, 2008; pp. 170–192. [Google Scholar]
- Smith, L.C.R. Surgical access to the nasal cavities in the horse. Equine Vet. Educ. 2016, 29, 70–71. [Google Scholar] [CrossRef]
- Pond, W.G.; Houpt, K.A. The Biology of the Pig; Comstock Pub. Associates: Ithaca, NY, USA, 1978. [Google Scholar]
- Watson, L. The Whole Hog: Exploring the Extraordinary Potential of Pigs; Smithsonian Books: Washington, DC, USA, 2004. [Google Scholar]
- Brunjes, P.C.; Feldman, S.; Osterberg, S.K. The pig olfactory brain: A primer. Chem. Senses 2016, 41, 415–425. [Google Scholar] [CrossRef]
- Nguyen, D.; Lee, K.; Choi, H.; Choi, M.; Le, M.; Song, N.; Kim, J.H.; Seo, H.; Oh, J.W.; Lee, K.; et al. The complete swine olfactory subgenome: Expansion of the olfactory gene repertoire in the pig genome. BMC Genom. 2012, 13, 584. [Google Scholar] [CrossRef]
- Paudel, Y.; Madsen, O.; Megens, H.J.; Frantz, L.A.F.; Bosse, M.; Crooijmans, R.P.M.A.; Groenen, M.A.M. Copy number variation in the speciation of pigs: A possible prominent role for olfactory receptors. BMC Genom. 2015, 16, 330. [Google Scholar] [CrossRef] [PubMed]
- Splivallo, R.; Ottonello, S.; Mello, A.; Karlovsky, P. Truffle volatiles: From chemical ecology to aroma biosynthesis. New Phytol. 2010, 189, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Food, health and agricultural importance of truffles: A review of current scientific literature. Curr. Trends Biotechnol. Pharm. 2012, 6, 15–27. [Google Scholar]
- Eshrah, E.A.; Kassab, A.A. Scanning electron microscopy and histomorphology of the epiglottis in dromedaries: A Study revealing unusual structure with the probable functional role. Microsc. Res. Techniq. 2019, 82, 1353–1358. [Google Scholar] [CrossRef]
- Barrios, A.W.; Sanchez Quinteiro, P.; Salazar, I. The nasal cavity of the sheep and its olfactory sensory epithelium. Microsc. Res. Tech. 2014, 77, 1052–1059. [Google Scholar] [CrossRef]
- Krysiak, K.; Świeżyński, K. Anatomia Zwierząt. 2, Narządy Wewnętrzne i Układ Krążenia; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2012. (In Polish) [Google Scholar]
- Metwally, M.A.; Hussieni, H.B.; Kassab, A.A.; Eshrah, E.A. Comparative anatomy of the nasal cavity in buffaloes, camels and donkeys. J. Adv. Vet. Res. 2019, 9, 69–75. [Google Scholar]
- Padodara, R. Olfactory sense in different animals. Indian J. Vet. Sci. 2014, 2, 1–14. [Google Scholar]
- Huber, W. Biometrische analyse der brachycephalie beim haushund. L’Année Biol. 1974, 13, 135–141. [Google Scholar]
- Pollinger, J.P. Selective sweep mapping of genes with large phenotypic effects. Genome Res. 2005, 15, 1809–1819. [Google Scholar] [CrossRef]
- Koch, A.D.; Arnold, S.; Hubler, M.; Montavon, M.P. Brachycephalic syndrome in dogs. Compend. Contin. Educ. Vet. 2003, 25, 48–55. [Google Scholar]
- Koch, A.D.; Wiestner, T.; Balli, A.; Montavon, M.P.; Michel, E.; Scharf, G.; Arnold, S. Proposal for a new radiological index to determine skull conformation in the dog. Schweiz. Arch. Tierheilkd. 2012, 154, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, A.; Barrs, V.; Awad, M.; Child, G.; Brunel, L.; Mooney, E.; Martinez-Taboada, F.; McDonald, B.; McGreevy, P. Consequences and management of canine brachycephaly in veterinary practice: Perspectives from australian veterinarians and veterinary specialists. Animal 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fecava.org/news-and-events/news/dutch-prohibition-of-the-breeding-of-dogs-with-too-short-muzzles/ (accessed on 5 February 2022).
- Liu, N.C.; Oechtering, G.U.; Adams, V.J.; Kalmar, L.; Sargan, D.R.; Ladlow, J.F. Outcomes and prognostic factors of surgical treatments for brachycephalic obstructive airway syndrome in 3 breeds. Vet. Surg. 2017, 46, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, J.; Moses, P. Brachycephalic airway syndrome surgery: A retrospective analysis of breeds and complications in 155 dogs. ACVSc Coll. Sci. Week 2010. [Google Scholar]
- Lindsay, B.; Cook, D.; Wetzel, J.; Siess, S.; Moses, P. Brachycephalic airway syndrome: Management of post-operative respiratory complications in 248 dogs. Aust. Vet. J. 2012, 34, E4. [Google Scholar] [CrossRef] [PubMed]
- Roedler, F.S.; Pohl, S.; Oechtering, G.U. How does severe brachycephaly affect dog’s lives? Results of a structured preoperative owner questionnaire. Vet. J. 2013, 198, 606–610. [Google Scholar] [CrossRef]
- Lecoindre, P.; Richard, S. Digestive disorders associated with the chronic obstructive respiratory syndrome of brachycephalic dogs: 30 cases (1999–2001). Rev. Med. Vet.-Toulouse 2004, 155, 141–146. [Google Scholar]
- Meola, S.D. Brachycephalic airway syndrome. Top. Companion Anim. Med. 2013, 28, 91–96. [Google Scholar] [CrossRef]
- Wykes, P.M. Brachycephalic airway obstructive syndrome. Probl. Vet. Med. 1991, 3, 188–197. [Google Scholar]
- Trappler, M.; Moore, K. Canine brachycephalic airway syndrome: Surgical management. Compendium 2011, 33, E1–E8. [Google Scholar]
- Pink, J.J.; Doyle, R.S.; Hughes, J.M.L.; Tobin, E.; Bellenger, C.R. Laryngeal collapse in seven brachycephalic puppies. J. Small Anim. Pract. 2006, 47, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.E. Inherited and congenital airway conditions. J. Small Anim. Pract. 1989, 30, 184–187. [Google Scholar] [CrossRef]
- Trainor, P.A.; Melton, K.R.; Manzanares, M. Origins and plasticity of neural crest cells and their roles in jaw and craniofacial evolution. Int. J. Dev. Biol. 2003, 47, 541–553. [Google Scholar] [PubMed]
- Creuzet, S.; Couly, G.; Douarin, N.M. Patterning the neural crest derivatives during development of the vertebrate head: Insights from avian studies. J. Anat. 2005, 207, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, D.; Gradner, G.; Kneissl, S.; Dupré, G. Nasopharyngeal dimensions from computed tomography of pugs and french bulldogs with brachycephalic airway syndrome. Vet. Surg. 2016, 45, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Oechtering, G.U.; Pohl, S.; Schlueter, C.; Lippert, J.P.; Alef, M.; Kiefer, I.; Ludewig, E.; Schuenemann, R. A novel approach to brachycephalic syndrome. Evaluation of anatomical intranasal airway obstruction. Vet. Surg. 2016, 45, 165–172. [Google Scholar] [CrossRef]
- Oechtering, G.U.; Pohl, S.; Schlueter, C.; Lippert, J.P.; Alef, M.; Kiefer, I.; Ludewig, E.; Schuenemann, R. A novel approach to brachycephalic syndrome. Laser-assisted turbinectomy (LATE). Vet. Surg. 2016, 45, 173–181. [Google Scholar] [CrossRef]

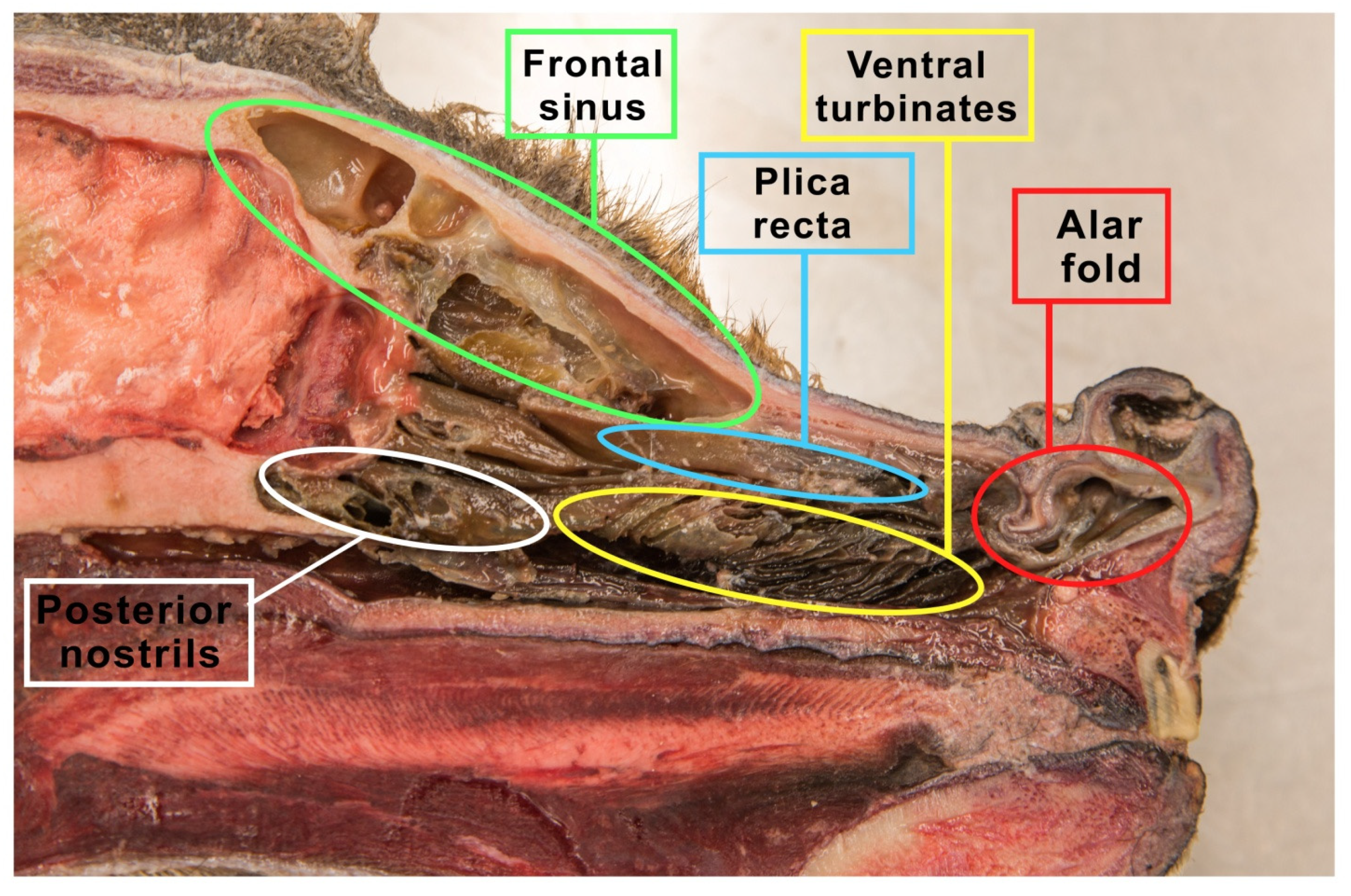
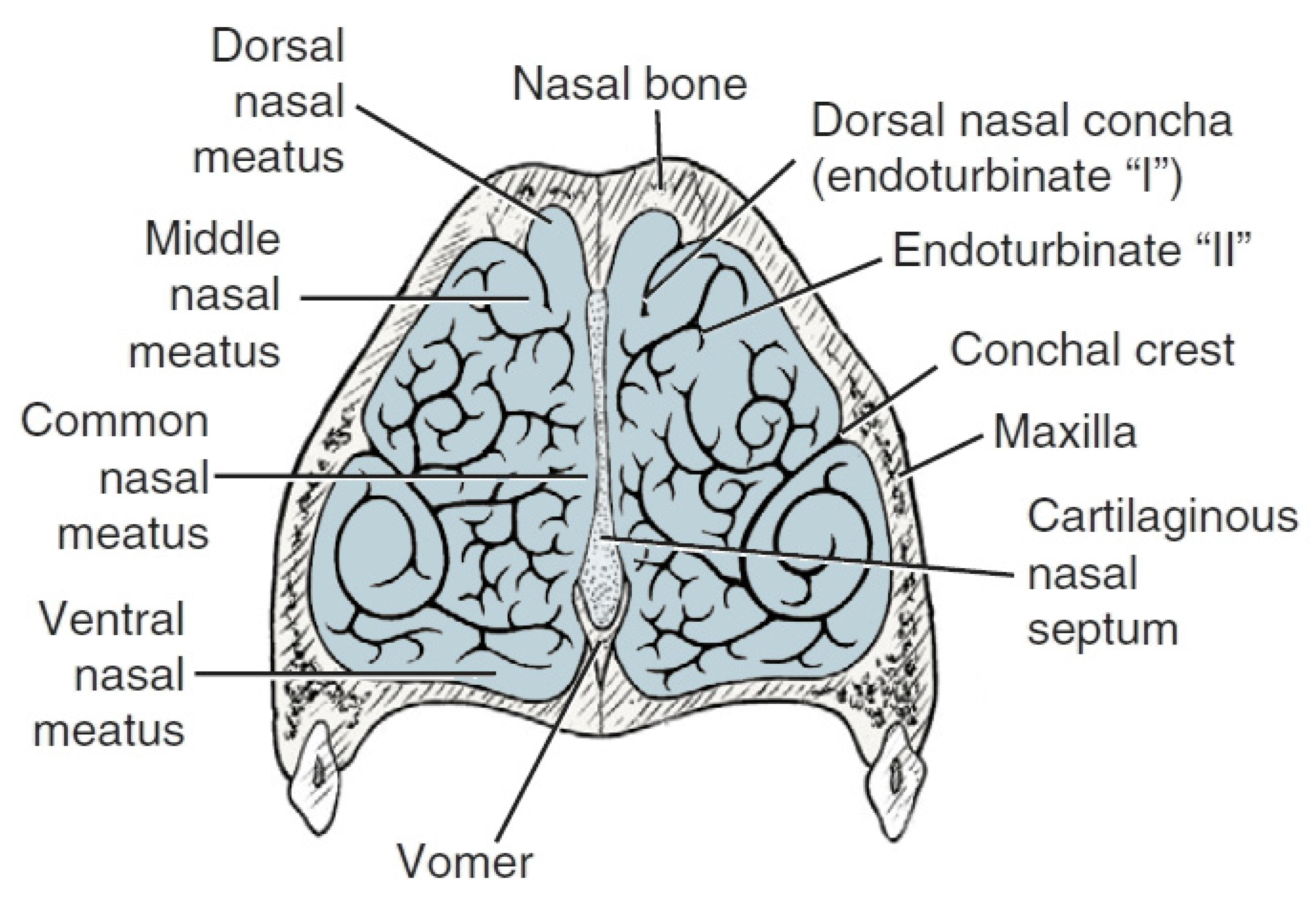
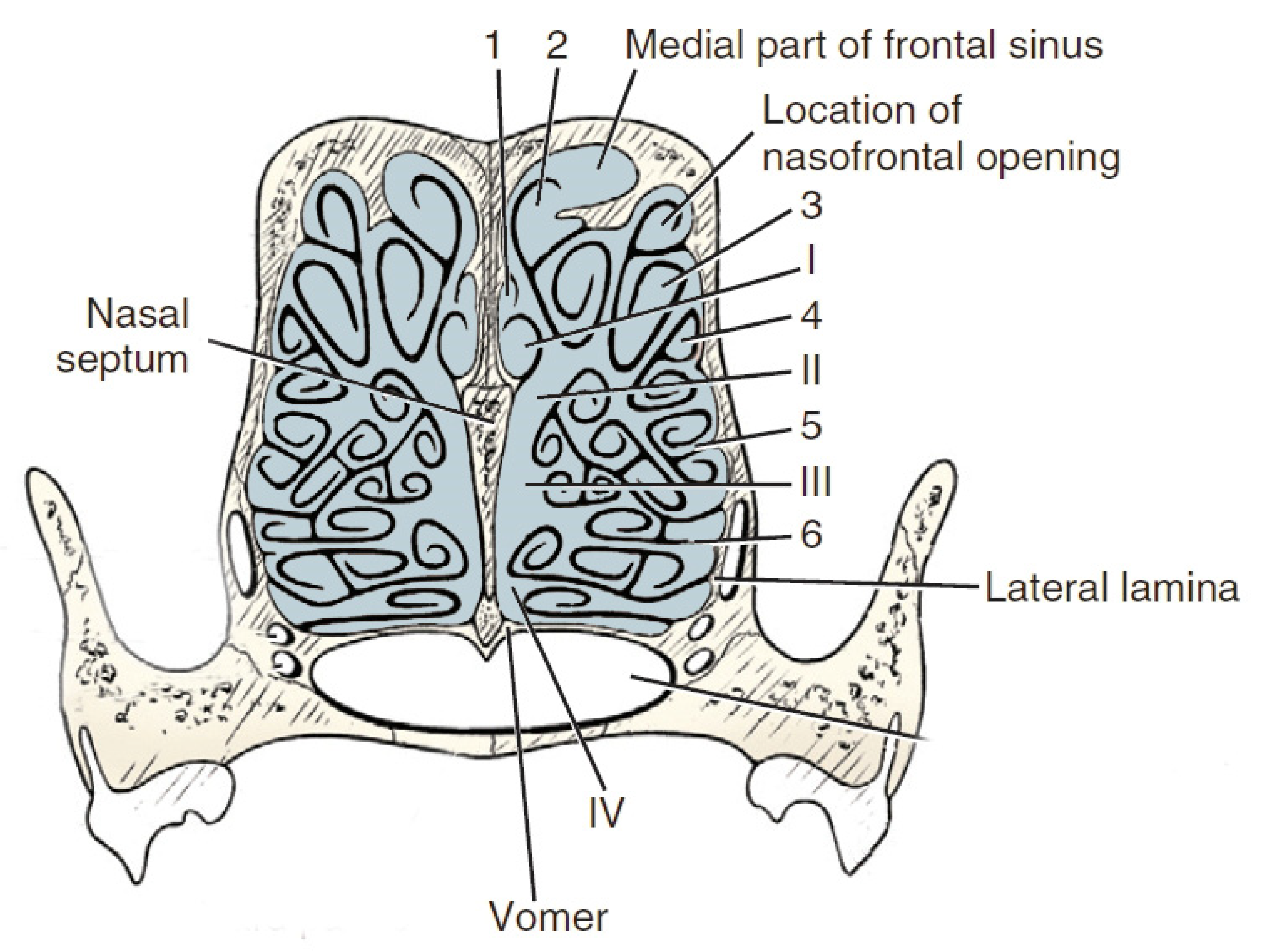

| Item | Ethmoid Turbinates in Domestic Animals | ||||
|---|---|---|---|---|---|
| Inner Ethmoid Turbinates | Outer Ethmoid Turbinates | ||||
| I | II | III | IV | Description | |
| Latin name | endoturbinale I | endoturbinale II | endoturbinale III | endoturbinale IV | ectoturbinale |
| Description | The longest; it lies the most dorsally, bone basis for the dorsal turbinate | Bone basis for the middle turbinate | Smaller than turbinates I and II, except for dogs—II, III and IV are particularly well developed in them | short | |
| Dogs | 4 inner ethmoid turbinates on one side | 6 turbinates on one side | |||
| Cats | 4 inner ethmoid turbinates on one side | 6 turbinates on one side | |||
| Horses | 6 inner ethmoid turbinates on one side | 25 turbinates in 2 rows (one-sided)—external medial and lateral ethmoid turbinates | |||
| Pigs | 7 inner ethmoid turbinates on one side | 20 turbinates on one side | |||
| Ruminants | 4 inner ethmoid turbinates on one side | 18 turbinates on one side | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzek, A.; Serwańska-Leja, K.; Zaworska-Zakrzewska, A.; Kasprowicz-Potocka, M. The Shape of the Nasal Cavity and Adaptations to Sniffing in the Dog (Canis familiaris) Compared to Other Domesticated Mammals: A Review Article. Animals 2022, 12, 517. https://doi.org/10.3390/ani12040517
Buzek A, Serwańska-Leja K, Zaworska-Zakrzewska A, Kasprowicz-Potocka M. The Shape of the Nasal Cavity and Adaptations to Sniffing in the Dog (Canis familiaris) Compared to Other Domesticated Mammals: A Review Article. Animals. 2022; 12(4):517. https://doi.org/10.3390/ani12040517
Chicago/Turabian StyleBuzek, Anna, Katarzyna Serwańska-Leja, Anita Zaworska-Zakrzewska, and Małgorzata Kasprowicz-Potocka. 2022. "The Shape of the Nasal Cavity and Adaptations to Sniffing in the Dog (Canis familiaris) Compared to Other Domesticated Mammals: A Review Article" Animals 12, no. 4: 517. https://doi.org/10.3390/ani12040517
APA StyleBuzek, A., Serwańska-Leja, K., Zaworska-Zakrzewska, A., & Kasprowicz-Potocka, M. (2022). The Shape of the Nasal Cavity and Adaptations to Sniffing in the Dog (Canis familiaris) Compared to Other Domesticated Mammals: A Review Article. Animals, 12(4), 517. https://doi.org/10.3390/ani12040517









