Environmental Drivers of an Intertidal Bryozoan Community in the Barents Sea: A Case Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
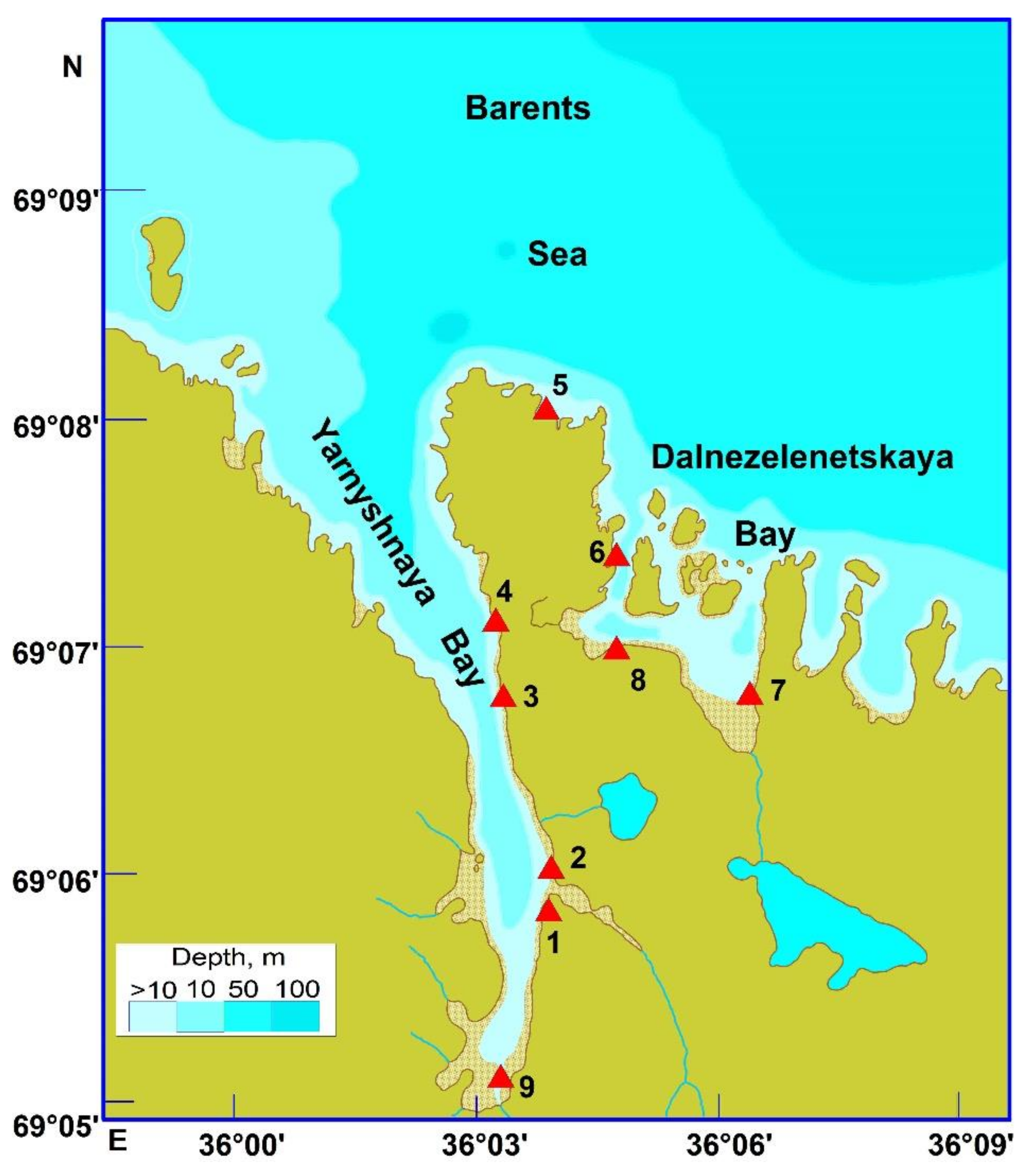
2.1. Study Area
2.2. Sampling and Processing
2.3. Statistical Analysis
3. Results
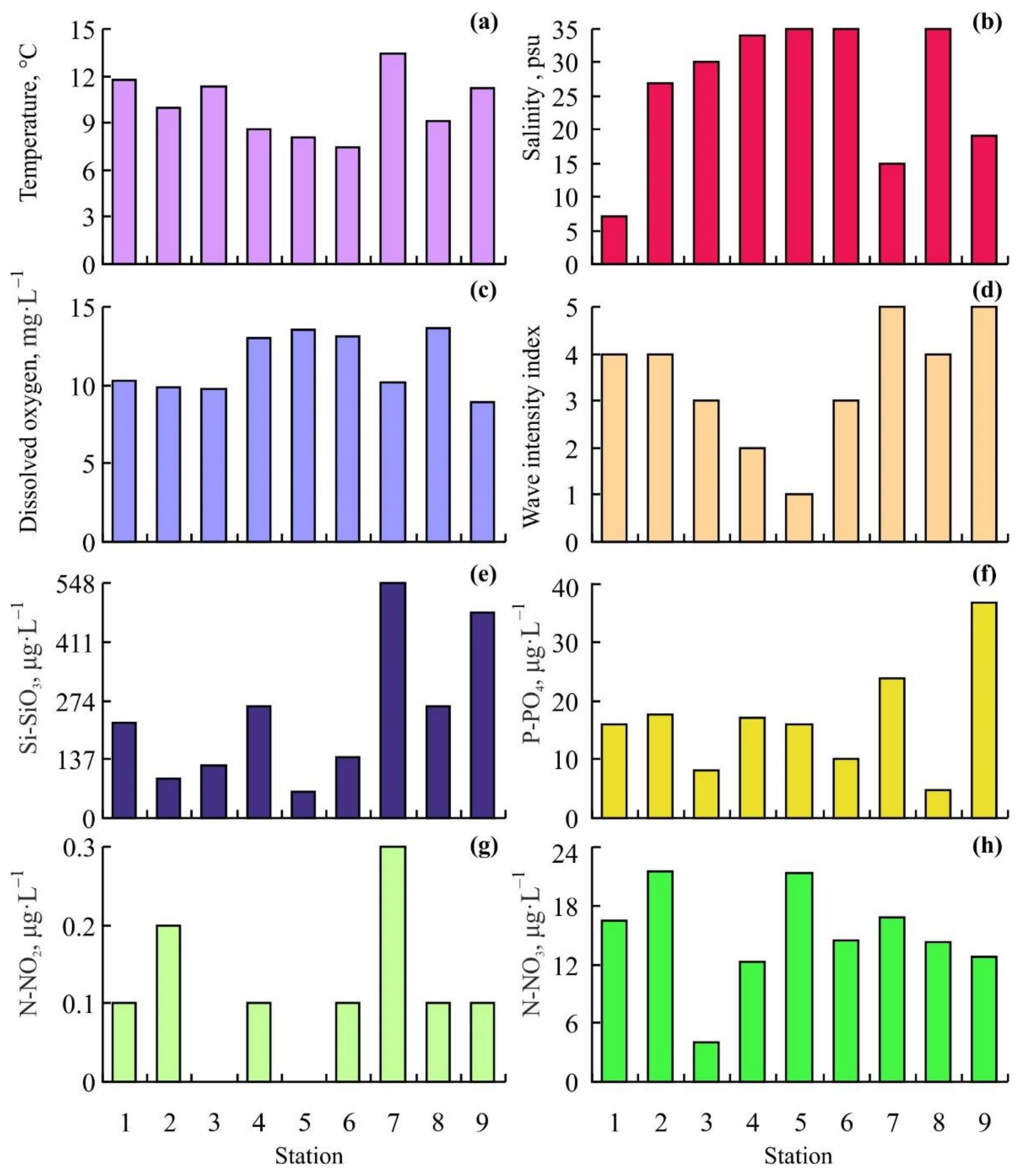
3.1. Environmental Conditions
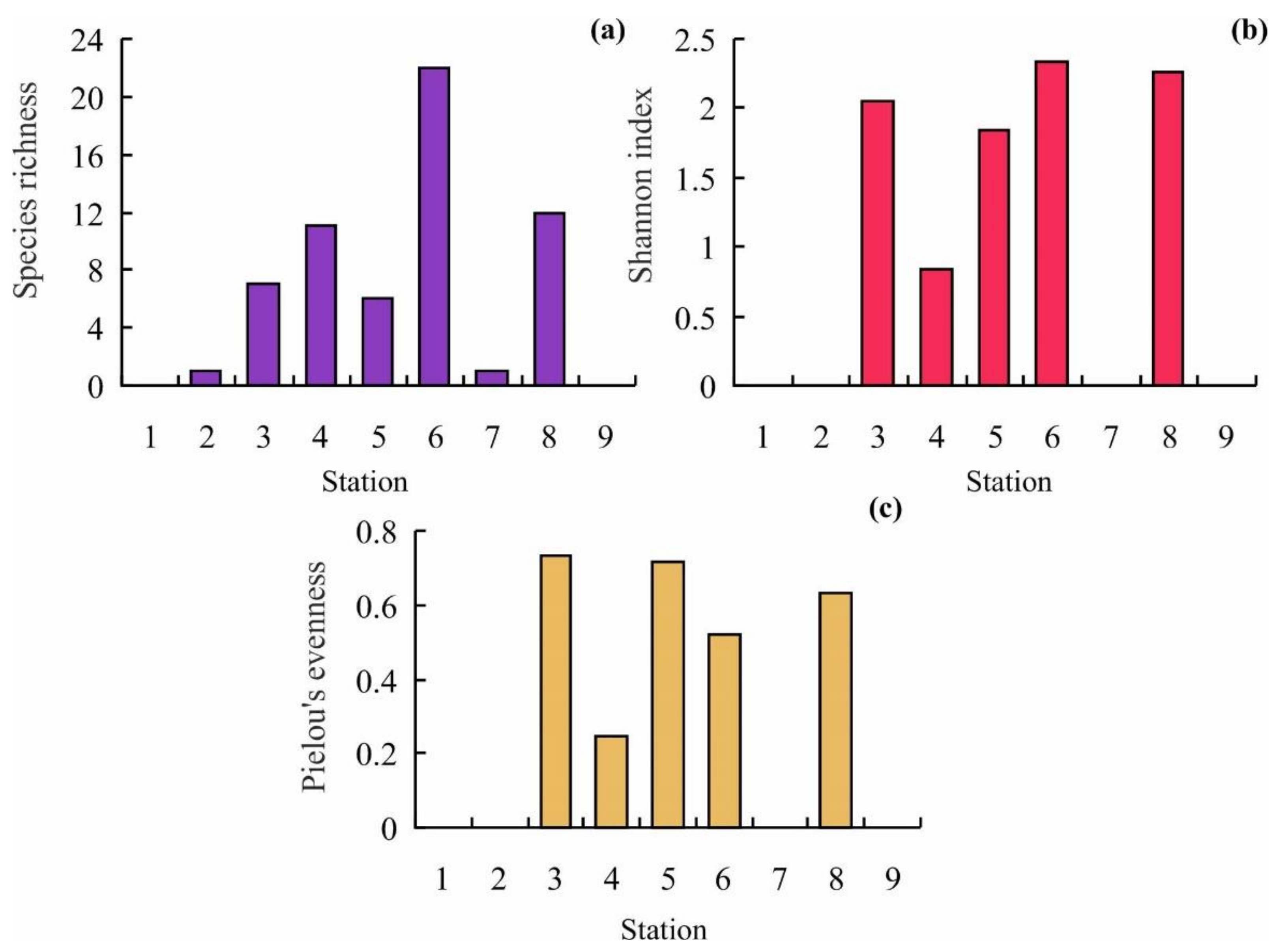
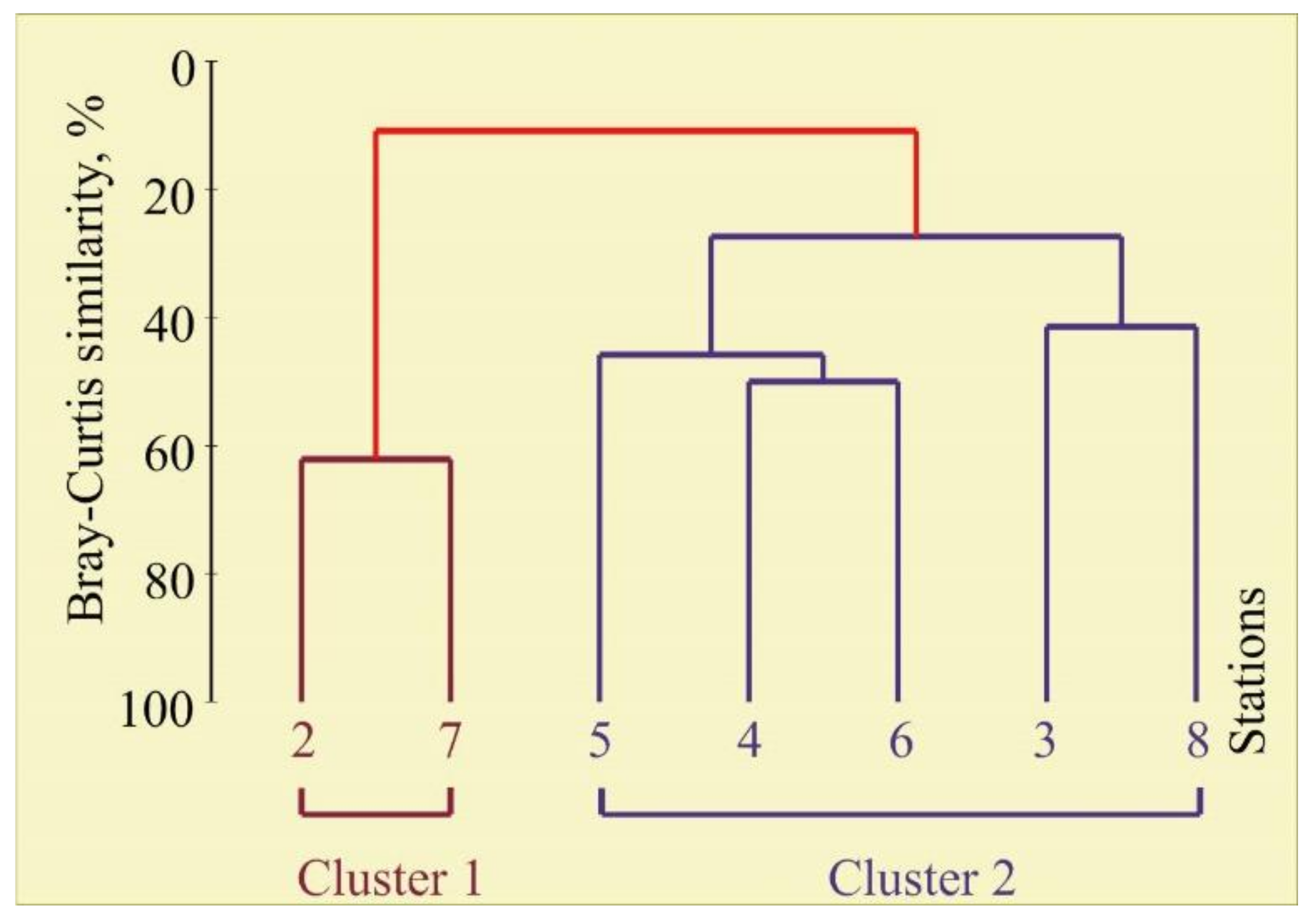
3.2. Bryozoan Diversity, Biomass, and Community
3.3. Relationships between Bryozoan Data and Environmental Variables
4. Discussion
4.1. Environmental Conditions
4.2. Bryozoan Diversity, Biomass, and Community
4.3. Relationships between Bryozoan Data and Environmental Variables
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakobsen, T.; Ozhigin, V. (Eds.) The Barents Sea Ecosystem: Russian-Norwegian Cooperation in Science and Management; Tapir Academic Press: Trondheim, Norway, 2011. [Google Scholar]
- Wassmann, P.; Reigstad, M.; Haug, T.; Rudels, B.; Carroll, M.L.; Hop, H.; Gabrielsen, G.W.; Falk-Petersen, S.; Denisenko, S.G.; Arashkevich, E.; et al. Food webs and carbon flux in the Barents Sea. Prog. Oceanogr. 2006, 71, 232–287. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Coastal mesozooplankton assemblages during spring bloom in the eastern Barents Sea. Biology 2022, 11, 204. [Google Scholar] [CrossRef]
- Loeng, H.; Drinkwater, K. An overview of the ecosystems of the Barents and Norwegian Seas and their response to climate variability. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 2478–2500. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Commercial fish and shellfish in the Barents Sea: Have introduced crab species affected the population trajectories of commercial fish? Rev. Fish Biol. Fisheries 2015, 25, 297–322. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Cucumaria in Russian waters of the Barents Sea: Biological aspects and aquaculture potential. Front. Mar. Sci. 2021, 8, 613453. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Archambault, P.; Assis, J.; Bartsch, I.; Bischof, K.; Filbee-Dexter, K.; Dunton, K.H.; Maximova, O.; Ragnarsdóttir, S.B.; Sejr, M.K.; et al. Imprint of climate change on Pan-Arctic marine vegetation. Front. Mar. Sci. 2020, 7, 617324. [Google Scholar] [CrossRef]
- Polyakov, I.V.; Alkire, M.B.; Bluhm, B.A.; Brown, K.A.; Carmack, E.C.; Chierici, M.; Danielson, S.L.; Ellingsen, I.; Ershova, E.A.; Gårdfeldt, K.; et al. Borealization of the Arctic Ocean in response to anomalous advection from sub-arctic seas. Front. Mar. Sci. 2020, 7, 491. [Google Scholar] [CrossRef]
- Pedersen, T.; Mikkelsen, N.; Lindstrøm, U.; Renaud, P.E.; Nascimento, M.C.; Blanchet, M.-A.; Ellingsen, I.H.; Jørgensen, L.L.; Blanchet, H. Overexploitation, recovery, and warming of the Barents Sea ecosystem during 1950–2013. Front. Mar. Sci. 2021, 8, 732637. [Google Scholar] [CrossRef]
- Sirenko, B.I. List of species of free-living invertebrates of Eurasian Arctic seas and adjacent deep waters. Explor. Fauna Seas 2001, 51, 1–76. [Google Scholar]
- Bader, B.; Schäfer, P. Bryozoans in polar latitudes: Arctic and Antarctic bryozoan communities and facies. Denisia 2005, 16, 263–282. [Google Scholar]
- Bock, P.E.; Gordon, D.P. Phylum Bryozoa Ehrenberg, 1831. Zootaxa 2013, 3703, 67–74. [Google Scholar] [CrossRef]
- Ryland, J.S. Bryozoa: An introductory overview. Denisia 2005, 19, 9–20. [Google Scholar]
- Figuerola, B.; Monleón-Getino, T.; Ballesteros, M.; Avila, C. Spatial patterns and diversity of bryozoan communities from the Southern Ocean: South Shetland Islands, Bouvet Island and Eastern Weddell Sea. Syst. Biodivers. 2012, 10, 109–123. [Google Scholar] [CrossRef]
- Hayward, P.J. Antarctic Cheilostomatous Bryozoa; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Rosso, A.; Di Martino, E. Bryozoan diversity in the Mediterranean Sea: An update. Mediterr. Mar. Sci. 2016, 17, 567–607. [Google Scholar] [CrossRef]
- Taylor, P.D.; Tan, S.H.A. Cheilostome Bryozoa from Penang and Langkawi, Malaysia. Eur. J. Taxon. 2015, 149, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.S.; Hyden, F.M.; Keane, S.L.; Leask, W.L.; Gordon, D.P. Application of Bryozoa zoarial growth-form studies in facies analysis of non-tropical carbonate deposits in New Zealand. Sediment. Geol. 1988, 60, 301–322. [Google Scholar] [CrossRef]
- Gontar, V.I.; Denisenko, N.V. Arctic Ocean Bryozoa. In The Arctic Seas Climatology, Oceanography, Geology, and Biology; Herman, Y., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1989; pp. 341–371. [Google Scholar]
- Kuklinski, P.; Gulliksen, B.; Lønne, O.J.; Weslawski, J.M. Composition of bryozoan assemblages related to depth in Svalbard fjords and sounds. Polar Biol. 2005, 28, 619–630. [Google Scholar] [CrossRef]
- Denisenko, N.V. Species richness and the level of knowledge of the bryozoan fauna of the Arctic region. Proc. Zool. Inst. RAS 2020, 324, 353–363. [Google Scholar] [CrossRef]
- Balazy, P.; Kuklinski, P.; Włodarska-Kowalczuk, M.; Barnes, D.; Kedra, M.; Legezynska, J.; Wesławski, J.M. Hermit crabs (Pagurus spp.) at their northernmost range: Distribution, abundance and shell use in the European. Arctic. Pol. Res. 2015, 34, 21412. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epifauna associated with the northern stone crab Lithodes maia in the Barents Sea. Polar Biol. 2008, 31, 1149–1152. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Fouling community of the red king crab, Paralithodes camtschaticus (Tilesius 1815), in a subarctic fjord of the Barents Sea. Polar Biol. 2009, 32, 1047–1054. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epifauna associated with an introduced crab in the Barents Sea: A 5-year study. ICES J. Mar. Sci. 2010, 67, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Dvoretsky, A.G. Epibionts of the great spider crab, Hyas araneus (Linnaeus, 1758), in the Barents Sea. Polar Biol. 2012, 35, 625–631. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibiotic communities of common crab species in the coastal Barents Sea: Biodiversity and infestation patterns. Diversity 2022, 14, 6. [Google Scholar] [CrossRef]
- Voskoboinikov, G.M.; Makarov, M.V.; Panteleeva, N.N. 2005. Problems and perspectives of biotechnology for brown algae cultivation in the Barents Sea. In Modern Information and Biological Technologies in Developing Resources of Shelf Seas; Matishov, G.G., Ed.; Nauka: Moscow, Russia, 2005; pp. 256–273. (In Russian) [Google Scholar]
- Kluge, G.A. Bryozoans of Northern Seas of USSR; ZIN AN USSR: Leningrad, Russia, 1962. (In Russian) [Google Scholar]
- Kluge, G.A. Bryozoa of the Northern Seas of the USSR; Amerind Publishing Co.: New Delhi, India, 1975. [Google Scholar]
- Denisenko, N.V. Bryozoan fauna of the Cheshskaya Bay of the Barents Sea. In Explorations of Zoobenthos of the Barents, Kara and White Seas; Matishov, G.G., Ed.; KSC RAS Press: Apatity, Russia, 2001; pp. 78–85. (In Russian) [Google Scholar]
- Denisenko, N.V. Bryozoans of the East Siberian Sea. Explor. Fauna Seas 2010, 66, 89–129. (In Russian) [Google Scholar]
- Denisenko, N.V. Bryozoans of the Kara Sea: Estimation of species diversity and patterns of biogeographic composition. Proc. Zool. Inst. RAS 2021, 325, 217–234. (In Russian) [Google Scholar] [CrossRef]
- Denisenko, N.V.; Grebmeier, J.M. Spatial patterns of bryozoan fauna biodiversity and issues of biogeographic regionalization of the Chukchi Sea. Oceanography 2015, 28, 134–145. [Google Scholar] [CrossRef]
- Kapkov, V.I.; Shoshina, E.V. Intertidal community of fucoid seaweeds in the Yarnyshnaya Bay, the Barents Sea. Vestn. MSTU 2021, 24, 149–159. (In Russian) [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. New echinoderm-crab epibiotic associations from the coastal Barents Sea. Animals 2021, 11, 917. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Aquaculture of green sea urchin in the Barents Sea: A brief review of Russian studies. Rev. Aquaculture 2020, 12, 1280–1290. [Google Scholar] [CrossRef]
- Guryanova, E.F.; Zaks, I.G.; Ushakov, P.V. Littoral of Kola Bay. Tr. Leningrad Soc. Nat. 1930, 60, 1–176. (In Russian) [Google Scholar]
- Ryzhik, I.V. Morpho-Functional Features of Commercial Algae from Different Biotopes of the Barents Sea. Ph.D. Thesis, MMBI KSC RAS Press, Murmansk, Russia, 2005. (In Russian). [Google Scholar]
- Malavenda, S.V. Evenness of species abundance in the littoral communities of the Murman. Iss. Modern Algol. 2021, 26, 38–45. (In Russian) [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Sapozhnikov, V.V.; Agatova, A.I.; Arzhanova, N.V.; Mordasova, N.V.; Lapina, N.M.; Zubarevich, V.L.; Lukyanova, O.N.; Morgunova, N.I. Guidelines for Chemical Analysis of Marine and Fresh Water in Environmental Monitoring of Fishery Waters and Fishery Prospective Areas of the World Ocean; VNIRO Press: Moscow, Russia, 2003. (In Russian) [Google Scholar]
- Bendschneider, K.; Robinson, R.J. A new spectrometric method for the determination of nitrite in the sea water. J. Mar. Res. 1952, 11, 87–96. [Google Scholar]
- Montgomery, H.A.C.; Thom, N.S.; Cockburn, A. Determination of dissolved oxygen by the winkler method and the solubility of oxygen in pure water and sea water. J. Appl. Chem. 1964, 14, 280–296. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, Version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Makarevich, P.; Druzhkova, E.; Larionov, V. Primary producers of the Barents Sea. In Diversity of Ecosystems; Mahamane, A., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 367–392. [Google Scholar]
- Cloern, J.E. Phytoplankton bloom dynamics in coastal ecosystems: A review with some general lessons from sustained investigation of San Francisco Bay, California. Rev. Geophys. 1996, 34, 127–168. [Google Scholar] [CrossRef]
- Makarevich, P.R.; Vodopianova, V.V.; Bulavina, A.S.; Vashchenko, P.S.; Ishkulova, T.G. Features of the distribution of chlorophyll-a concentration along the western coast of the Novaya Zemlya Archipelago in spring. Water 2021, 13, 3648. [Google Scholar] [CrossRef]
- Voronkov, P.P.; Uralov, N.S.; Chernovskaya, E.N. Common aspects of hydrochemical regime of the coastal zone of the Barents Sea near Central Murman. Tr. Murm. Biol. Stat. 1948, 1, 39–101. (In Russian) [Google Scholar]
- Ilyin, G.V.; Garkavaya, G.P. Biogenic elements of coastal waters of the Eastern Murmansk. In Regularities of Bioproduction Processes in the Barents Sea; Bryazgin, V.F., Ed.; Kola Branch of the USSR Academy of Sciences: Apatity, Russia, 1978; pp. 13–27. (In Russian) [Google Scholar]
- Ilyin, G.V.; Matishov, G.G.; Mironov, O.G.; Pavlova, L.G.; Petrov, V.S. Chemical Processes in Ecosystems of Northern Seas (Hydrochemistry, Geochemistry, Oil Contamination); KSC RAS Press: Apatity, Russia, 1997. (In Russian) [Google Scholar]
- Denisenko, N.V. Species composition and distribution of bryozoans on littoral of some bays of the Eastern Murmansk. In Benthos of the Barents Sea. Distribution, Ecology and Structure of Populations; Semenov, V.N., Ed.; Kola Branch of the USSR Academy of Sciences: Apatity, Russia, 1984; pp. 71–79. (In Russian) [Google Scholar]
- Denisenko, N.V. Bryozoans (Bryozoa) of Yarnyshnaya Bay (East Murmansk): Fauna, distribution, seasonal changes. In Current Benthos of the Barents and the Kara Seas; Matishov, G.G., Ed.; KSC RAS Press: Apatity, Russia, 2000; pp. 203–219. (In Russian) [Google Scholar]
- Kuklinski, P.; Taylor, P.D. A new genus and some cryptic species of Arctic and boreal calloporid cheilostome bryozoans. J. Mar. Biol. Ass. UK 2006, 86, 1035–1046. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Effects of environmental factors on the abundance, biomass, and individual weight of juvenile red king crabs in the Barents Sea. Front. Mar. Sci. 2020, 7, 726. [Google Scholar] [CrossRef]
- Chan, F.T.; Stanislawczyk, K.; Sneekes, A.C.; Dvoretsky, A.; Gollasch, S.; Minchin, D.; David, M.; Jelmert, A.; Albretsen, J.; Bailey, S.A. Climate change opens new frontiers for marine species in the Arctic: Current trends and future invasion risks. Glob. Chang. Biol. 2019, 25, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Evseyeva, O.Y. Fauna of bryozoans (Bryozoa) in the Isfjorden Bay (Spitsbergen Island, Svalbard). In Complex Investigations of Spitsbergen and Offshore Nature, Proceedings of the XV Scientific Conference, Murmansk, Russia, 28–30 October 2020; Makarevich, P.R., Ed.; Publ. FRC Kola Science Centre RAS: Apatity, Russia, 2020; pp. 30–32. (In Russian) [Google Scholar]
- Akhmetchina, O.Y.; Zakharov, D.V. Species composition and distribution of bryozoans (Bryozoa) along the transect “Kola meridian” (Barents Sea) in 2010–2011. Vestn. MSTU 2013, 16, 415–424. (In Russian) [Google Scholar]
- Akhmetchina, O.Y. Formation of the structure of moss animals fauna (Bryozoa) off the Franz Josef Land Archipelago. In Perspective Directions in Investigations of Ecosystems of Russia’s Arctic and Southern Seas, Proceedings of the XXXIV Conference for Young Scientists Dedicated to the 50th Anniversary of the Birth of RAS Correspondent-Member Dmitry G. Matishov, Murmansk, Russia, 15 May 2016; Moiseev, D.V., Ed.; MMBI KSC RAS: Murmansk, Russia, 2016; pp. 9–14. (In Russian) [Google Scholar]
- Evseeva, O.Y. Bryozoa of the northern part of Barents Sea: Species composition, distribution, ecology (based on the materials of expeditions MMBI 2016–2017). Trans. Kola Sci. Cent. 2020, 5, 54–68. (In Russian) [Google Scholar] [CrossRef]
- Kvach, A.Y.; Varfolomeeva, M.A.; Kotenko, O.N.; Sukhotin, A.A.; Kutiumov, V.A.; Grischenko, A.V.; Granovitch, A.I.; Ostrovsky, A.N. Life history of the ctenostome bryozoan Flustrellidra hispida in the White Sea. Invert. Zool. 2019, 16, 343–360. [Google Scholar] [CrossRef]
- Akhmetchina, O.Y. Structure and distribution of bryozoans (Bryozoa) in the shallow zone of the Gulf of Gren Fjord. In Proceedings of the Complex Studies of the Nature of Svalbard and the Adjacent Shelf: Materials of the All-Russian Scientific Conference with International Participation, Murmansk, Russia, 2–4 November 2016; Matishov, G.G., Tarasov, G.A., Eds.; SSC RAS Press: Rostov-on-Don, Russia, 2016; pp. 31–37. (In Russian). [Google Scholar]
- Bayer, M.M.; Todd, C.D.; Hoyle, J.E.; Wilson, J.F.B. Wave-related abrasion induces formation of extended spines in a marine bryozoan. Proc. R. Soc. Lond. B 1997, 264, 1605–1611. [Google Scholar] [CrossRef]
- Cowen, R.; Rider, J. Functional analysis of fenestellid bryozoan colonies. Lethaia 1972, 5, 147–164. [Google Scholar] [CrossRef]
- Eckman, J.E.; Okamura, B. A model of particle capture by bryozoans in turbulent flow: Significance of colony form. Am. Nat. 1998, 152, 861–880. [Google Scholar] [CrossRef]
- Ben Ismail, D.; Rabaoui, K.; Diawara, M.; Ben Hassine, O.K. The Bryozoan assemblages and their relationship with certain environmental factors along the shallow and subtidal Tunisian coasts. Cah. Biol. Mar. 2012, 53, 231–242. [Google Scholar]
- Kuklinski, P.; Balazy, P.; Nowak, M.; Bielecka, L. Factors controlling initial development of Polar bryozoan assemblages. Studi Trent. Sci. Nat. 2014, 94, 145–151. [Google Scholar]
- Santagata, S.; Ade, V.; Mahon, A.R.; Wisocki, P.A.; Halanych, K.M. Compositional differences in the habitat-forming bryozoan communities of the Antarctic Shelf. Front. Ecol. Evol. 2018, 6, 116. [Google Scholar] [CrossRef] [Green Version]

| Taxa | Origin | Construction Form | Orientation Form | Biomass, g·m−2 | Stations | |||
|---|---|---|---|---|---|---|---|---|
| Min | Max | X | SE | |||||
| Cyclostomatida | ||||||||
| Crisia eburnea (Linnaeus, 1758) | BA | Ca | Er | 0 | 1.214 | 0.213 | 0.141 | 4,5,6 |
| Crisiella producta (Smitt, 1865) | BA | Ca | Er | 0 | 0.72 | 0.087 | 0.079 | 4,5,6 |
| Filicrisia geniculata (Milne Edwards, 1838) | B | Ca | Er | 0 | 0.002 | 0.000 | 0.000 | 6 |
| Oncousoecia diastoporides (Norman, 1869) | BA | Ca | En | 0 | 0.011 | 0.001 | 0.001 | 6 |
| Patinella verrucaria (Linnaeus, 1758) | BA | Ca | En | 0 | 0.002 | 0.000 | 0.000 | 6 |
| Ctenostomatida | ||||||||
| Alcyonidioides mytili (Dalyell, 1848) | BA | Fl | En | 0 | 0.002 | 0.000 | 0.000 | 4,6,8 |
| Amathia gracilis (Leidy, 1855) | B | Fl | Er | 0 | 0.162 | 0.018 | 0.018 | 6 |
| Flustrellidra hispida (O. Fabricius, 1780) | B | Fl | En | 0 | 0.525 | 0.150 | 0.073 | 2,3,4,7 |
| Walkeria uva (Linnaeus, 1758) | B | Fl | Er | 0 | 0.001 | 0.000 | 0.000 | 6,8 |
| Cheilostomatida | ||||||||
| Aquiloniella scabra (van Beneden, 1848) | BA | Ca | Er | 0 | 0.009 | 0.001 | 0.001 | 6 |
| Callopora lineata (Linnaeus, 1767) | BA | Ca | En | 0 | 0.012 | 0.002 | 0.001 | 6,8 |
| Callopora weslawski Kuklinski and Taylor, 2006 | A | Ca | En | 0 | 0.817 | 0.134 | 0.089 | 3,4,5,6,8 |
| Celleporella hyalina (Linnaeus, 1767) | BA | Ca | En | 0 | 0.023 | 0.004 | 0.002 | 3,4,5,6,8 |
| Cribrilina cryptooecium Norman, 1903 | B | Ca | En | 0 | 1.373 | 0.198 | 0.149 | 3,4,6,8 |
| Cylindroporella tubulosa (Norman, 1868) | BA | Ca | En | 0 | 0.011 | 0.002 | 0.001 | 6,8 |
| Dendrobeania murrayana (Bean, in Johnston, 1847) | BA | Ca | Er | 0 | 0.004 | 0.000 | 0.000 | 3 |
| Electra pilosa (Linnaeus, 1767) | BA | Ca | En | 0 | 0.356 | 0.103 | 0.048 | 3,4,5,6 |
| Escharella immersa (Fleming, 1828) | BA | Ca | En | 0 | 0.092 | 0.010 | 0.010 | 6,8 |
| Eucratea loricata (Linnaeus, 1758) | BA | Ca | Er | 0 | 4.68 | 1.009 | 0.602 | 4,5,6 |
| Harmeria scutulata (Busk, 1855) | A | Ca | En | 0 | 1.676 | 0.269 | 0.194 | 3,4,6,8 |
| Juxtacribrilina annulata (O. Fabricius, 1780) | BA | Ca | En | 0 | 0.17 | 0.024 | 0.019 | 6,8 |
| Microporella arctica Norman, 1903 | B | Ca | En | 0 | 0.14 | 0.016 | 0.016 | 4,6 |
| Porella alba Nordgaard, 1906 | BA | Ca | En | 0 | 0.003 | 0.000 | 0.000 | 6 |
| Tegella arctica (d’Orbigny, 1853) | BA | Ca | En | 0 | 0.08 | 0.009 | 0.009 | 6,8 |
| Tegella unicornis (Fleming, 1828) | B | Ca | En | 0 | 0.007 | 0.001 | 0.001 | 8 |
| Species | Average Dissimilarity, % | Contribution, % | Cumulative Contribution, % |
|---|---|---|---|
| Eucratea loricata | 19.31 | 21.67 | 21.67 |
| Electra pilosa | 10.52 | 11.81 | 33.48 |
| Callopora weslawski | 10.05 | 11.28 | 44.77 |
| Cribrilina cryptooecium | 9.93 | 11.14 | 55.91 |
| Flustrellidra hispida | 8.71 | 9.77 | 65.68 |
| Crisia eburnea | 8.57 | 9.62 | 75.30 |
| Harmeria scutulata | 6.82 | 7.65 | 82.94 |
| Crisiella producta | 3.81 | 4.28 | 87.22 |
| Juxtacribrilina annulata | 2.07 | 2.33 | 89.55 |
| Diversity | Biomass | ||||||
|---|---|---|---|---|---|---|---|
| Variable | LambdaA | F | p | Variable | LambdaA | F | p |
| S | 68 | 14.77 | 0.002 | T | 52 | 7.52 | 0.001 |
| P-PO4 | 9 | 2.26 | 0.140 | O2 | 8 | 1.15 | 0.341 |
| Si-SiO3 | 5 | 1.35 | 0.276 | WFII | 7 | 1.05 | 0.385 |
| T | 5 | 1.84 | 0.235 | N-NO2 | 7 | 1.15 | 0.354 |
| N-NO3 | 4 | 1.23 | 0.33 | N-NO3 | 11 | 2.27 | 0.163 |
| N-NO2 | 3 | 1.19 | 0.394 | S | 6 | 1.24 | 0.376 |
| WFII | 2 | 0.53 | 0.586 | Si-SiO3 | 3 | 0.47 | 0.687 |
| O2 | 4 | 0 | 1 | P-PO4 | 6 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evseeva, O.Y.; Ishkulova, T.G.; Dvoretsky, A.G. Environmental Drivers of an Intertidal Bryozoan Community in the Barents Sea: A Case Study. Animals 2022, 12, 552. https://doi.org/10.3390/ani12050552
Evseeva OY, Ishkulova TG, Dvoretsky AG. Environmental Drivers of an Intertidal Bryozoan Community in the Barents Sea: A Case Study. Animals. 2022; 12(5):552. https://doi.org/10.3390/ani12050552
Chicago/Turabian StyleEvseeva, Olga Yu., Tatyana G. Ishkulova, and Alexander G. Dvoretsky. 2022. "Environmental Drivers of an Intertidal Bryozoan Community in the Barents Sea: A Case Study" Animals 12, no. 5: 552. https://doi.org/10.3390/ani12050552







