Effect of Steroid Hormones, Prostaglandins (E2 and F2α), Oxytocin, and Tumor Necrosis Factor Alpha on Membrane Progesterone (P4) Receptors Gene Expression in Bovine Myometrial Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Isolation and Culture of Myometrial Cells
2.3. PGE2 Determination
2.4. Total RNA Isolation and cDNA Synthesis
2.5. Real-Time PCR
2.6. Statistical Analysis
3. Results
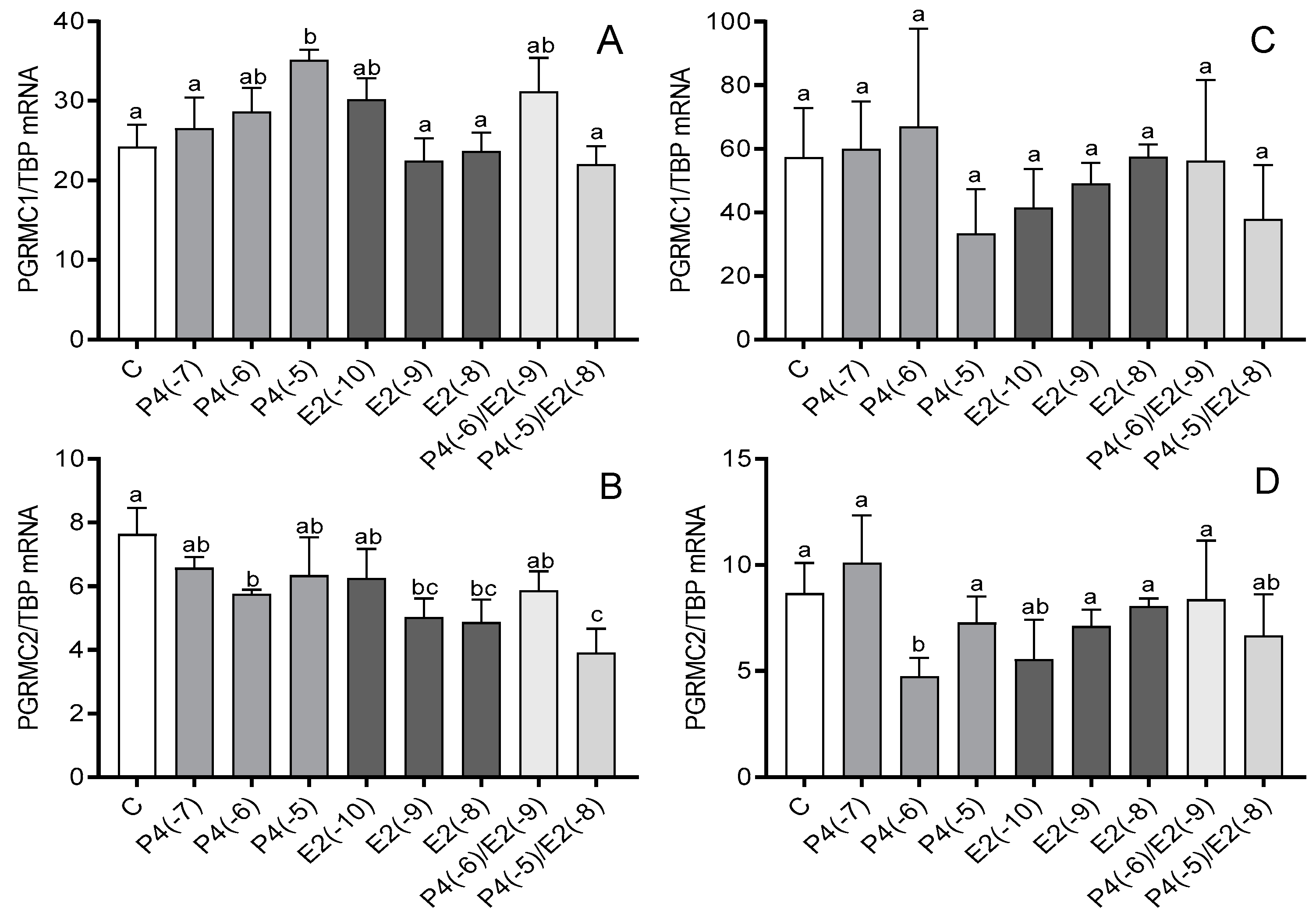
3.1. The Effect of Steroid Hormones on the mRNA Expression of PGRMC1 and PGRMC2
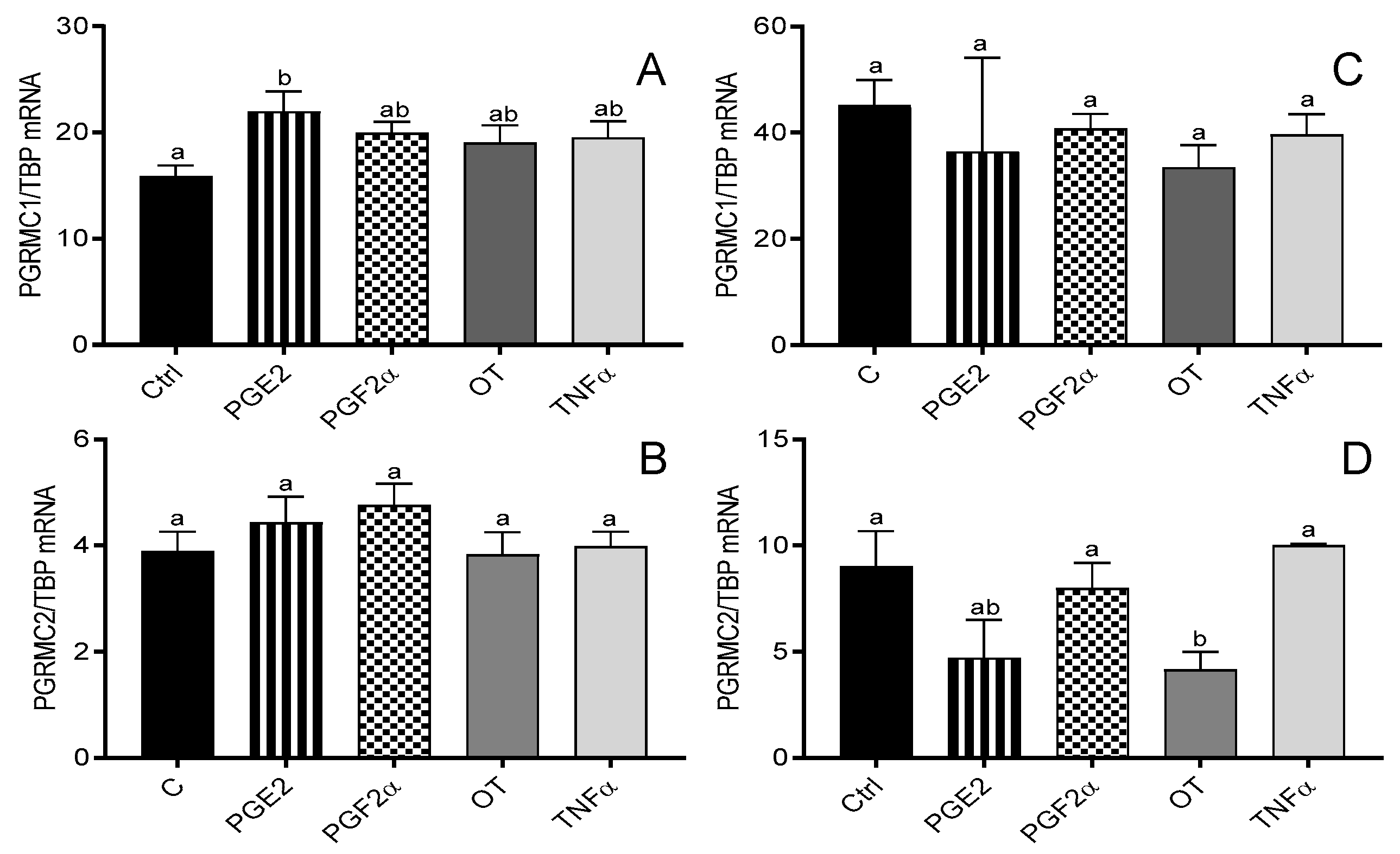
3.2. The Effect of Prostaglandins (E2 and F2α), OT, and TNFα on the mRNA Expression of PGRMC1 and PGRMC2
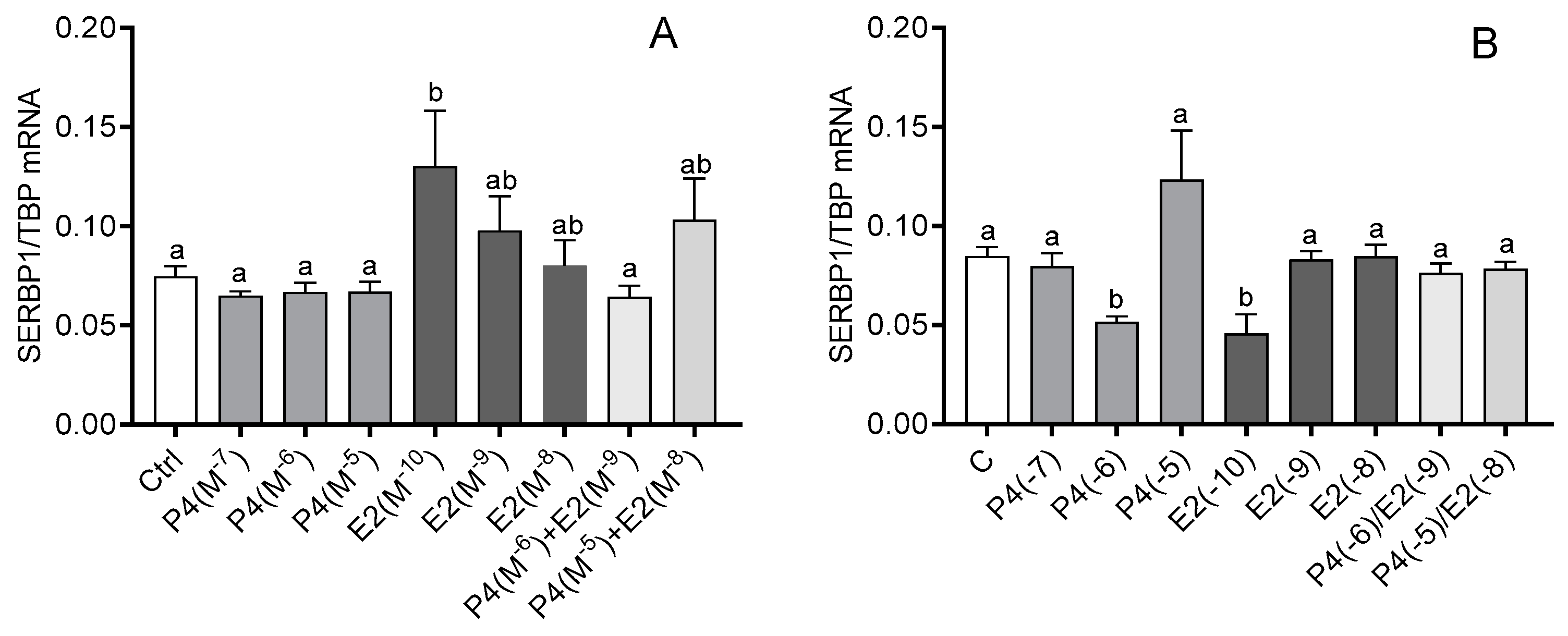
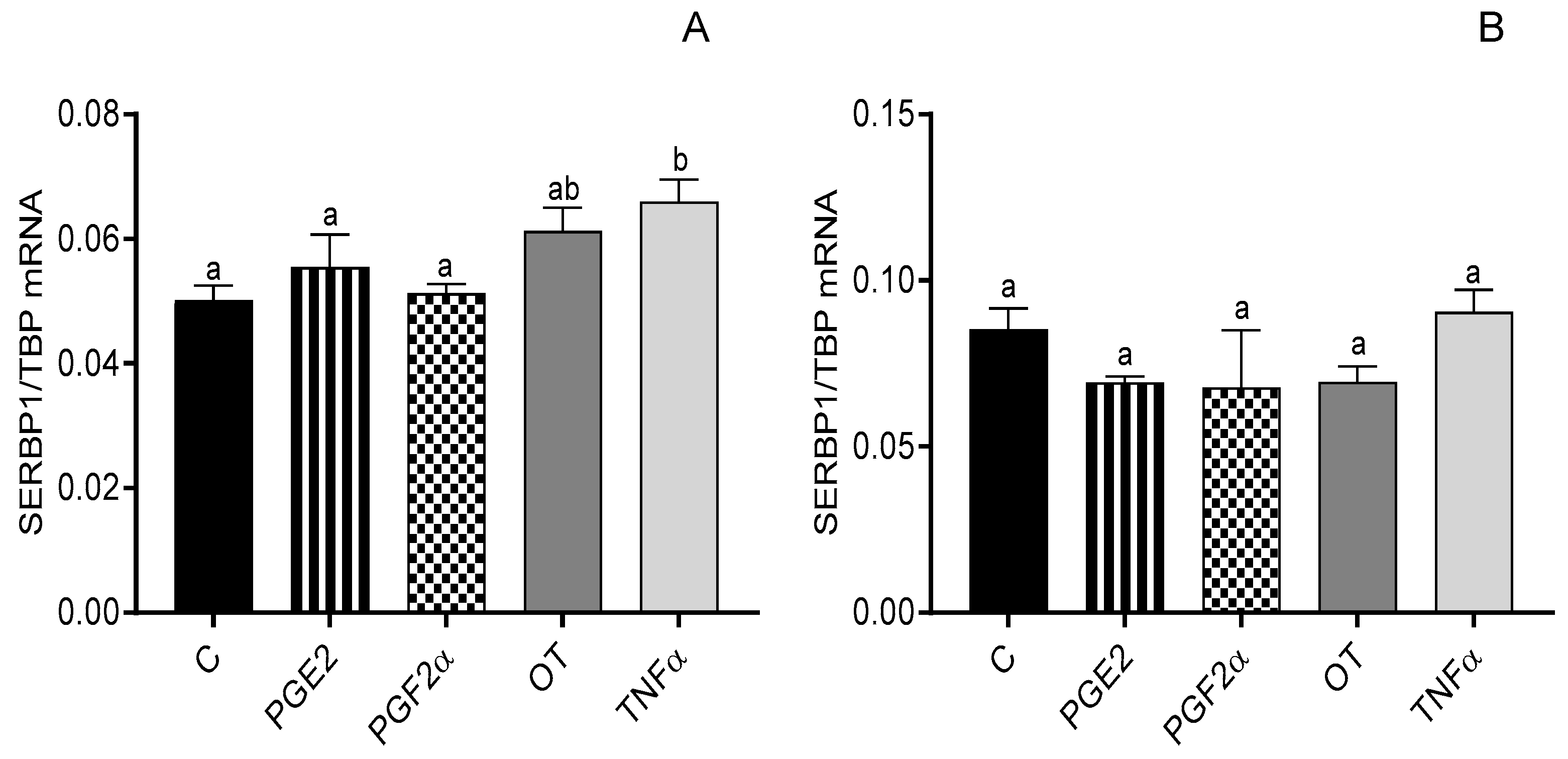
3.3. The Effect of Steroid Hormones, Prostaglandins (E2 and F2α), OT, and TNFα on the mRNA Expression of SERBP1
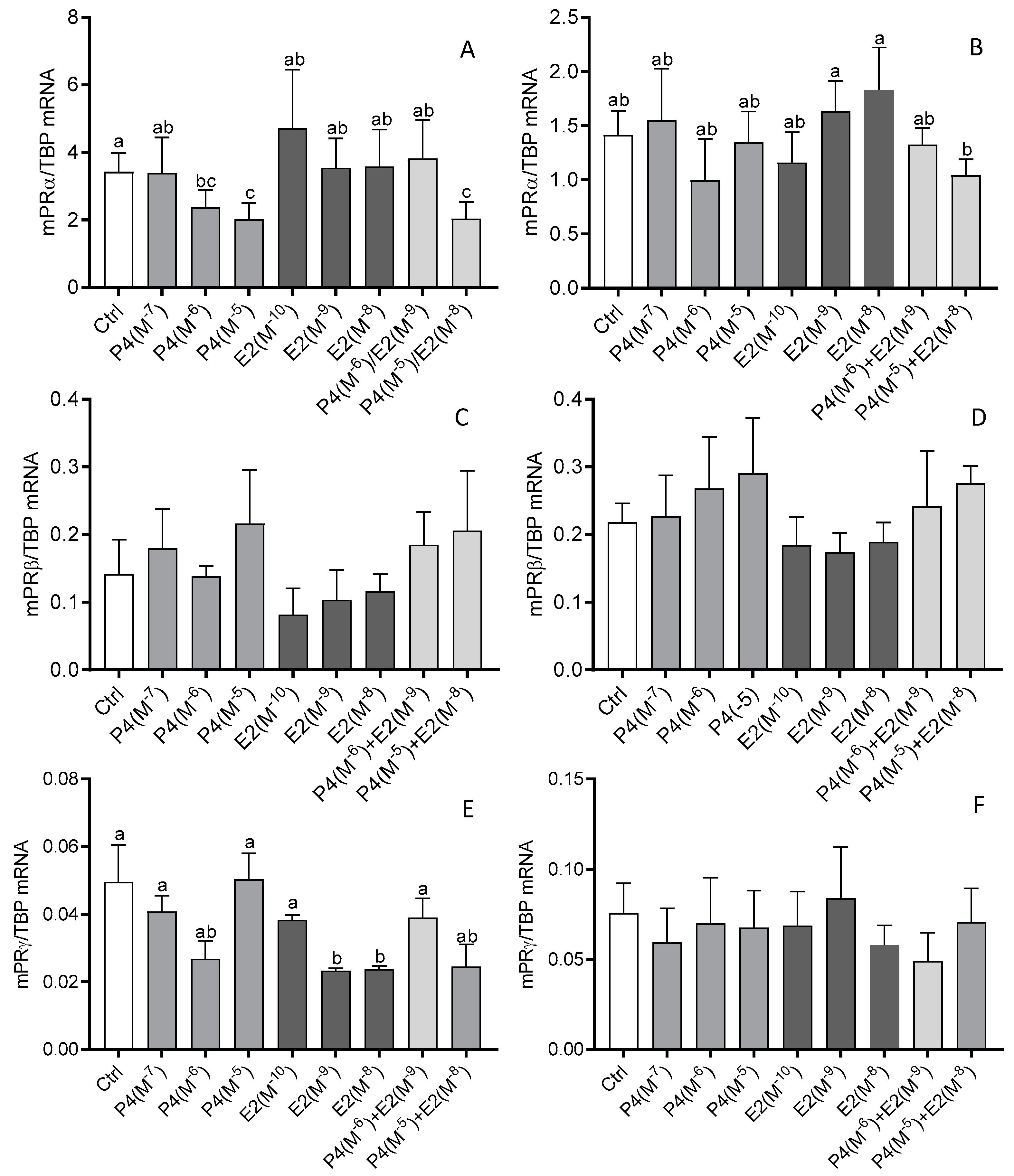
3.4. The Effect of Steroid Hormones, Prostaglandins (E2 and F2α), OT, and TNFα on the mRNA Expression of mPRα, mPRβ, and mPRγ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms Controlling the Function and Life Span of the Corpus Luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Diep, C.H.; Daniel, A.R.; Mauro, L.J.; Knutson, T.P.; Lange, C.A. Progesterone Action in Breast, Uterine, and Ovarian Cancers. J. Mol. Endocrinol. 2015, 54, R31–R53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulac-Jericevic, B.; Conneely, O.M. Reproductive Tissue Selective Actions of Progesterone Receptors. Reproduction 2004, 128, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoncini, T.; Genazzani, A.R. Non-Genomic Actions of Sex Steroid Hormones. Eur. J. Endocrinol. 2003, 148, 281–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peluso, J.J. Multiplicity of Progesterone’s Actions and Receptors in the Mammalian Ovary. Biol. Reprod. 2006, 75, 2–8. [Google Scholar] [CrossRef]
- Wehling, M.; Lösel, R. Non-Genomic Steroid Hormone Effects: Membrane or Intracellular Receptors? J. Steroid Biochem. Mol. Biol. 2006, 102, 180–183. [Google Scholar] [CrossRef]
- Cahill, M.A. Progesterone Receptor Membrane Component 1: An Integrative Review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef]
- Gellersen, B.; Fernandes, M.S.; Brosens, J.J. Non-Genomic Progesterone Actions in Female Reproduction. Hum. Reprod. Update 2009, 15, 119–138. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Rice, C.D.; Pang, Y.; Pace, M.; Thomas, P. Cloning, Expression, and Characterization of a Membrane Progestin Receptor and Evidence It Is an Intermediary in Meiotic Maturation of Fish Oocytes. Proc. Natl. Acad. Sci. USA 2003, 100, 2231–2236. [Google Scholar] [CrossRef] [Green Version]
- Charles, N.J.; Thomas, P.; Lange, C.A. Expression of Membrane Progesterone Receptors (MPR/PAQR) in Ovarian Cancer Cells: Implications for Progesterone-Induced Signaling Events. Horm. Cancer 2010, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Kowalik, M.K.; Slonina, D.; Rekawiecki, R.; Kotwica, J. Expression of Progesterone Receptor Membrane Component (PGRMC) 1 and 2, Serpine MRNA Binding Protein 1 (SERBP1) and Nuclear Progesterone Receptor (PGR) in the Bovine Endometrium during the Estrous Cycle and the First Trimester of Pregnancy. Reprod. Biol. 2013, 13, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Luciano, A.M.; Corbani, D.; Lodde, V.; Tessaro, I.; Franciosi, F.; Peluso, J.J.; Modina, S. Expression of Progesterone Receptor Membrane Component-1 in Bovine Reproductive System during Estrous Cycle. Eur. J. Histochem. 2011, 55, e27. [Google Scholar] [CrossRef] [PubMed]
- Slonina, D.; Kowalik, M.K.; Kotwica, J. Expression of Progesterone Receptor Membrane Component 1, Serpine MRNA Binding Protein 1 and Nuclear Progesterone Receptor Isoforms A and B in the Bovine Myometrium during the Estrous Cycle and Early Pregnancy. J. Reprod. Dev. 2012, 58, 288–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Dizier, M.; Sandra, O.; Ployart, S.; Chebrout, M.; Constant, F. Expression of Nuclear Progesterone Receptor and Progesterone Receptor Membrane Components 1 and 2 in the Oviduct of Cyclic and Pregnant Cows during the Post-Ovulation Period. Reprod. Biol. Endocrinol. 2012, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.K.; Martyniak, M.; Rekawiecki, R.; Kotwica, J. Expression and Immunolocalization of Membrane Progesterone Receptors in the Bovine Oviduct. Domest. Anim. Endocrinol. 2016, 55, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.K.; Rekawiecki, R.; Kotwica, J. Expression and Localization of Progesterone Receptor Membrane Component 1 and 2 and Serpine MRNA Binding Protein 1 in the Bovine Corpus Luteum during the Estrous Cycle and the First Trimester of Pregnancy. Theriogenology 2014, 82, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Suchanek, M.; Radzikowska, A.; Thiele, C. Photo-Leucine and Photo-Methionine Allow Identification of Protein-Protein Interactions in Living Cells. Nat. Methods 2005, 2, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Powell, D.W.; Bard, M.; Eckstein, J.; Barbuch, R.; Link, A.J.; Espenshade, P.J. Dap1/PGRMC1 Binds and Regulates Cytochrome P450 Enzymes. Cell Metab. 2007, 5, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Shi, S.-Q.; Huang, H.-J.; Balducci, J.; Garfield, R.E. Changes in PGRMC1, a Potential Progesterone Receptor, in Human Myometrium during Pregnancy and Labour at Term and Preterm. Mol. Hum. Reprod. 2011, 17, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, I.M.; Garcia-Herreros, M.; O’Shea, L.C.; Hensey, C.; Lonergan, P.; Fair, T. Expression, Regulation, and Function of Progesterone Receptors in Bovine Cumulus Oocyte Complexes during in Vitro Maturation. Biol. Reprod. 2011, 84, 910–921. [Google Scholar] [CrossRef]
- Shankar, R.; Johnson, M.P.; Williamson, N.A.; Cullinane, F.; Purcell, A.W.; Moses, E.K.; Brennecke, S.P. Molecular Markers of Preterm Labor in the Choriodecidua. Reprod. Sci. 2010, 17, 297–310. [Google Scholar] [CrossRef] [Green Version]
- Kowalik, M.K.; Rekawiecki, R.; Kotwica, J. Expression of Membrane Progestin Receptors (MPRs) in the Bovine Corpus Luteum during the Estrous Cycle and First Trimester of Pregnancy. Domest. Anim. Endocrinol. 2018, 63, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.K.; Dobrzyn, K.; Rekawiecki, R.; Kotwica, J. Expression of Membrane Progestin Receptors (MPRs) α, β and γ in the Bovine Uterus during the Oestrous Cycle and Pregnancy. Theriogenology 2019, 140, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, A.; Keren-Tal, I.; Aharoni, D.; Dantes, A.; Land-Bracha, A.; Rimon, E.; Sasson, R.; Hirsh, L. Steroidogenesis and Apoptosis in the Mammalian Ovary. Steroids 2003, 68, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.B.; Lu, S.S.; Ji, K.L.; Song, X.M.; Lu, Y.Q.; Zhang, M.; Lu, K.H. Membrane Progestin Receptor Beta (MPR-Beta): A Protein Related to Cumulus Expansion that Is Involved in In Vitro Maturation of Pig Cumulus-Oocyte Complexes. Steroids 2008, 73, 1416–1423. [Google Scholar] [CrossRef]
- Nutu, M.; Weijdegård, B.; Thomas, P.; Bergh, C.; Thurin-Kjellberg, A.; Pang, Y.; Billig, H.; Larsson, D.G.J. Membrane Progesterone Receptor Gamma: Tissue Distribution and Expression in Ciliated Cells in the Fallopian Tube. Mol. Reprod. Dev. 2007, 74, 843–850. [Google Scholar] [CrossRef]
- Nutu, M.; Weijdegård, B.; Thomas, P.; Thurin-Kjellberg, A.; Billig, H.; Larsson, D.G.J. Distribution and Hormonal Regulation of Membrane Progesterone Receptors Beta and Gamma in Ciliated Epithelial Cells of Mouse and Human Fallopian Tubes. Reprod. Biol. Endocrinol. 2009, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Ashley, R.L.; Clay, C.M.; Farmerie, T.A.; Niswender, G.D.; Nett, T.M. Cloning and Characterization of an Ovine Intracellular Seven Transmembrane Receptor for Progesterone that Mediates Calcium Mobilization. Endocrinology 2006, 147, 4151–4159. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Pierron, V.; Michalovich, D.; Astle, S.; Thornton, S.; Peltoketo, H.; Lam, E.W.-F.; Gellersen, B.; Huhtaniemi, I.; Allen, J.; et al. Regulated Expression of Putative Membrane Progestin Receptor Homologues in Human Endometrium and Gestational Tissues. J. Endocrinol. 2005, 187, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Dressing, G.E.; Goldberg, J.E.; Charles, N.J.; Schwertfeger, K.L.; Lange, C.A. Membrane Progesterone Receptor Expression in Mammalian Tissues: A Review of Regulation and Physiological Implications. Steroids 2011, 76, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Karteris, E.; Zervou, S.; Pang, Y.; Dong, J.; Hillhouse, E.W.; Randeva, H.S.; Thomas, P. Progesterone Signaling in Human Myometrium through Two Novel Membrane G Protein-Coupled Receptors: Potential Role in Functional Progesterone Withdrawal at Term. Mol. Endocrinol. 2006, 20, 1519–1534. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Sheehan, P.M.; Brennecke, S.P. Changes in Myometrial Expression of Progesterone Receptor Membrane Components 1 and 2 Are Associated with Human Parturition at Term. Reprod. Fertil. Dev. 2016, 28, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kanda, Y.; Roberts, D.J.; Ecker, J.L.; Losel, R.; Wehling, M.; Peluso, J.J.; Pru, J.K. Expression of Progesterone Receptor Membrane Component 1 and Its Partner Serpine 1 MRNA Binding Protein in Uterine and Placental Tissues of the Mouse and Human. Mol. Cell. Endocrinol. 2008, 287, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Fields, M.J.; Fields, P.A. Morphological Characteristics of the Bovine Corpus Luteum during the Estrous Cycle and Pregnancy. Theriogenology 1996, 45, 1295–1325. [Google Scholar] [CrossRef]
- Ireland, J.J.; Murphee, R.L.; Coulson, P.B. Accuracy of Predicting Stages of Bovine Estrous Cycle by Gross Appearance of the Corpus Luteum. J. Dairy Sci. 1980, 63, 155–160. [Google Scholar] [CrossRef]
- Wróbel, M.; Kotwica, J. Effect of Polychlorinated Biphenyls (PCBs) on Basal and OT-Stimulated Calcium Concentrations in Myometrial Cells in Cows. Reprod. Biol. 2005, 5, 321–330. [Google Scholar]
- Slonina, D.; Kowalik, M.K.; Subocz, M.; Kotwica, J. The Effect of Ovarian Steroids on Oxytocin-Stimulated Secretion and Synthesis of Prostaglandins in Bovine Myometrial Cells. Prostaglandins Other Lipid Mediat. 2009, 90, 69–75. [Google Scholar] [CrossRef]
- Rekawiecki, R.; Kowalik, M.K.; Kotwica, J. Luteotropic and Luteolytic Factors Regulate MRNA and Protein Expression of Progesterone Receptor Isoforms A and B in the Bovine Endometrium. Reprod. Fertil. Dev. 2016, 28, 907–913. [Google Scholar] [CrossRef]
- Rekawiecki, R.; Kowalik, M.K.; Kotwica, J. Onapristone (ZK299) and Mifepristone (RU486) Regulate the Messenger RNA and Protein Expression Levels of the Progesterone Receptor Isoforms A and B in the Bovine Endometrium. Theriogenology 2015, 84, 348–357. [Google Scholar] [CrossRef]
- Rekawiecki, R.; Kowalik, M.K.; Kotwica, J. Validation of Housekeeping Genes for Studying Differential Gene Expression in the Bovine Myometrium. Acta Vet. Hung. 2013, 61, 505–516. [Google Scholar] [CrossRef]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Ashley, R.L.; Arreguin-Arevalo, J.A.; Nett, T.M. Binding Characteristics of the Ovine Membrane Progesterone Receptor Alpha and Expression of the Receptor during the Estrous Cycle. Reprod. Biol. Endocrinol. 2009, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Krebs, C.J.; Jarvis, E.D.; Chan, J.; Lydon, J.P.; Ogawa, S.; Pfaff, D.W. A Membrane-Associated Progesterone-Binding Protein, 25-Dx, Is Regulated by Progesterone in Brain Regions Involved in Female Reproductive Behaviors. Proc. Natl. Acad. Sci. USA 2000, 97, 12816–12821. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, H.; Clare, S.E.; Wozny, W.; Schwall, G.P.; Poznanovic, S.; Stegmann, W.; Vogel, U.; Sotlar, K.; Wallwiener, D.; Kurek, R.; et al. Breast Cancer Proteomics Reveals Correlation between Estrogen Receptor Status and Differential Phosphorylation of PGRMC1. Breast Cancer Res. 2008, 10, R85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastner, P.; Krust, A.; Turcotte, B.; Stropp, U.; Tora, L.; Gronemeyer, H.; Chambon, P. Two Distinct Estrogen-Regulated Promoters Generate Transcripts Encoding the Two Functionally Different Human Progesterone Receptor Forms A and B. EMBO J. 1990, 9, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.K.; Fortune, J.E. Estradiol-17 Beta Has a Biphasic Effect on Oxytocin Secretion by Bovine Granulosa Cells. Biol. Reprod. 1993, 48, 1404–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, K.; Sakumoto, R.; Okamoto, N.; Acosta, T.J.; Abe, H.; Okada, H.; Sinowatz, F.; Skarzynski, D.J. Cellular Localization of Genes and Proteins for Tumor Necrosis Factor-α (TNF), TNF Receptor Types I and II in Bovine Endometrium. Mol. Cell Endocrinol. 2010, 330, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Skarzynski, D.J.; Okuda, K. Is Tumor Necrosis Factor Alpha a Trigger for the Initiation of Endometrial Prostaglandin F(2alpha) Release at Luteolysis in Cattle? Biol. Reprod. 2000, 62, 1109–1115. [Google Scholar] [CrossRef]
- Woclawek-Potocka, I.; Deptula, K.; Bah, M.M.; Lee, H.Y.; Okuda, K.; Skarzynski, D.J. Effects of Nitric Oxide and Tumor Necrosis Factor-Alpha on Production of Prostaglandin F2alpha and E2 in Bovine Endometrial Cells. J. Reprod. Dev. 2004, 50, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Murakami, S.; Miyamoto, Y.; Skarzynski, D.J.; Okuda, K. Effects of Tumor Necrosis Factor-Alpha on Secretion of Prostaglandins E2 and F2alpha in Bovine Endometrium throughout the Estrous Cycle. Theriogenology 2001, 55, 1667–1678. [Google Scholar] [CrossRef]
- Okuda, K.; Miyamoto, Y.; Skarzynski, D.J. Regulation of Endometrial Prostaglandin F2α Synthesis during Luteolysis and Early Pregnancy in Cattle. Domest. Anim. Endocrinol. 2002, 23, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Valadez-Cosmes, P.; Germán-Castelán, L.; González-Arenas, A.; Velasco-Velázquez, M.A.; Hansberg-Pastor, V.; Camacho-Arroyo, I. Expression and Hormonal Regulation of Membrane Progesterone Receptors in Human Astrocytoma Cells. J. Steroid Biochem. Mol. Biol. 2015, 154, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, K.A.; Petersen, S.L. 17β-Estradiol and Progesterone Regulate Multiple Progestin Signaling Molecules in the Anteroventral Periventricular Nucleus, Ventromedial Nucleus and Sexually Dimorphic Nucleus of the Preoptic Area in Female Rats. Neuroscience 2011, 176, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Stocco, C. Expression and Regulation of Progestin Membrane Receptors in the Rat Corpus Luteum. Endocrinology 2005, 146, 5522–5532. [Google Scholar] [CrossRef] [PubMed]
- Zachariades, E.; Mparmpakas, D.; Pang, Y.; Rand-Weaver, M.; Thomas, P.; Karteris, E. Changes in Placental Progesterone Receptors in Term and Preterm Labour. Placenta 2012, 33, 367–372. [Google Scholar] [CrossRef]
| Gene | Primers Forward (F)/Reverse (R) | Amplicon Length (bp) | GenBank Accession No. |
|---|---|---|---|
| PGRMC1 | F: TCTTCAGGGGTGTGTGTGAA R: CATTGTCCTGTGCTCTTTGG | 266 | NM_001075133 |
| PGRMC2 | F: TGCCTCTTTGCCTCGTATGA R: GAGGCATCCCTACCAGCAAAT | 179 | NM_001099060 |
| SERBP1 | F: AGCTCAGACCAACTCCAATGC R: CGGCTCAGACCTTCTTTCTTCA | 149 | NM_001046449 |
| mPRα | F: CCGGCGGTCCATCTATGA R: CCACCCCCTTCACTGAGTCTT | 159 | NM_001038553 |
| mPRβ | F: TGCCCCTGCTCGTCTATGTC R: CCCACGTAGTCCACGAAGTAGAA | 120 | NM_001101135 |
| mPRγ | F: GGTTCTTCTCGTGGAGGTTTGT R: GTTCCTGGACATGGAGCTGAA | 151 | XM_005193580 |
| TBP | F: CAGAGAGCTCCGGGATCGT R: CACCATCTTCCCAGAACTGAATAT | 194 | NM_001075742.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalik, M.K.; Dobrzyn, K.; Mlynarczuk, J.; Rekawiecki, R. Effect of Steroid Hormones, Prostaglandins (E2 and F2α), Oxytocin, and Tumor Necrosis Factor Alpha on Membrane Progesterone (P4) Receptors Gene Expression in Bovine Myometrial Cells. Animals 2022, 12, 519. https://doi.org/10.3390/ani12040519
Kowalik MK, Dobrzyn K, Mlynarczuk J, Rekawiecki R. Effect of Steroid Hormones, Prostaglandins (E2 and F2α), Oxytocin, and Tumor Necrosis Factor Alpha on Membrane Progesterone (P4) Receptors Gene Expression in Bovine Myometrial Cells. Animals. 2022; 12(4):519. https://doi.org/10.3390/ani12040519
Chicago/Turabian StyleKowalik, Magdalena K., Karolina Dobrzyn, Jaroslaw Mlynarczuk, and Robert Rekawiecki. 2022. "Effect of Steroid Hormones, Prostaglandins (E2 and F2α), Oxytocin, and Tumor Necrosis Factor Alpha on Membrane Progesterone (P4) Receptors Gene Expression in Bovine Myometrial Cells" Animals 12, no. 4: 519. https://doi.org/10.3390/ani12040519






