First Attempt to Infer Sound Hearing and Its Paleoenvironmental Implications in the Extinct Insular Canid Cynotherium sardous Studiati, 1857 (Sardinia, Italy)
Abstract
Simple Summary
Abstract
1. Introduction
Cynotherium sardous
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Microtomography
2.2.2. Morphometry
3. Results
4. Discussion
4.1. Hearing Ability in Cynotherium sardous
4.2. Inferences about Evolutionary Processes of Cynotherium in the Sardinian Insular Environment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Wysocki, J. Dimensions of the vestibular and tympanic scalae of the cochlea in selected mammals. Hear. Res. 2001, 161, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A. Evolutionary paths to mammalian cochleae. JARO 2012, 13, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.; Pearson, O.; Marean, C.W.; Chen, X.J. Bone density studies in zooarchaeology. J. Archaeol. Sci. 2003, 30, 1701–1708. [Google Scholar] [CrossRef]
- Heine, P.A. Anatomy of the ear. Vet. Clin. 2004, 34, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, C.; Nagel, D.; Gunnell, G.; Weber, G.W.; Kriwet, J.; Morlo, M.; Bastl, K. Palaeobiology of Hyaenodon exiguus (Hyaenodonta, Mammalia) based on morphometric analysis of the bony labyrinth. J. Anat. 2017, 230, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ekdale, E.G.; Rowe, T. Morphology and variation within the bony labyrinth of zhelestids (Mammalia, Eutheria) and other therian mammals. J. Vertebr. Paleontol. 2011, 31, 658–675. [Google Scholar] [CrossRef]
- Grohée, C.; Tseng, Z.J.; Lebrun, R.; Boistel, R.; Flynn, J.J. Bony labyrinth shape variation in extant Carnivora: A case study of Musteloidea. J. Anat. 2016, 228, 366–383. [Google Scholar] [CrossRef]
- Costeur, L.; Grohée, C.; Aguirre-Fernández, G.; Ekdale, E.; Schulz, G.; Müller, B.; Mennecart, B. The bony labyrinth of toothed whales reflects both phylogeny and habitat preferences. Sci. Rep. 2018, 8, 7841. [Google Scholar] [CrossRef]
- Schwab, J.A.; Kriwet, J.; Weber, G.W.; Pfaff, C. Carnivoran hunting style and phylogeny reflected in bony labyrinth morphometry. Sci. Rep. 2019, 9, 70. [Google Scholar] [CrossRef]
- Braga, J.; Bouvier, P.; Dherbeya, J.R.; Balaresquea, P.; Risser, L.; Loubes, J.-M.; Dumoncel, J.; Duployer, B.; Tenailleau, C. Echoes from the past: New insights into the early hominin cochlea from a phylo-morphometric approach. C. R. Palevol 2017, 16, 508–520. [Google Scholar] [CrossRef]
- Cox, P.G.; Jeffrey, N. Semicircular canals and agility: The influence of size and shape measures. J. Anat. 2010, 216, 37–47. [Google Scholar] [CrossRef] [PubMed]
- David, R.; Droulez, J.; Allain, R.; Berthoz, A.; Janvier, P.; Bennequin, D. Motion from the past. A new method to infer vestibular capacities of extinct species. C. R. Palevol 2010, 9, 397–410. [Google Scholar] [CrossRef]
- Mennecart, B.; DeMiguel, D.; Bibi, F.; Rössner, G.E.; Métais, G.; Neenan, J.M.; Wang, S.; Shulz, G.; Müller, B.; Costeur, L. Bony labyrinth morphology clarifies the origin and evolution of deer. Sci. Rep. 2017, 7, 13176. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, C.; Martin, T.; Ruf, I. Bony labyrinth morphometry indicates locomotor adaptations in the squirrel-related clade (Rodentia, Mammalia). Proc. R. Soc. B 2015, 282, 20150744. [Google Scholar] [CrossRef]
- Gray, A. The Labyrinth of Animals: Including Mammals, Birds, Reptiles and Amphibian; J. & A. Churchill: London, UK, 1907; Volume 1, pp. 1–198. [Google Scholar]
- Manley, G.A. Cochlear mechanisms from a phylogenetic viewpoint. Proc. Natl. Acad. Sci. USA 2000, 97, 11736–11743. [Google Scholar] [CrossRef]
- Macrini, T.E.; Flynn, J.J.; Ni, X.; Croft, D.A.; Wyss, A.R. Comparative study of notoungulate (Placentalia, Mammalia) bony labyrinths and new phylogenetically informative inner ear characters. J. Anat. 2013, 223, 442–461. [Google Scholar] [CrossRef]
- Hadzelimovic, H.; Savkovic, L.J. Appearance of semicircular canals in birds in relation to mode of life. Acta Anat. 1964, 57, 306–315. [Google Scholar] [CrossRef]
- Pereira, E.; Salotti, M. Nouvelles données sur le peuplement mammalien endémique du Pléistocène de Corse. Mammalia 2002, 66, 423–438. (In French) [Google Scholar] [CrossRef]
- Palombo, M.R. Insular mammalian fauna dynamics and Paleogeography: A lesson from the Western Mediterranean islands. Integr. Zool. 2018, 13, 2–20. [Google Scholar] [CrossRef]
- Eisenmann, V. Caractères juveniles et affinités systematiques du crâne de Cynotherium sardous, canide endémique Pléistocène de Sardaigne. C. R. Acad. Sci. Paris 1990, 310, 433–439. (In French) [Google Scholar]
- Malatesta, A. Cynotherium sardous Studiati, an extinct canid from the Pleistocene of Sardinia. Mem. Ist. Ital. Paleontol. Um. 1970, 1, 1–72. [Google Scholar]
- Eisenmann, V.; van der Geer, B. The Cynotherium from Corbeddu (Sardinia): Comparative biometry with extant and fossil canids. Deinsea 1999, 7, 147–168. [Google Scholar]
- Caloi, L.; Palombo, M.R. Il cranio di Cynotherium sardous Studiati di Dragonara (Alghero, Sardegna Nord-occidentale). In 1° Workshop Nazionale di Paleontologia dei Vertebrati “Tutela e Valorizzazione dei Siti paleontologici a Vertebrati”; Bonfiglio, L., Ed.; University of Messina: Messina, Italy, 2000; pp. 19–20. [Google Scholar]
- Abbazzi, L.; Arca, M.; Tuveri, C.; Rook, L. The endemic canid Cynotherium (Mammalia: Carnivora) from the Pleistocene depo sits of Monte Tutta vista (Nuoro, Eastern Sardinia). Riv. Ital. Paleontol. Stratigr. 2005, 111, 493–507. [Google Scholar]
- Lyras, G.A.; van der Geer, A.A.E. External brain anatomy in relation to the phylogeny of Caninae (Carnivora: Canidae). Zool. J. Linn. Soc. 2003, 138, 505–522. [Google Scholar] [CrossRef]
- Lyras, G.A.; van der Geer, A.A.E.; Dermitzakis, M.; De Vos, J. Cynotherium sardous, an insular canid (Mammalia: Carnivora) from the Pleistocene of Sardinia (Italy), and its origin. J. Vertebr. Paleontol. 2006, 26, 735–745. [Google Scholar] [CrossRef]
- Palombo, M.R. Biochronology, paleobiogeography and faunal turnover in western Mediterranean Cenozoic mammals. Integr. Zool. 2009, 4, 367–386. [Google Scholar] [CrossRef]
- Melis, R.T.; Ghaleb, B.; Boldrini, R.; Palombo, M.R. The Grotta dei Fiori (Sardinia, Italy) stratigraphical successions: A key for inferring palaeoenvironment evolution and updating the biochronology of the Pleistocene mammalian fauna from Sardinia. Quat. Int. 2013, 288, 81–96. [Google Scholar] [CrossRef]
- Madurell-Malapeira, J.; Palombo, M.R.; Sotnikova, M. Cynotherium malatestai, sp. nov. (Carnivora, Canidae), from the early Middle Pleistocene deposits of Grotta dei Fiori (Sardinia, western Mediterranean). J. Vertebr. Paleontol. 2015, 35, e943400. [Google Scholar] [CrossRef]
- Ciucani, M.M.; Jensen, J.K.; Sinding, M.H.S.; Smith, O.; Lucenti, S.B.; Rosengren, E.; Rook, L.; Tuveri, C.; Arca, M.; Capellini, E.; et al. Evolutionary history of the extinct Sardinian dhole. Curr. Biol. 2021, 31, 5571–5579. [Google Scholar] [CrossRef]
- Klein Hofmeijer, G. Late Pleistocene deer fossils from Corbeddu cave. Implications for human colonization of the island of Sardinia. Ph.D. Thesis, University Utrecht, Utrecht, The Netherlands, 1996. [Google Scholar]
- Van Valkenburgh, B. Interative evolution of hypercanivory in Canids (Mammalia: Carnivora): Evolutionary interactions among sympatric predators. Paleobiology 1991, 17, 340–362. [Google Scholar] [CrossRef]
- Bonifay, M.F. Les carnivores de la Grotte de Macinaggio (Haute-Corse). Bull. Société Sci. Hist. Nat. Corse 1994, 668, 97–113. [Google Scholar]
- Novelli, M.; Palombo, M.R. Hunter Schreger Bands in Cynotherium sardous Studiati 1857 from Dragonara cave (Late Pleistocene, North-Western Sardinia). In Proceedings of the Giornate di Paleontologia 2005, Urbino, Italy, 20–22 May 2005; Coccioni, R., Marsili, A., Eds.; Grzybowski Foundation Special Publication: Krakow, Poland, 2007; Volume 12, pp. 61–71. [Google Scholar]
- Novelli, M.; Palombo, M.R.; Arca, M.; Tuveri, C. Hunter Schreger Bands in Cynotherium sardous Studiati 1857, from Monte Tuttavista (Orosei, Sardinia). Geol. Romana 2008, 41, 65–70. [Google Scholar]
- Palombo, M.R. Mandibular force profiles and implications for the feeding behaviour of the endemic dog Cynotherium. In Proceedings of the XIV Annual Meeting of the European Association of Vertebrate Palaeontologists, EAVP, Haarlem, The Netherlands, 6–10 July 2016; p. 145. [Google Scholar]
- Palombo, M.R.; Antonioli, F.; Lo Presti, V.; Mannino, M.A.; Melis, R.T.; Orru, P.; Stocchi, P.; Talamo, S.; Quarta, G.; Calcagnile, L.; et al. The late Pleistocene to Holocene palaeogeographic evolution of the Porto Conte area: Clues for a better understanding of human colonization of Sardinia and faunal dynamics during the last 30 ka. Quat. Int. 2017, 439, 117–140. [Google Scholar] [CrossRef]
- Manoussaki, D.; Dimitriadis, E.K.; Chadwick, R.S. Cochleas graded curvature effect on low frequency waves. Phys. Rev. Lett. 2006, 96, 088701. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, A.V.; Lebrun, R.; Wilson, L.A.B.; Costeur, L.; Schmelzle, T.; Sánchez-Villagra, M.R. Size Variation under Domestication: Conservatism in the inner ear shape of wolves, dogs and dingoes. Sci. Rep. 2017, 7, 13330. [Google Scholar] [CrossRef] [PubMed]
- Manoussaki, D.; Chadwick, R.S.; Ketten, D.R.; Arruda, J.; Dimitriadis, E.K.; O’Malley, J.T. The influence of cochlear shape on low-frequency hearing. Proc. Natl. Acad. Sci. USA 2008, 105, 6162–6166. [Google Scholar] [CrossRef]
- Le Maître, A.; Grunstra, N.D.S.; Pfaff, C.; Mitteroecker, P. Evolution of the mammalian ear: An evolvability hypothesis. Evol. Biol. 2020, 47, 187–192. [Google Scholar] [CrossRef]
- Ekdale, E.G. Comparative anatomy of the bony labyrinth (inner ear) of placental mammals. PLoS ONE 2013, 8, e66624. [Google Scholar]
- Edge, R.M.; Evans, B.N.; Pearce, C.P.; Ruchter, C.P.; Hu, X.; Dallos, P. Morphology of the unfixed cochlea. Hear. Res. 1998, 124, 1–16. [Google Scholar] [CrossRef]
- Thorne, M.; Salt, A.N.; De Mott, J.E.; Henson, M.M.; Henson, O.W.; Gewalt, S.L., Jr. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope 1999, 9, 1661–1668. [Google Scholar] [CrossRef]
- Coleman, M.N.; Colbert, M. Correlations between auditory structures and hearing sensitivity in nonhuman primates. J. Morphol. 2010, 27, 511–532. [Google Scholar]
- Coleman, M.N.; Kay, R.F.; Colbert, M.W. Auditory morphology and hearing sensitivity in fossil new world monkeys. Anat. Rec. 2010, 293, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Braga, J.; Loubes, J.-M.; Descouens, D.; Thackeray, J.F.; Dumoncel, J.; Kahn, J.-L.; de Beer, F.; Riberon, A.; Hoffman, K.; Balaresque, P.; et al. Disproportionate cochlear length in genus Homo shows a high phylogenetic signal during apes’ hearing evolution. PLoS ONE 2015, 10, e0127780. [Google Scholar] [CrossRef] [PubMed]
- Ekdale, E.G. Form and function of the mammalian inner ear. J. Anat. 2016, 228, 324–337. [Google Scholar] [CrossRef]
- Coleman, M.N.; Boyer, D.M. The inner ear evolution in primates through the Cenozoic: Implications for the evolution of hearing. Anat. Rec. 2012, 295, 615–631. [Google Scholar] [CrossRef] [PubMed]
- West, C.D. The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J. Acoust. Soc. Am. 1985, 77, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, G. Hearing in extinct cetaceans as determined by cochlear structure. J. Paleontol. 1976, 50, 133–152. [Google Scholar]
- Geisler, J.H.; Luo, Z.-X. The petrosal and inner ear of Herpetocetus sp. (Mammalia: Cetacea) and their implications for the phylogeny and hearing of archaic mysticetes. J. Paleontol. 1996, 70, 1045–1066. [Google Scholar] [CrossRef]
- Court, N. Cochlea anatomy of Numidotherium koholense: Auditory acuity in the oldest known proboscidean. Lethaia 1992, 25, 211–215. [Google Scholar] [CrossRef]
- Luo, Z.-X.; Eastman, E.R. Petrosal and inner ear of a squalodontoid whale: Implications for evolution of hearing in odontocetes. J. Vertebr. Paleontol. 1995, 15, 431–442. [Google Scholar] [CrossRef]
- Luo, Z.-X.; Marsh, K.K. Petrosal (periotic) and inner ear of a Pliocene kogiine whale (Kogiinae, Odontoceti): Implications on relationships and hearing evolution of toothed whales. J. Vertebr. Paleontol. 1996, 16, 328–348. [Google Scholar] [CrossRef]
- Janssens, L.A.; Gunz, P.; Stenger, T.E.; Fischer, M.S.; Boone, M.; Stoessel, A. Bony labyrinth shape differs distinctively between modern wolves and dogs. Zoomorphology 2019, 138, 409–417. [Google Scholar] [CrossRef]
- Mellet, J.S. Paleobiology of North American Hyaenodon (Mammalia, Creodonta). In Contributions to Vertebrate Evolution; Hecht, M.K., Szalay, F.S., Eds.; Karger Press: Basel, Switzerland, 1977; Volume 1, pp. 1–133. [Google Scholar]
- Echteler, S.M.; Fay, R.R.; Popper, A.N. Comparative Hearing: Mammals; Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 1994; pp. 134–171. [Google Scholar]
- Barone, R. Anatomie Comparée Des Mammifaires Domestiques. Tome 7, Neurologie II; Vigot: Paris, France, 2010. [Google Scholar]
- Benichoux, V.; Rébillat, M.; Brette, R. On the variation of interaural time differences with frequency. J. Acoust. Soc. Am. 2016, 139, 1810. [Google Scholar] [CrossRef] [PubMed]
- Lister, A.M. Rapid dwarfing of red deer on Jersey in the last interglacial. Nature 1989, 30, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rozzi, R.; Lomolino, M.V. Rapid Dwarfing of an Insular Mammal—The Feral Cattle of Amsterdam Island. Sci. Rep. 2017, 18, 8820. [Google Scholar] [CrossRef]
- Foster, J.B. The evolution of mammals on islands. Nature 1964, 202, 234–235. [Google Scholar] [CrossRef]
- Van Valen, L. A new evolutionary law. Evol. Theory 1973, 1, 1–33. [Google Scholar]
- De Vos, J. The endemic Pleistocene deer of Crete. In Verhandelingen der Koninklijke Nederlandse Akademie van Wetenschappen, afd. Natuurkunde. Eerste Reeks 31; North Holland Publishing Company: Amsterdam, The Netherlands; Oxford, UK; New York, NY, USA, 1984; 100p. [Google Scholar]
- Palombo, M.R.; Köhler, M.; Solà, S.M.; Giovinazzo, C. Brain versus body mass in endemic ruminant artiodactyls: A case studied of Myotragus balearicus and smallest Candiacervus species from Mediterranean Islands. Quat. Int. 2008, 182, 160–183. [Google Scholar] [CrossRef]
- Jianu, C.M.; Weishampel, D.B. The smallest of the largest: A new look at possible dwarfing in sauropod dinosaurs. Geol. Mijnb. 1999, 78, 335–343. [Google Scholar] [CrossRef]
- Stein, K.; Csiki, Z.; Curry Rogers, K.; Weishampel, D.B.; Redelstorff, R.; Jose, L.; Carballido, J.L.; Sandera, P.M. Small body size and extreme cortical bone remodeling indicate phyletic dwarfism in Magyarosaurus dacus (Sauropoda: Titanosauria). Proc. Natl. Acad. Sci. USA 2010, 107, 9258–9263. [Google Scholar] [CrossRef]
- Csiki-Sava, Z.; Vremir, M.; Meng, J.; Brusatte, S.L.; Norell, M.A. Dome-headed, small-brained island mammal from the Late Cretaceous of Romania. Proc. Natl. Acad. Sci. USA 2018, 115, 4857–4862. [Google Scholar] [CrossRef]
- Falk, D.; Hildebolt, C.; Smith, K.; Morwood, M.J.; Sutikna, T.; Brown, P.; Saptomo, J.E.W.; Brunsden, B.; Prior, F. The brain of LB1, Homo floresiensis. Science 2005, 308, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Moyà-Solà, S. Reduction of brain and sense organs in the fossil insular bovid Myotragus. Brain Behav. Evol. 2004, 63, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Weston, E.M.; Lister, A.M. Insular dwarfism in hippos and a model for brain size reduction in Homo floresiensis. Nature 2009, 459, 85–88. [Google Scholar] [CrossRef]
- Lyras, G.A.; Giannakopoulou, A.; Lillis, T.; van der Geer, A.A.E. Paradise lost: Evidence for a devastating metabolic bone disease in an insular Pleistocene deer. Int. J. Paleopathol. 2019, 24, 213–226. [Google Scholar] [CrossRef]
- Palombo, M.R.; Giovinazzo, C. Elephas falconeri from Spinagallo Cave (South-Eastern Sicily, Hyblean Plateau, Siracusa): A preliminary report on brain to body weight comparison. In Proceedings of the International Symposium Insular Vertebrate Evolution: The Palaeontological Approach, 12; Alcover, J.A., Bover, P., Eds.; Monografies de la Societat d’Historia Natural de les Balears (SHNB): Palma de Mallorca, Spain, 2005; pp. 255–264. [Google Scholar]
- Angelelli, F. Adaptive and evolutionary characters inferred from an endocranial cast of Nesolutria trinacriae Burgio & Fiore, an endemic otter of the Sicilian middle-upper Pleistocene. Atti Acc. Peloritana dei Pericolanti, Classe I di Scienze Fis. Mat. E Nat. 1991, 67, 279–295. [Google Scholar]
- Angelelli, F. Peculiar sensorimotor characters inferred from an endocranial cast of Nesolutra ichnusae Malatesta, an endemic Pleistocenic otter from Sardinia. Geol. Romana 1995, 31, 15–20. [Google Scholar]
- Palombo, M.R.; Giovinazzo, C. Brain Weight Versus Body Mass in Late Pleistocene Cynotherium Sardous Studiati, 1847 from Dragonara Cave (North-Western Sardinia); Giornate di Paleontologia: Bolzano, Italy, 2004; Volume 44. [Google Scholar]
- Dawson, M. Osteology of Prolagus sardus, a Quaternary ochotonid (Mammalia, Lagomorpha). Palaeovertebrata 1969, 2, 157–191. [Google Scholar] [CrossRef]
- Volodin, I.A.; Matrosova, V.A.; Frey, R.; Julia, D.; Kozhevnikova, J.D.; Isaeva, I.L.; Volodina, E.V. Altai pika (Ochotona alpina) alarm calls: Individual acoustic variation and the phenomenon of call-synchronous ear folding behavior. Sci. Nat. 2018, 105, 40. [Google Scholar] [CrossRef]
- Smith, A.T. World of pikas. In Lagomorph Biology Evolution, Ecology, and Conservation; Alves, P.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 89–102. [Google Scholar]
- Frey, R.; Volodin, I.A.; Fritsch, G.; Volodina, E.V. Potential Sources of High Frequency and Biphonic Vocalization in the Dhole (Cuon alpinus). PLoS ONE 2016, 11, e0146330. [Google Scholar] [CrossRef]

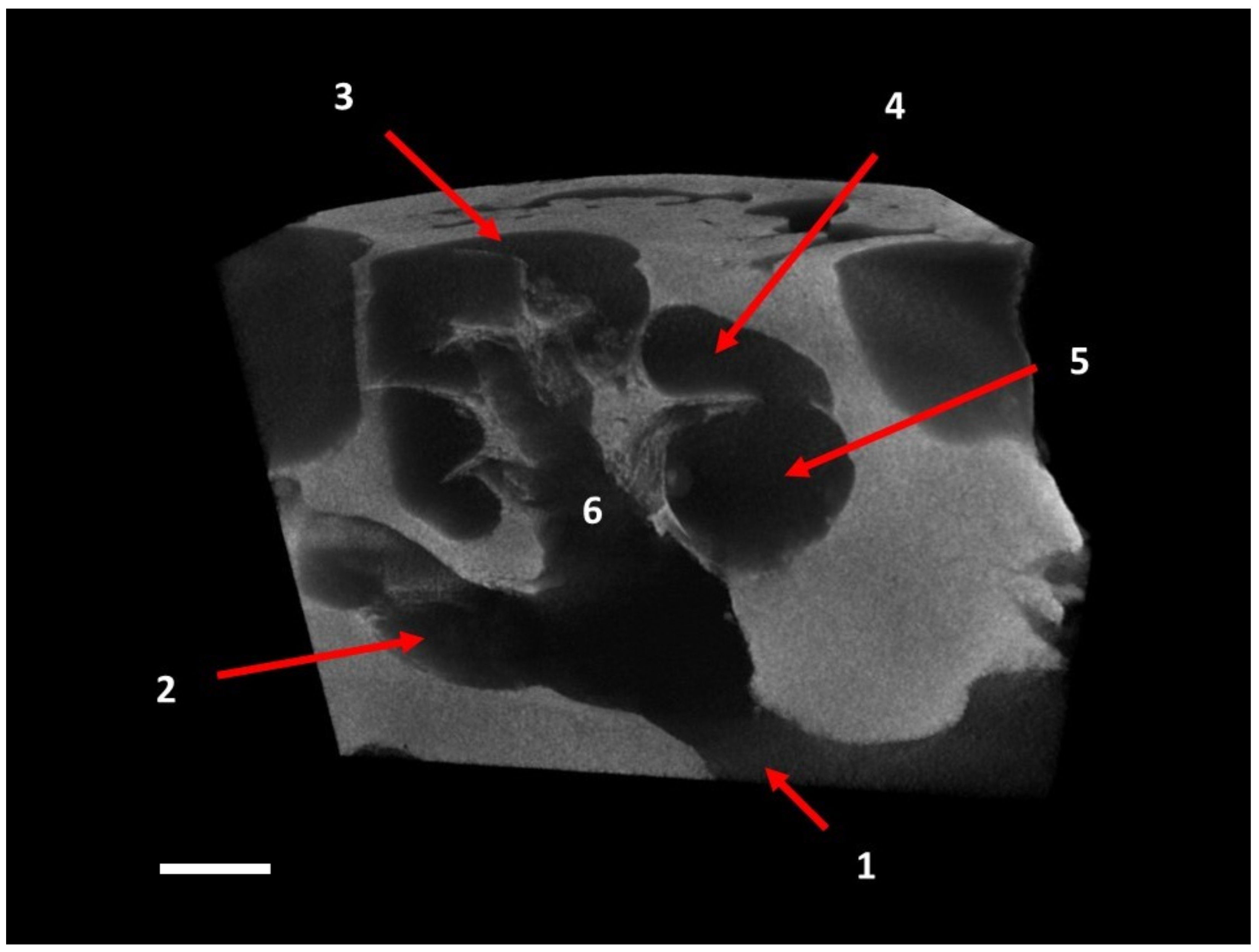

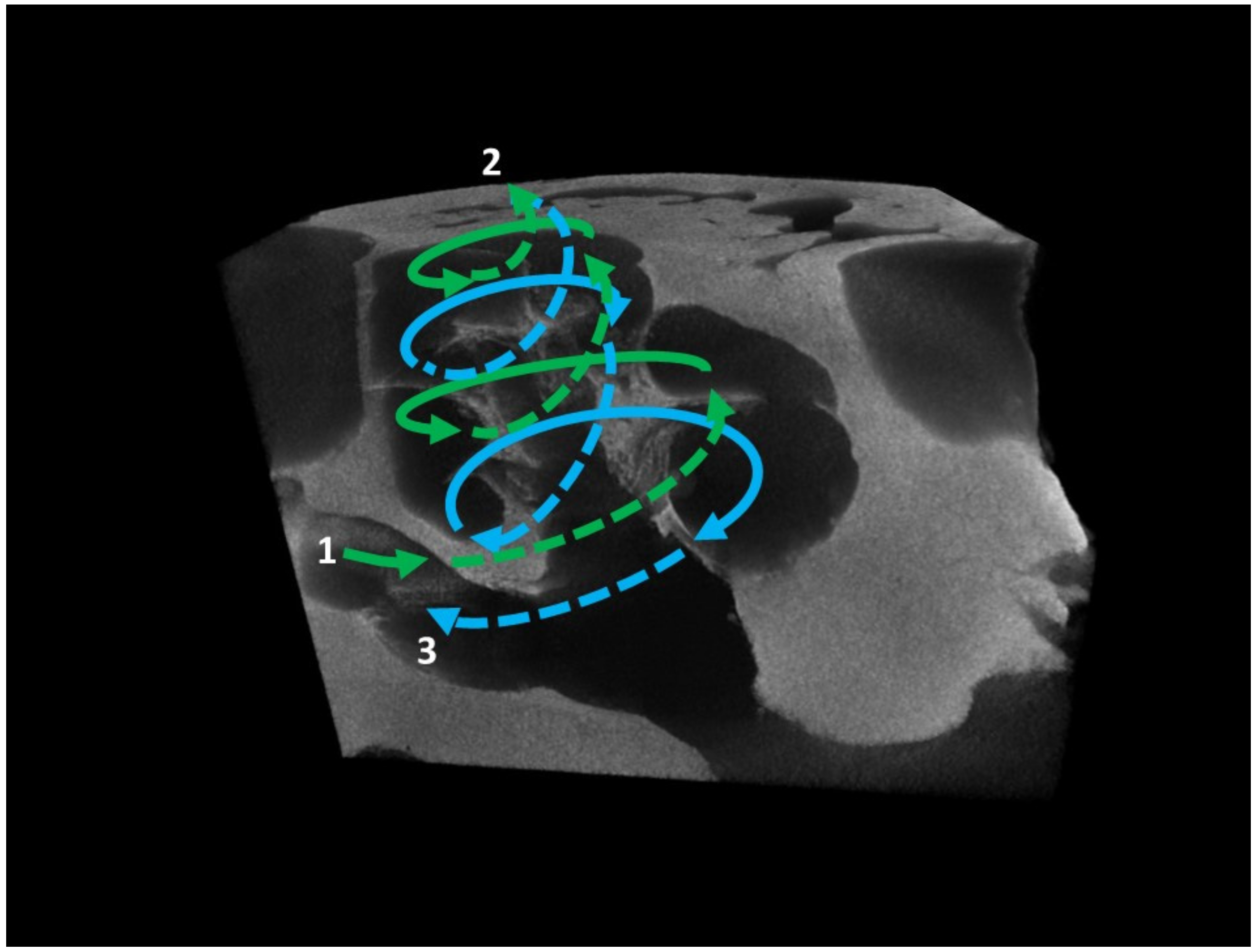
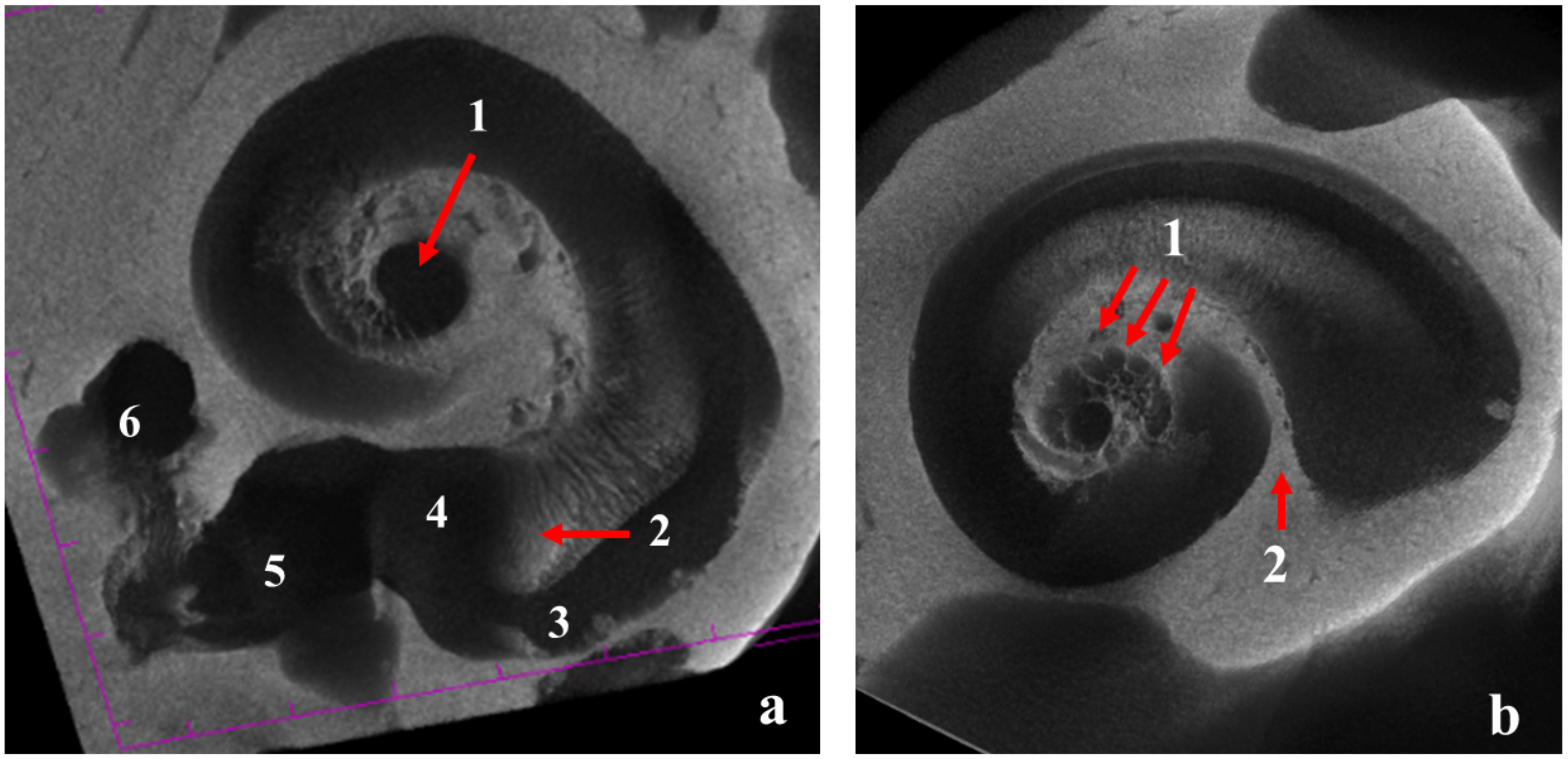
| Taxa | Cow | Coh | Con | Col | Rbasal | Rapex | ρ | Scw | Bmw |
|---|---|---|---|---|---|---|---|---|---|
| Cynotherium sardous | 4.7 | 4.1 | 2.25 | 25.8 | 2.35 | 0.7 | 3.35 | 1 turn 1.6 2 turn 0.8 3 turn 0.3 | 0.4 |
| other Carnivora (mean values) | 7.1 * | 3.71 * | 3.25 ** | 34.7 * | 3.55 * | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedda, M.; Brunetti, A.; Palombo, M.R. First Attempt to Infer Sound Hearing and Its Paleoenvironmental Implications in the Extinct Insular Canid Cynotherium sardous Studiati, 1857 (Sardinia, Italy). Animals 2022, 12, 833. https://doi.org/10.3390/ani12070833
Zedda M, Brunetti A, Palombo MR. First Attempt to Infer Sound Hearing and Its Paleoenvironmental Implications in the Extinct Insular Canid Cynotherium sardous Studiati, 1857 (Sardinia, Italy). Animals. 2022; 12(7):833. https://doi.org/10.3390/ani12070833
Chicago/Turabian StyleZedda, Marco, Antonio Brunetti, and Maria Rita Palombo. 2022. "First Attempt to Infer Sound Hearing and Its Paleoenvironmental Implications in the Extinct Insular Canid Cynotherium sardous Studiati, 1857 (Sardinia, Italy)" Animals 12, no. 7: 833. https://doi.org/10.3390/ani12070833
APA StyleZedda, M., Brunetti, A., & Palombo, M. R. (2022). First Attempt to Infer Sound Hearing and Its Paleoenvironmental Implications in the Extinct Insular Canid Cynotherium sardous Studiati, 1857 (Sardinia, Italy). Animals, 12(7), 833. https://doi.org/10.3390/ani12070833







