Effects of Seasonal Heat Stress during Late Gestation on Growth Performance, Metabolic and Immuno-Endocrine Parameters of Calves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Experimental Design
2.2. Growth Measures and Sample Collection
2.3. Statistical Analyses
3. Results
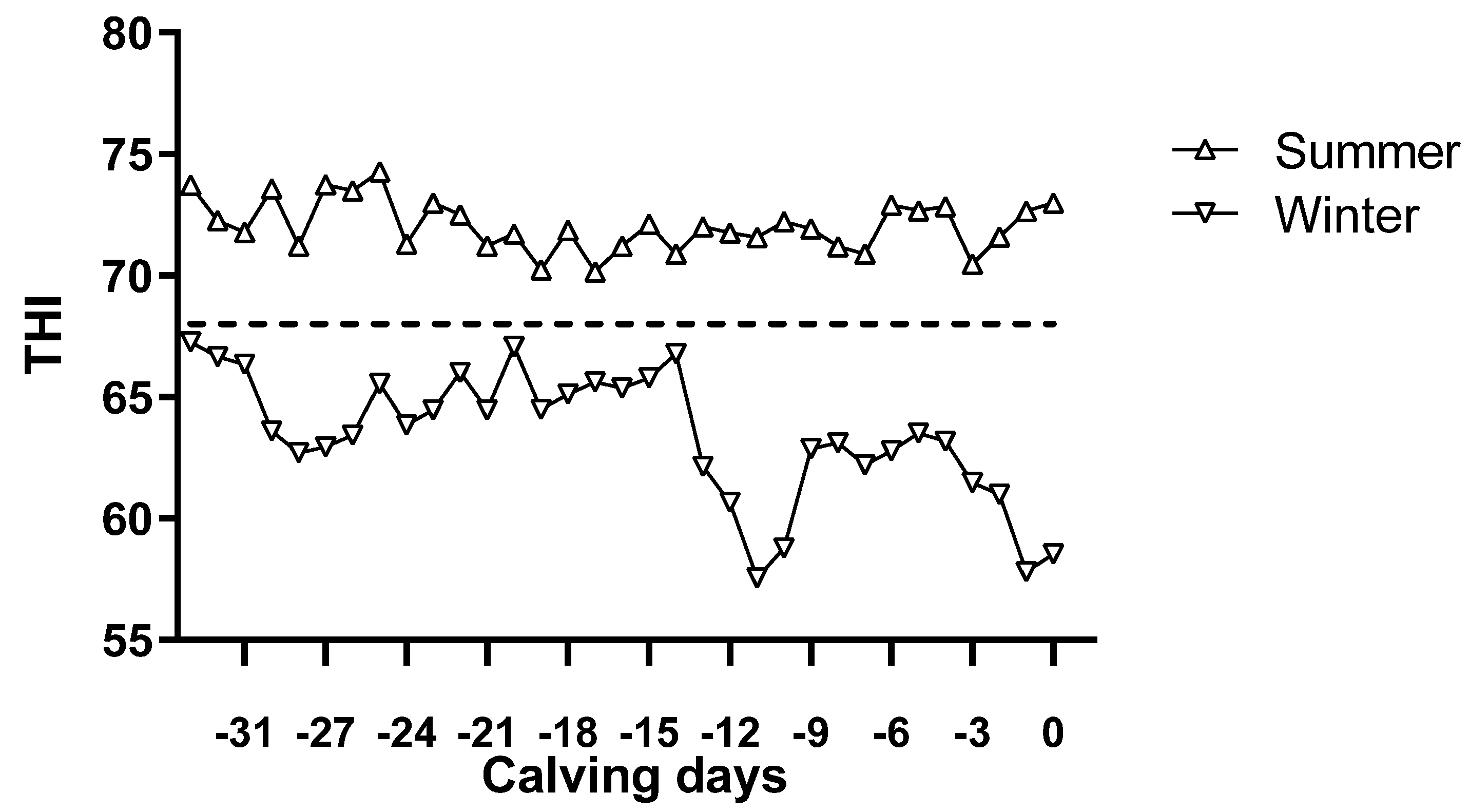
3.1. Environmental Data, Dam Physiological Parameters, and Qualified Rate of Colostrum
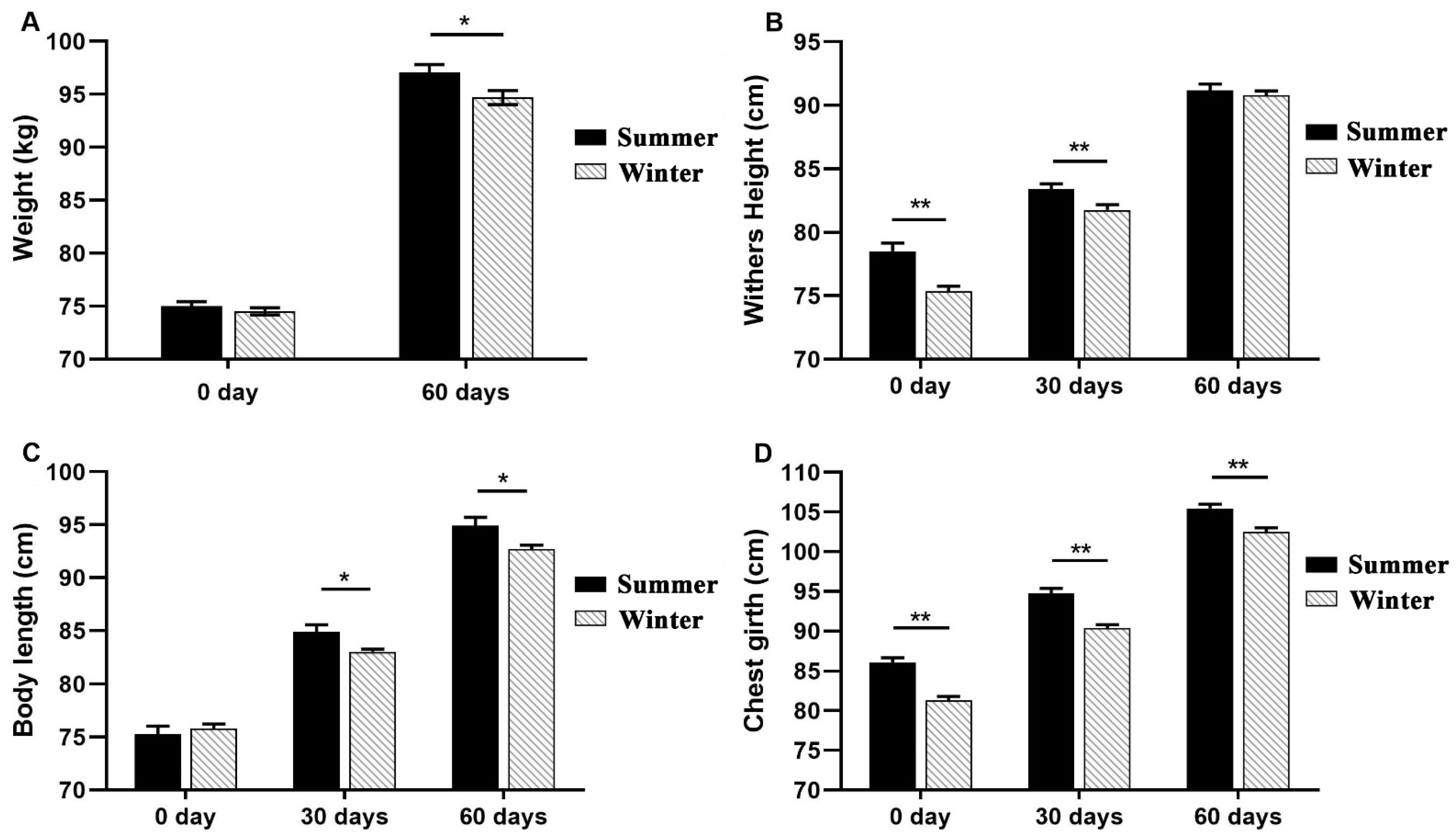
3.2. The Growth Performances of Calves
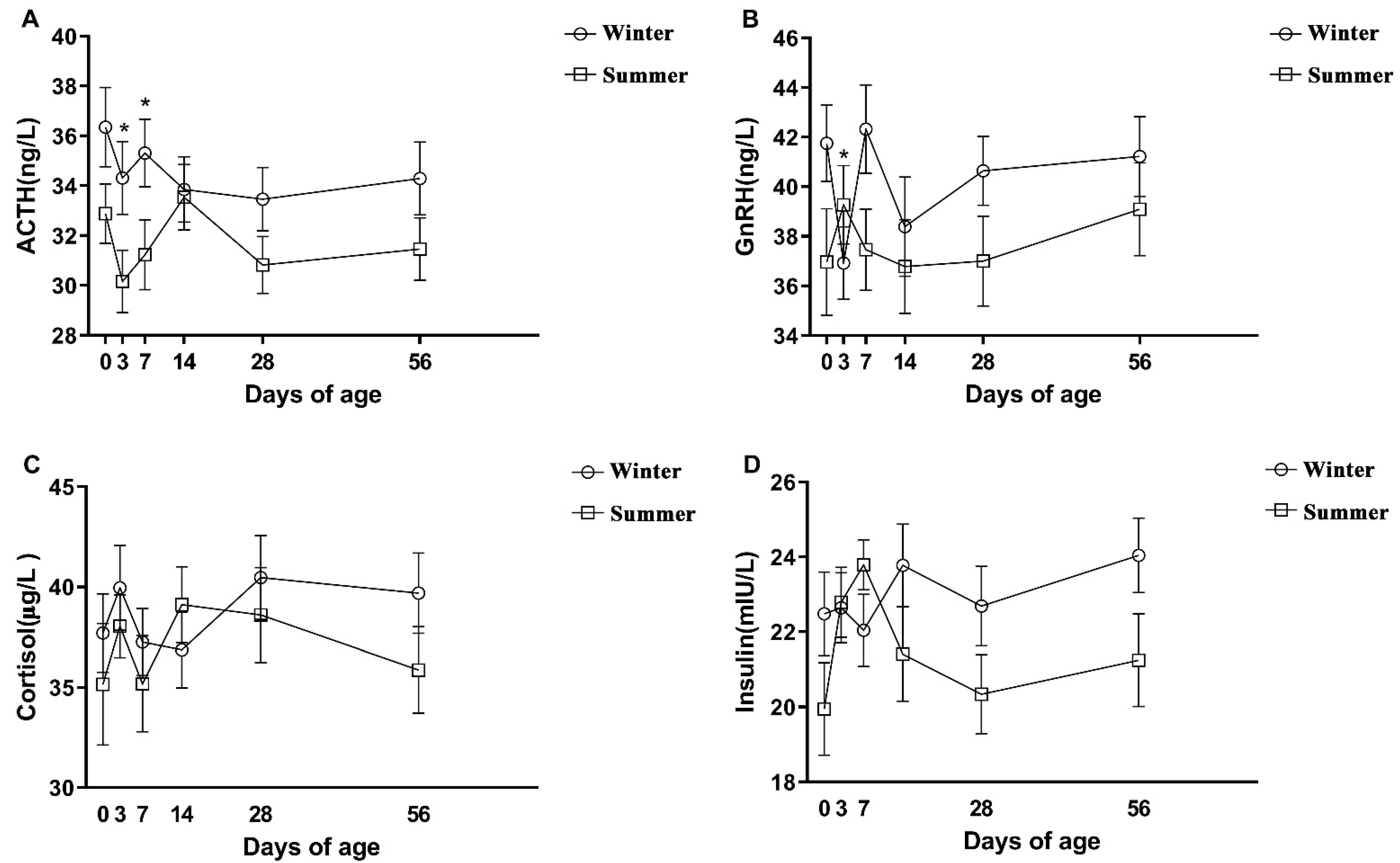
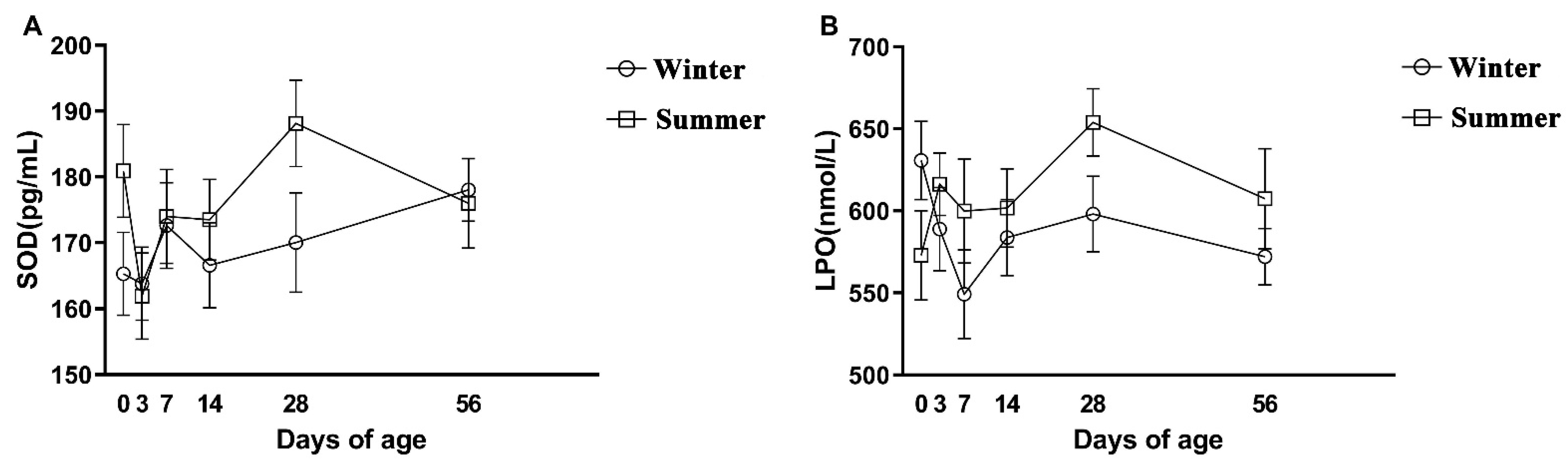
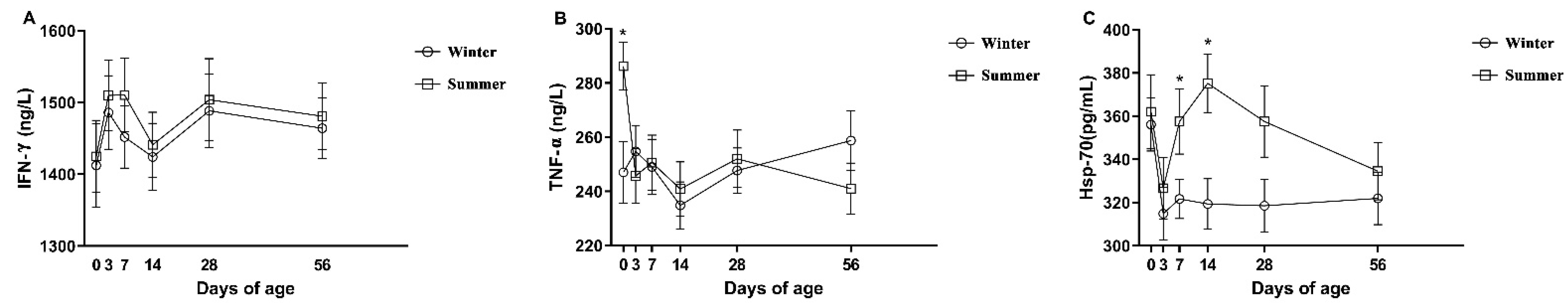
3.3. The Hormonal, Oxidative Stress, and Immune Parameters of Calves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Baumgard, L.H.; Rhoads, R.J. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Dahl, G.E. Invited review: Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Broucek, J.; Kisac, P.; Uhrincat, M. Effect of hot temperatures on the hematological parameters, health and performance of calves. Int J Biometeorol 2009, 53, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E.; Tao, S.; Monteiro, A.P.A. Effects of late-gestation heat stress on immunity and performance of calves1. J Dairy Sci 2016, 99, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- NASA|NOAA. Find 2014 Warmest Year in Modern Record; PR Newswire Association LLC: New York, NY, USA, 2015. [Google Scholar]
- Renaudeau, D.; Collin, A.; Yahav, S.; de Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Safa, S.; Kargar, S.; Moghaddam, G.A.; Ciliberti, M.G.; Caroprese, M. Heat stress abatement during the postpartum period: Effects on whole lactation milk yield, indicators of metabolic status, inflammatory cytokines, and biomarkers of the oxidative stress. J. Anim. Sci. 2019, 97, 122–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skibiel, A.L.; Peñagaricano, F.; Amorín, R.; Ahmed, B.M.; Dahl, G.E.; Laporta, J. In Utero Heat Stress Alters the Offspring Epigenome. Sci. Rep. 2018, 8, 14609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindström, J. Early Development and Fitness in Birds and Mammals; Elsevier Ltd.: Oxford, UK, 1999; Volume 14, pp. 343–348. [Google Scholar]
- Mcmillen, I.C.; Robinson, J.S. Developmental Origins of the Metabolic Syndrome: Prediction, Plasticity, and Programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Giussani, D.A.; Forhead, A.J. Intrauterine Programming of Physiological Systems: Causes and Consequences. Physiology 2006, 21, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A.W.M.B. Chronic heat stress and prenatal development in sheep: I. Conceptus growth and maternal plasma hormones and metabolites. J. Anim. Sci. 1989, 67, 3289–3299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flouris, A.D.; Spiropoulos, Y.; Sakellariou, G.J.; Koutedakis, Y. Effect of seasonal programming on fetal development and longevity: Links with environmental temperature. Am. J. Hum. Biol. 2009, 21, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.P.A.; Guo, J.R.; Weng, X.S.; Ahmed, B.M.; Hayen, M.J.; Dahl, G.E.; Bernard, J.K.; Tao, S. Effect of maternal heat stress during the dry period on growth and metabolism of calves. J. Dairy Sci. 2016, 99, 3896–3907. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.H.A.E. Growth-and breed-related changes of fetal development in cattle. Asian-Australas. J. Anim. Sci. 2008, 21, 640–647. [Google Scholar] [CrossRef]
- Bauman, D.E.; Bruce Currie, W. Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Do, A.B.; Connor, E.E.; Tao, S.; Hayen, M.J.; Bubolz, J.W.; Dahl, G.E. Heat stress abatement during the dry period influences metabolic gene expression and improves immune status in the transition period of dairy cows. J. Dairy Sci. 2011, 94, 86–96. [Google Scholar] [CrossRef]
- Sangild, P.T.; Schmidt, M.; Jacobsen, H.; Fowden, A.L.; Forhead, A.; Avery, B.; Greve, T. Blood Chemistry, Nutrient Metabolism, and Organ Weights in Fetal and Newborn Calves Derived from In Vitro-Produced Bovine Embryos1. Biol. Reprod. 2000, 62, 1495–1504. [Google Scholar] [CrossRef] [Green Version]
- Trifkovic, J.; Jovanovic, L.; Duric, M.; Stevanovic-Dordevic, S.; Milanovic, S.; Lazarevic, M.; Sladojevic, Z.; Kirovski, D. Influence of different seasons during late gestation on Holstein cows’ colostrum and postnatal adaptive capability of their calves. Int. J. Biometeorol. 2018, 62, 1097–1108. [Google Scholar] [CrossRef]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.; Dahl, G.E. Effect of heat stress during late gestation on immune function and growth performance of calves: Isolation of altered colostral and calf factors. J. Dairy Sci. 2014, 97, 6426–6439. [Google Scholar] [CrossRef]
- Tao, S.; Monteiro, A.P.A.; Thompson, I.M.; Hayen, M.J.; Dahl, G.E. Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J. Dairy Sci. 2012, 95, 7128–7136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikmen, S.; Alava, E.; Pontes, E.; Fear, J.M.; Dikmen, B.Y.; Olson, T.A.; Hansen, P.J. Differences in Thermoregulatory Ability Between Slick-Haired and Wild-Type Lactating Holstein Cows in Response to Acute Heat Stress. J. Dairy Sci. 2008, 91, 3395–3402. [Google Scholar] [CrossRef] [PubMed]
- Zimbelman, R.B.; Rhoads, R.P.; Rhoads, M.L.; Duff, G.C.; Baumgard, L.H.; Collier, R.J. A re-evaluation of the impact of temperature humidity index (THI) and black globe humidity index (BGHI) on milk production in high producing dairy cows. In Proceedings of the Southwest Nutrition Conference, Savoy, IL, USA, 26–27 February 2009; Collier, R.J., Ed.; pp. 158–169. [Google Scholar]
- Fabris, T.F.; Laporta, J.; Corra, F.N.; Torres, Y.M.; Kirk, D.J.; McLean, D.J.; Chapman, J.D.; Dahl, G.E. Effect of nutritional immunomodulation and heat stress during the dry period on subsequent performance of cows. J. Dairy Sci. 2017, 100, 6733–6742. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Bani, P.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of Short- and Long-Term Exposure to a Hot Environment on Rumen Passage Rate and Diet Digestibility by Friesian Heifers1. J. Dairy Sci. 1999, 82, 967–973. [Google Scholar] [CrossRef]
- Hough, R.L.; McCarthy, F.D.; Kent, H.D.; Eversole, D.E.; Wahlberg, M.L. Influence of nutritional restriction during late gestation on production measures and passive immunity in beef cattle. J. Anim. Sci. 1990, 68, 2622. [Google Scholar] [CrossRef] [PubMed]
- Janovick, N.A.; Drackley, J.K. Prepartum dietary management of energy intake affects postpartum intake and lactation performance by primiparous and multiparous Holstein cows. J. Dairy Sci. 2010, 93, 3086–3102. [Google Scholar] [CrossRef] [PubMed]
- Dreiling, C.E.; Carman, F.R.; Brown, D.E. Maternal endocrine and fetal metabolic responses to heat stress. J. Dairy Sci. 1991, 74, 312–327. [Google Scholar] [CrossRef]
- Regnault, T.R.H.; Vrijer, B.; Galan, H.L.; Davidsen, M.L.; Trembler, K.A.; Battaglia, F.C.; Wilkening, R.B.; Anthony, R.V. The relationship between transplacental O2 diffusion and placental expression of PlGF, VEGF and their receptors in a placental insufficiency model of fetal growth restriction. J. Physiol. 2003, 550, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Early, R.J.; McBride, B.W.; Vatnick, I.; Bell, A.W. Chronic heat stress and prenatal development in sheep: II. Placental cellularity and metabolism. J. Anim. Sci. 1991, 69, 3610–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, D.T.; Green, A.S.; Limesand, S.W. Catecholamines mediate multiple fetal adaptations during placental insufficiency that contribute to intrauterine growth restriction: Lessons from hyperthermic sheep. J. Pregnancy 2011, 2011, 740408. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Fahy, A.L.; Green, A.S.; Anderson, M.J.; Rhoads, R.P.; Limesand, S.W. beta2-Adrenergic receptor desensitization in perirenal adipose tissue in fetuses and lambs with placental insufficiency-induced intrauterine growth restriction. J. Physiol. 2010, 588, 3539–3549. [Google Scholar] [CrossRef] [PubMed]
- Limesand, S.W.; Rozance, P.J.; Zerbe, G.O.; Hutton, J.C.; Hay, W.J. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 2006, 147, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A.; Gatford, K.L.; De Blasio, M.J.; Edwards, L.J.; McMillen, I.C.; Fowden, A.L. Restriction of placental growth in sheep impairs insulin secretion but not sensitivity before birth. J. Physiol. 2007, 584, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Limesand, S.W.; Rozance, P.J.; Macko, A.R.; Anderson, M.J.; Kelly, A.C.; Hay, J.W.W. Reductions in insulin concentrations and β-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E516–E523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.; Monteiro, A.P.A.; Hayen, M.J.; Dahl, G.E. Short communication: Maternal heat stress during the dry period alters postnatal whole-body insulin response of calves. J. Dairy Sci. 2014, 97, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Senger, P.L. Pathways to Pregnancy and Parturition; Current Conceptions. Inc.: Pullman, WA, USA, 2003; p. 144. [Google Scholar]
- Ranade, R.; Talukder, S.; Muscatello, G.; Celi, P. Assessment of oxidative stress biomarkers in exhaled breath condensate and blood of dairy heifer calves from birth to weaning. Vet. J. 2014, 202, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Tüzün, A.; Erdil, A.; İnal, V.; Aydın, A.; Bağcı, S.; Yeşilova, Z.; Sayal, A.; Karaeren, N.; Dağalp, K. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin. Biochem. 2002, 35, 569–572. [Google Scholar] [CrossRef]
- Celi, P.T.U.O. The role of oxidative stress in small ruminants’ health and production. Rev. Bras. Zootec. 2010, 39, 348–363. [Google Scholar] [CrossRef] [Green Version]
- Mohamed Ibrahim, H.M. Cytokine Response and Oxidative Stress Status in Dairy Cows with Acute Clinical Mastitis. J. Dairy Vet. Anim. Res. 2015, 3, 00064. [Google Scholar] [CrossRef] [Green Version]
- Min, L.; Zheng, N.; Zhao, S.; Cheng, J.; Yang, Y.; Zhang, Y.; Yang, H.; Wang, J. Long-term heat stress induces the inflammatory response in dairy cows revealed by plasma proteome analysis. Biochem. Biophys. Res. Commun. 2016, 471, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V. IFN-λs. Curr. Opin. Immunol. 2011, 23, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Apostolaki, M.; Armaka, M.; Victoratos, P.; Kollias, G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr. Dir. Autoimmun. 2010, 11, 1. [Google Scholar] [PubMed]
- Nenci, A.; Becker, C.; Wullaert, A.; Gareus, R.; van Loo, G.; Danese, S.; Huth, M.; Nikolaev, A.; Neufert, C.; Madison, B.; et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007, 446, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.; Kotlyarov, A.; Carballo, E.; Alexopoulou, L.; Blackshear, P.J.; Gaestel, M.; Davis, R.; Flavell, R.; Kollias, G. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 2001, 20, 3760–3770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neurath, M.F.; Fuss, I.; Pasparakis, M.; Alexopoulou, L.; Haralambous, S.; Meyer, Z.B.K.; Strober, W.; Kollias, G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur. J. Immunol. 1997, 27, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Powrie, F.; Leach, M.W.; Mauze, S.; Menon, S.; Barcomb Caddle, L.; Coffman, R.L. Inhibition of Thl responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1994, 1, 553–562. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hue, N.T.; Tran, H.T.; Phan, T.; Nakamura, J.; Iwata, T.; Harano, K.; Ishibashi, Y.; Yuasa, T.; Iwaya-Inoue, M. Hsp90 and reactive oxygen species regulate thermotolerance of rice seedlings via induction of heat shock factor A2 (OsHSFA2) and galactinol synthase 1 (OsGolS1). Agric. Sci. 2013, 4, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Kapila, N.; Kishore, A.; Sodhi, M.; Sharma, A.; Mohanty, A.K.; Kumar, P.; Mukesh, M. Temporal changes in mRNA expression of heat shock protein genes in mam-mary epithelial cells of riverine buffalo in response to heat stress in vitro. Int. J. Anim. Biotechnol. 2013, 3, 5–9. [Google Scholar]
- Deb, R.; Sajjanar, B.; Singh, U.; Kumar, S.; Singh, R.; Sengar, G.; Sharma, A. Effect of heat stress on the expression profile of Hsp90 among Sahiwal (Bos indicus) and Frieswal (Bos indicus×Bos taurus) breed of cattle: A comparative study. Gene 2014, 536, 435–440. [Google Scholar] [CrossRef]
- Belhadj, S.I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Diets Composition and Nutritional Level | Dry Cows in Summer | Dry Cows in Winter |
|---|---|---|
| Diets Composition | ||
| Corn silage | 28.77 | 30.44 |
| Oat grass | 34.44 | 34.44 |
| Perinatal concentrated feeding stuff | 34.47 | 33.09 |
| Sugarcane molasses | 2.32 | 2.03 |
| Nutritional Level | ||
| DM | 37.45 | 41.79 |
| CP | 22.75 | 23.84 |
| NDF | 38.42 | 40.04 |
| ADF | 23.77 | 24.94 |
| NEL(MJ/kg) | 1.42 | 1.55 |
| Variable | Summer | Winter | SEM | p-Value |
|---|---|---|---|---|
| Rectal temperature (°C) | 39.02 | 38.95 | 0.02 | <0.01 |
| Gestation length (d) | 274.00 | 275.44 | 1.51 | 0.33 |
| Variable | Summer | Winter |
|---|---|---|
| Colostrum qualified rate (%) | 80.77 | 100 |
| Diarrhea rate of calves (%) | 57.14 | 21.74 |
| Variable | Summer | Winter | SEM | p-Value |
|---|---|---|---|---|
| IFN-γ (ng·L−1) | 1480.93 | 1454.42 | 28.36 | 0.633 |
| SOD (pg·mL−1) | 175.24 | 169.42 | 3.77 | 0.058 |
| ACTH (ng·L−1) | 31.61 | 34.60 | 0.77 | <0.001 |
| GnRH (ng·L−1) | 37.83 | 40.21 | 1.00 | 0.034 |
| Cortisol (μg·L−1) | 37.08 | 38.66 | 1.19 | 0.142 |
| HSP-70 (pg·mL−1) | 351.26 | 325.43 | 7.81 | 0.002 |
| GH (μg·L−1) | 23.56 | 23.92 | 0.57 | 0.625 |
| Insulin (mIU·L−1) | 21.67 | 22.95 | 0.61 | 0.038 |
| LPO (nmol·L−1) | 609.88 | 587.13 | 14.22 | 0.204 |
| TNF-α (ng·L−1) | 251.62 | 248.69 | 5.83 | 0.369 |
| IgG (μg·mL−1) | 2828.32 | 2747.20 | 66.75 | 0.829 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Liang, Y.; Guo, J.; Wang, M.; Li, M.; Zhang, H.; Arbab, A.A.I.; Karrow, N.A.; Yang, Z.; Mao, Y. Effects of Seasonal Heat Stress during Late Gestation on Growth Performance, Metabolic and Immuno-Endocrine Parameters of Calves. Animals 2022, 12, 716. https://doi.org/10.3390/ani12060716
Tang C, Liang Y, Guo J, Wang M, Li M, Zhang H, Arbab AAI, Karrow NA, Yang Z, Mao Y. Effects of Seasonal Heat Stress during Late Gestation on Growth Performance, Metabolic and Immuno-Endocrine Parameters of Calves. Animals. 2022; 12(6):716. https://doi.org/10.3390/ani12060716
Chicago/Turabian StyleTang, Cheng, Yan Liang, Jiahe Guo, Mengqi Wang, Mingxun Li, Huimin Zhang, Abdelaziz Adam Idriss Arbab, Niel A. Karrow, Zhangping Yang, and Yongjiang Mao. 2022. "Effects of Seasonal Heat Stress during Late Gestation on Growth Performance, Metabolic and Immuno-Endocrine Parameters of Calves" Animals 12, no. 6: 716. https://doi.org/10.3390/ani12060716






