Differential Expression of MSTN Isoforms in Muscle between Broiler and Layer Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. IACUC Approval and Animal Source
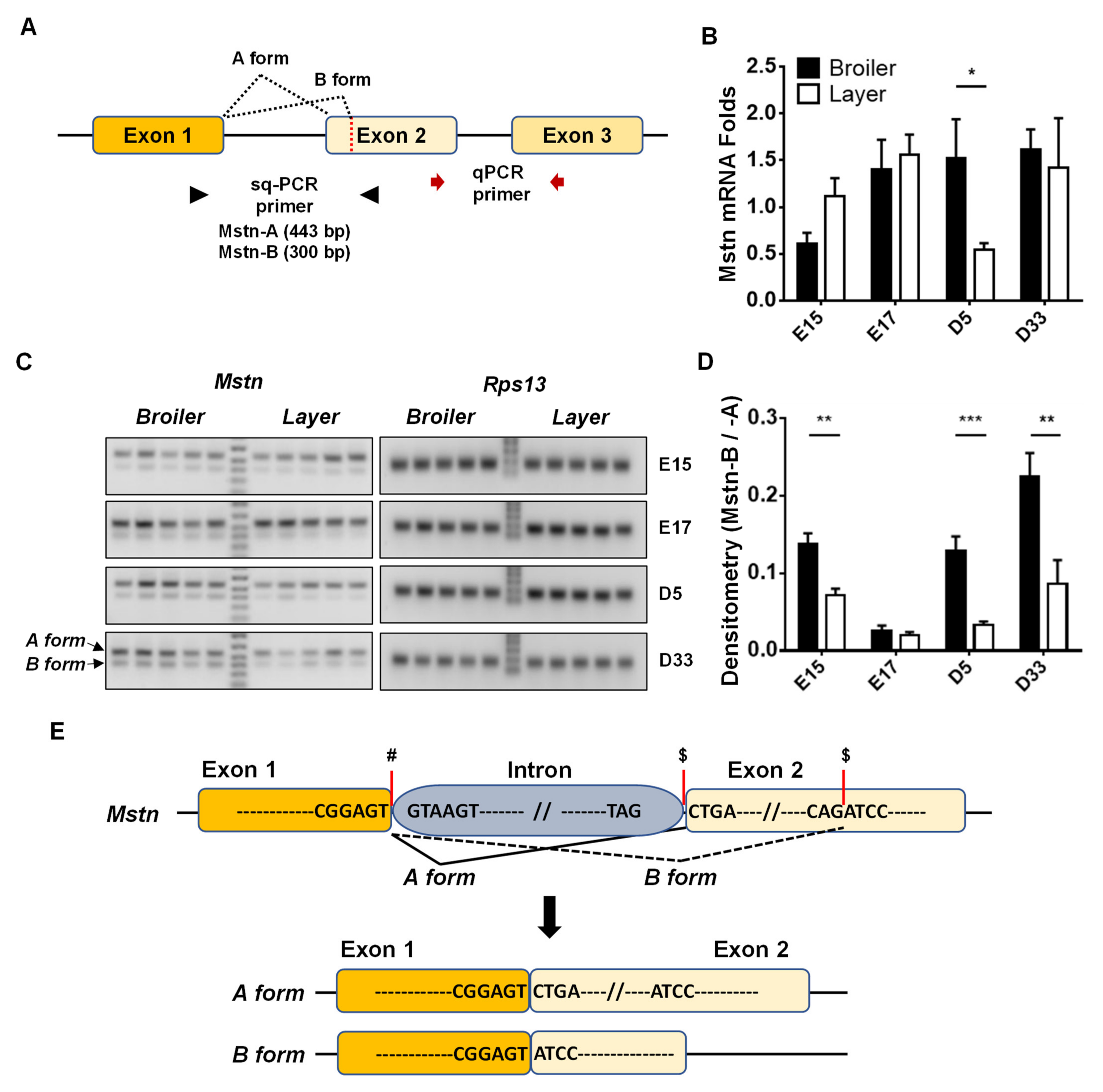
2.2. Quantitative Real-Time PCR (qPCR) and Semi-Quantitative PCR (sqPCR)
2.3. Analysis of MSTN Alternative Splicing in Chicken
2.4. Histological Processing and Measurement of the Muscle Cell Size
2.5. Statistical Analysis
3. Results
3.1. Expression Levels of Mstn Isoforms in Broiler and Layer Chickens
3.2. Histological Comparisons of Pectoralis Major Muscle at D5 and D33 in Broiler and Layer Chickens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grobet, L.; Martin, L.J.R.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ḿnissier, F.; Massabanda, J.; et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Kim, D.H.; Lee, K. Muscle hyperplasia in Japanese quail by single amino acid deletion in MSTN propeptide. Int. J. Mol. Sci. 2020, 21, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.D.; Lee, J.H.; Song, S.; Kim, S.W.; Han, J.S.; Shin, S.P.; Park, B.C.; Park, T.S. Generation of myostatin-knockout chickens mediated by D10A-Cas9 nickase. FASEB J. 2020, 34, 5688–5696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Song, Y.; Ahn, J.; Kim, E.; Chen, P.; Yang, S.; Suh, Y.; Lee, K. A novel mechanism of myostatin regulation by its alternative splicing variant during myogenesis in avian species. Am. J. Physiol.-Cell Physiol. 2015, 309, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.R.; Suh, Y.; Shin, S.; Woodfint, R.M.; Hwang, S.; Lee, K. Exogenous expression of an alternative splicing variant of myostatin prompts leg muscle fiber hyperplasia in Japanese quail. Int. J. Mol. Sci. 2019, 20, 4617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Choi, Y.M.; Suh, Y.; Shin, S.; Lee, J.; Hwang, S.; Lee, S.S.; Lee, K. Research Note: Increased myostatin expression and decreased expression of myogenic regulatory factors in embryonic ages in a quail line with muscle hypoplasia. Poult. Sci. 2021, 100, 100978. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.E.; Cheng, C. Methods for Characterization of Alternative RNA Splicing. Methods Mol. Biol. 2016, 1402, 229–241. [Google Scholar] [PubMed] [Green Version]
- Mott, I.; Ivarie, R. Expression of myostatin is not altered in lines of poultry exhibiting myofiber hyper- and hypoplasia. Poult. Sci. 2002, 81, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Dou, T.F.; Li, Q.H.; Rong, H.; Tong, H.Q.; Xu, Z.Q.; Huang, Y.; Gu, D.H.; Chen, X.B.; Ge, C.R.; et al. Myostatin mRNA expression and its association with body weight and carcass traits in Yunnan Wuding chicken. Genet. Mol. Res. 2016, 15, gmr15048967. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-L.; Wang, J.-W.; Han, C.-C.; Liu, H.-H.; Li, L.; Dai, F.; Pan, Z.; Xu, F.; He, H.; Xu, H. Developmental expression and alternative splicing of the duck myostatin gene. Comp. Biochem. Physiol.-Part D Genomics Proteomics 2011, 6, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Burge, C.B. Splicing regulation: From a parts list of regulatory elements to an integrated splicing code. RNA 2008, 14, 802–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheuermann, G.N.; Bilgili, S.F.; Tuzun, S.; Mulvaney, D.R. Comparison of chicken genotypes: Myofiber number in pectoralis muscle and myostatin ontogeny. Poult. Sci. 2004, 83, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Teuscher, F.; Ender, K.; Wegner, J. Growth- and breed-related changes of marbling characteristics in cattle1. J. Anim. Sci. 2006, 84, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Nestor, K.E.; Bacon, W.L.; Lambio, A.L. Divergent Selection for Body Weight and Yolk Precursor in Coturnix coturnix japonica. Poult. Sci. 1982, 61, 12–17. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, Y.M.; Suh, Y.; Shin, S.; Lee, J.; Hwang, S.; Lee, K. Research Note: Association of temporal expression of myostatin with hypertrophic muscle growth in different Japanese quail lines. Poult. Sci. 2020, 99, 2926–2930. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Choi, Y.M.; Lee, J.; Shin, S.; Kim, S.; Suh, Y.; Lee, K. Differential Expression of MSTN Isoforms in Muscle between Broiler and Layer Chickens. Animals 2022, 12, 539. https://doi.org/10.3390/ani12050539
Kim D-H, Choi YM, Lee J, Shin S, Kim S, Suh Y, Lee K. Differential Expression of MSTN Isoforms in Muscle between Broiler and Layer Chickens. Animals. 2022; 12(5):539. https://doi.org/10.3390/ani12050539
Chicago/Turabian StyleKim, Dong-Hwan, Young Min Choi, Joonbum Lee, Sangsu Shin, Sanggu Kim, Yeunsu Suh, and Kichoon Lee. 2022. "Differential Expression of MSTN Isoforms in Muscle between Broiler and Layer Chickens" Animals 12, no. 5: 539. https://doi.org/10.3390/ani12050539
APA StyleKim, D. -H., Choi, Y. M., Lee, J., Shin, S., Kim, S., Suh, Y., & Lee, K. (2022). Differential Expression of MSTN Isoforms in Muscle between Broiler and Layer Chickens. Animals, 12(5), 539. https://doi.org/10.3390/ani12050539





