Simple Summary
This paper describes a new species, Demodex pusillus, inhabiting the hairy skin of Nyctalus noctula, which is one of the smallest arthropods. New data on the coexistence of skin mites from the sister families Demodecidae and Psorergatidae in bats are also included, as well as an updated global checklist and data on their occurrence, including location (topography) within the hosts.
Abstract
The bat skin mites from the closely-related Demodecidae and Psorergatidae families occur synhospitally, populating the same host species and perhaps neighboring microhabitats. However, data on their occurrence and parasitism are fragmentary and dispersed. Thus far, 27 Demodecidae and 18 Psorergatidae species have been described, but the coexistence of mites from both families was only demonstrated in six species of bats. This article presents a description of Demodex pusillus sp. nov. from Nyctalus noctula, including a new host record (first observation of demodecid mites in Nyctalus) and a new record concerning the occurrence of Psorergatoides kerivoluae in Plecotus auritus. It also includes an updated global checklist of the occurrence of Demodecidae and Psorergatidae in Chiroptera, including data on their records/distribution and location in their hosts. In both studied families, the mites exhibit preferences, and even topographic specificity, colonizing different microhabitats in the host, including the eye region (e.g., Meibomian glands of the eyes, corneal surface and eyelid vault), wing membranes and hairy skin on the body. Such colonization of separate microhabitats enables different species to co-occur within the same host, while the total number of parasites determines the level of parasite load, with higher levels being associated with the incidence of disease symptoms. It is worth mentioning that Demodex pusillus sp. nov. is the smallest known representative of the Demodecidae family and one of the smallest animals (70–80 micrometers in length).
1. Introduction
Over 20 mite families are associated with bats. Within this group is a separate ecological group comprising the skin mites, which are stationary parasites whose entire life cycle takes place within the host body [1,2]. This subgroup includes the closely-related sister families Demodecidae and Psorergatidae, whose members are mostly characterized by high host specificity, being monoxenic or oligoxenic parasites [3,4,5]. It is probable that representatives of these families can occur synhospitally, i.e., colonizing different habitats in the same hosts. However, data on their occurrence and parasitism are fragmentary and dispersed. Thus far, they have been observed in relatively few bats from different parts of the world, and only six bat species have been found to have representatives of both mite families [4,5]. However, these relationships have never been analyzed at individual level and it remains unknown whether mites from either family can occur concomitantly, or if they compete for the same, or similar, microhabitats, and the presence of one group excludes colonization by representatives of the other.
No such competition has been observed for the Demodecidae, where individual species inhabit distinct microhabitats within the host at the same time. Six species of bat have been found to harbor one or more Demodecidae species [5], and such co-occurrence has also been noted at the individual level, e.g., individual bats were found to demonstrate both Demodex chiropteralis (hairy skin of the body, head) and D. plecoti (wing membranes) [6]. Similar examples of co-occurrence have also been observed for D. mystacina and D. novazelandica, inhabiting adjacent microhabitats within the eyelids of Mystacina tuberculata, and for D. neopisthosomae and D. spelaea, which both inhabit the Meibomian glands of Eonycteris spelaea eyelids [7,8].
The lack of data on the co-occurrence of Demodecidae and Psorergatidae is related to the methodological difficulties in their research, more precisely, their minuscule size, identification issues and secretive lifestyle. While these mites are more easily detected when their presence elicits disease symptoms, this is a rare phenomenon, and they typically occur asymptomatically [9]. Analyses of asymptomatic cases are highly labor-intensive and they only include select locations or part of the body surface (cuttings/skin fragments), low number of hosts (difficult to obtain) and typically mites from one group/family.
The present study analyzed the occurrence of skin mites from both families in the common noctule Nyctalus noctula, as well as brown long-eared bat Plecotus auritus. All studied bats had previously been confirmed to demonstrate the asymptomatic presence of Demodecidae or Psorergatidae. The study presents new data on the occurrence of these mites, including the discovery of a new species described as Demodex pusillus sp. nov.
2. Material and Methods
2.1. Detection of Skin Mites in Bats
Six specimens of dead Nyctalus noctula (Poland, Pomerania, Redzikowo near Słupsk, 54°28′21.48″ N/17°06′13.27″ E) collected from November 2007 and six specimens of dead Plecotus auritus (Poland, Pomeranian Voivodeship, Gdańsk, 54°25′32″ N/18°29′29″ E, 1 bat; Gdynia, 54°31′57″ N/18°27′11″ E, 54°29′01″ N/18°32′27″ E, 2 bats; Skrzeszewo, 54°17′53.38″ N/18°20′24.02″ E, 2 bats; Zbysław, 54°14′43.79″ N/17°28′31.64″ E, 1 bat), collected from February 2012–August 2018, were examined for Demodecidae and Psorergatidae mites. All N. noctula drowned during rain after falling into the gutter on residential block and were found a day later, while P. auritus were found during routine winter bat census in the underground roosts and thus, probably died during hibernation.
The skin mites were isolated using skin digestion methods [10], with modifications to suit the examined host. For analyzing the topography (microhabitats) of mites, skin fragments of 1 cm2 were examined from several body regions, including the head (around the eyes, ear pinnae, nose, lips, chin, cheeks, vertex), neck, abdomen, back, wing membranes, limbs and genital–anal area. Skin samples were preserved in 70% ethanol and digested in 10% KOH solution. The obtained samples were decanted (the examination of 1 cm2 of skin was equal to that of approximately 100 wet preparations) and examined using phase-contrast microscopy (Nikon Eclipse 50i, Nikon Corporation, Tokyo, Japan). Mites were placed in polyvinyl–lactophenol solution and subjected to morphometric examination. All measurements are in micrometers and were obtained as follows: total body length = length of gnathosoma, podosoma and opisthosoma; gnathosomal width = width at base; podosomal and opisthosomal width = maximum width.
Specimen depositories are cited using the abbreviation UGDIZP, University of Gdańsk, Department of Invertebrate Zoology and Parasitology, Gdańsk, Poland [11].
The description of the species adopted the nomenclature commonly used for the family Demodecidae [12] and was completed with the nomenclature proposed by Bochkov [3] for the superfamily Cheyletoidea, and by Izdebska and Rolbiecki [13].
The prevalences were calculated to determine the level of host infection [14].
2.2. The Checklist Structure
The checklist was drawn up based on manuscripts published during the period 1859–2019. It also contains own unpublished data, marked in the table as the present study. Demodecidae and Psorergatidae species have been arranged in systematic order, and in alphabetical order within the genera. The list includes all formally described species and information on dates of host species, as well as the occurrence have been included.
The scientific and common names of the hosts follow Wilson and Reeder [15] and the Integrated Taxonomic Information System [16].
3. Results
3.1. Descriptions
Demodex pusillus Izdebska, Cierocka, Rolbiecki et Ciechanowski, 2022
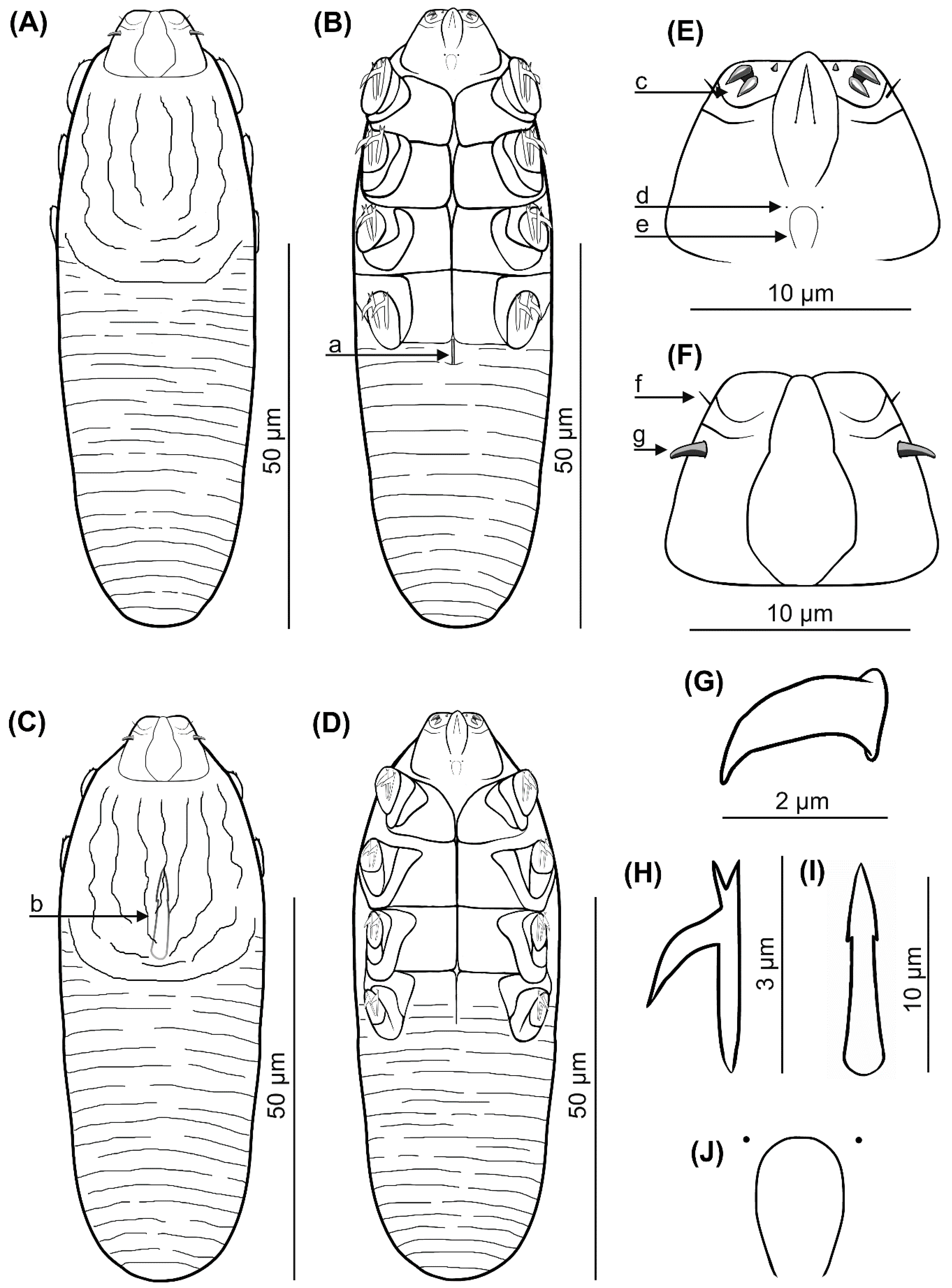
Female (n = 1 holotype and 27 paratypes): The female body is stocky, cylindrical with short gnathosoma and podosoma similar in length and width to opisthosoma, 80 (70–93) long and 25 (23–30) wide (holotype, 78 × 24) (Table 1, Figure 1 and Figure 2). Gnathosoma trapezoidal are shorter than the base width. On the dorsal side at the external edge, a pair of hook-shaped supracoxal spines (setae elc.p) present, ca. 2.0 long (holotype, 2.0) and are directed outwardly. Palps 3-segmented terminate in three spines (two larger, curved and one small, conical) on the tibio–tarsus; also, setae v”F on the middle segment (trochanter–femur–genu) present. On the ventral part of gnathosoma, horseshoe-shaped pharyngeal bulb, with a pair of small subgnathosomal setae (setae n) are situated anterior on both sides. The podosoma rectangular. Four pairs of short legs, with coxa integrated into the ventral idiosomal wall and five free, overlapping segments (trochanter-tarsus); two forked claws, ca. 3.0 long (holotype, 3.0), with large, hooked spur on each tarsus. Epimeral plates (coxal fields) are trapezoidal and sclerotized; all epimeral plates connect medially; posterior edges of pair IV form a triangular incision. On the dorsal side of the podosoma, a podosomal shield is present, reaching the anterior level of legs III. The opisthosoma oval constitutes 45 (41–49) of body length (holotype, 45). Whole opisthosoma is distinctly annulated; annuli relatively wide ca. 1.0–1.5. The opisthosomal organ is absent. The vulva 4 (3–5) long (holotype, 3.0) is located in incision of IV epimeral plate.

Table 1.
Body size (mean, range and SD, in μm) for adults of Demodex pusillus sp. nov.
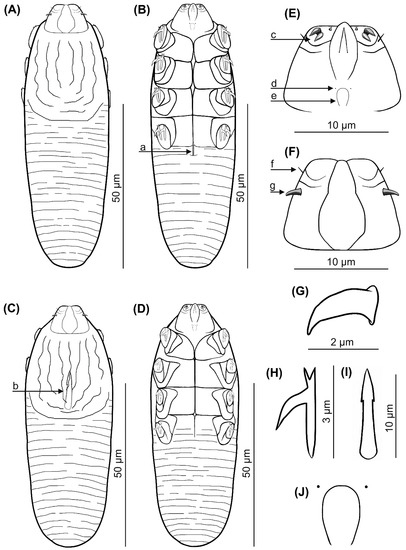
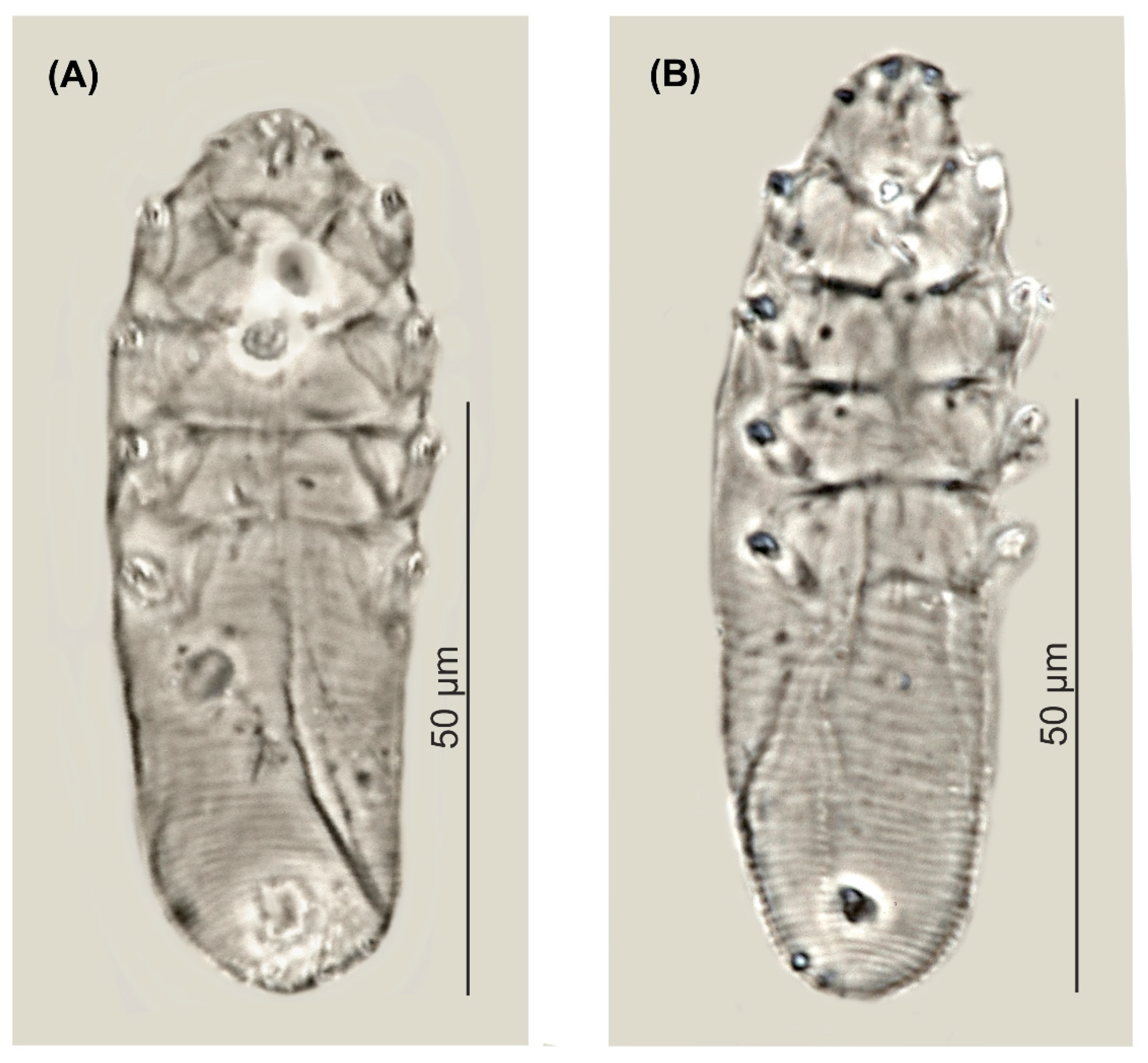
Figure 1.
Demodex pusillus sp. nov.: female, dorsal view (A), female, ventral view (B), male, dorsal view (C), male, ventral view (D), gnathosoma, female, ventral view (E), gnathosoma, female, dorsal view (F), supracoxal spine, lateral view (G), claw on the leg (H), aedeagus (I); pharyngeal bulbs with subgnathosomal setae (J); a: vulva, b: aedeagus, c: spines on palps, d: subgnathosomal seta (seta n), e: pharyngeal bulb, f: seta v”F, g: supracoxal spine (seta elc.p).
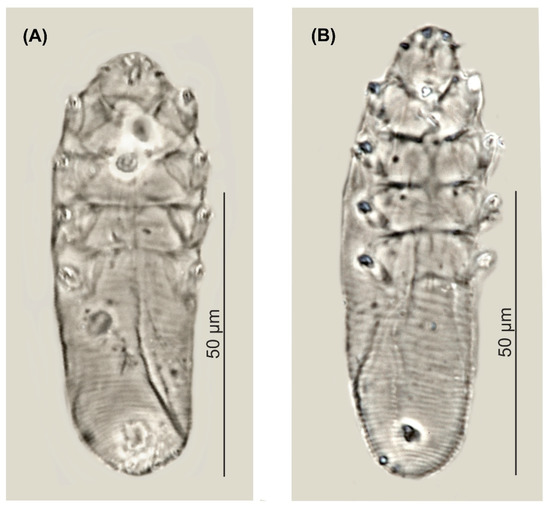
Figure 2.
Demodex pusillus sp. nov.: male (A) and female, holotype (B).
Male (11 paratypes): On average, males are slightly smaller than the females, 75 (68–83) long and 24 (23–28) wide. Gnathosoma trapezoidal are shorter than the base width. Pharyngeal bulb and morphological details of gnathosoma are similar to those in females. The shape of podosoma and legs are similar to those in the females, but the posterior edge of epimeral plate IV is without a triangular incision. A podosomal shield is present, also reaching the anterior level of legs III. The opisthosoma constitutes 47 (43–56) of body length, is oval, clearly annulated and with relatively wide annuli, ca. 1.0–1.5. The opisthosomal organ is absent. The aedeagus 11 (9–13) long, on the dorsal side, located between epimeral plates II and III. The genital opening is located on the dorsal surface, at the level of anterior edge of the epimeral plate II.
Type of material: The female holotype (reg. no. UGDIZPVNnDDp01f) was from Nyctalus noctula (reg. no. MCVNn01/2007-06/2007), Redzikowo near Słupsk, Pomeranian Voivodeship, Poland, November 2007, parasites coll. K. Cierocka, J.N. Izdebska, L. Rolbiecki; host coll. M. Ciechanowski; 27 female paratypes (reg. nos. UGDIZPVNnDDp02–28f) and 11 male paratypes (reg. nos. UGDIZPVNnDDp01–11m) were from Nyctalus noctula (reg. nos. MCVNn01/2007-06/2007), Redzikowo near Słupsk, Pomeranian Voivodeship, Poland, November 2007; the collectors are the same.
Type of material deposition: The whole-type material (mounted microscope slides with the demodecid mites) was deposited in the scientific collections within the framework of the Collection of Extant Invertebrates in Department of Invertebrate Zoology and Parasitology, University of Gdańsk, Poland (UGDIZP).
Infection and location in the host: Demodex pusillus sp. nov. was found in all examined common noctule (100%); 39 specimens in total were found (11 males, 28 females). The demodecid mites were found on the hairy skin of the body (head—6 individuals, abdomen—6, back—27). The observed mites did not cause any lesions in examined common noctule.
Etymology: The specific epithet pusillus refers to the small size of this demodecid mite.
3.2. Differential Diagnosis
Among the known Demodecidae, D. pusillus sp. nov. is close to D. plecoti, and described from another European representative of the Vespertilionidae, namely the brown long-eared bat (Table 1 and Table 2). However, D. pusillus sp. nov. is smaller, has different body proportions (D. pusillus sp. nov. is cylindrical, while D. plecoti – fusiform) and does not exhibit sexual dysmorphism typical of D. plecoti; in D. pusillus sp. nov., males are typically slightly smaller than females; in D. plecoti, males are clearly smaller than females and their epimeral plates are shaped and arranged differently to females. The gnathosoma of D. pusillus sp. nov. is trapezoid shaped and oval in D. plecoti. Supracoxal spines in both species are conical, hook-shaped/curved, but in D. pusillus sp. nov. they are located in the anterior part of the coxal (basal) palp segment, near the edge and directed outwards, while in D. plecoti, they are located in the middle of the coxal palp segment and directed downwards, to the inside (posteromedially). The terminal segment of the palpi has three spines in D. pusillus sp. nov. (two larger, curved, one small, conical), and two large spines in D. plecoti (one bifurcated and other simple, conical). Subgnathosomal setae in D. pusillus sp. nov. are situated at the level of the anterior edge of the pharyngeal bulb, but are situated lower in D. plecoti. In both sexes, all epimeral plates connect medially in D. pusillus sp. nov., but are separated in D. plecoti (pairs I and IV of epimeral plates partly come into contact in females, but only pair I in males). In D. pusillus sp. nov., the tarsi of the legs are equipped with forked claws, with a long spur, while in D. plecoti, the claws are also forked, but lack the spur. The aedeagus of the male D. pusillus sp. nov. is situated at the level of the pair II–III epimeral plates (genital orifice at the level of anterior edge of II epimeral plate), while it is located between plates III and IV in D. plecoti (genital orifice—at the level of border between plates II and III). The vulva is located in an incision between pair IV epimeral plates in the female D. pusillus sp. nov., but below the posterior edge of pair IV in D. plecoti. The typical microhabitat is also different: D. pusillus sp. nov. was found in the hairy skin of the body, and D. plecoti in the wing membranes.

Table 2.
Morphometric comparison between Demodex pusillus sp. nov. and Demodex plecoti.
3.3. A New Record of Psorergatidae
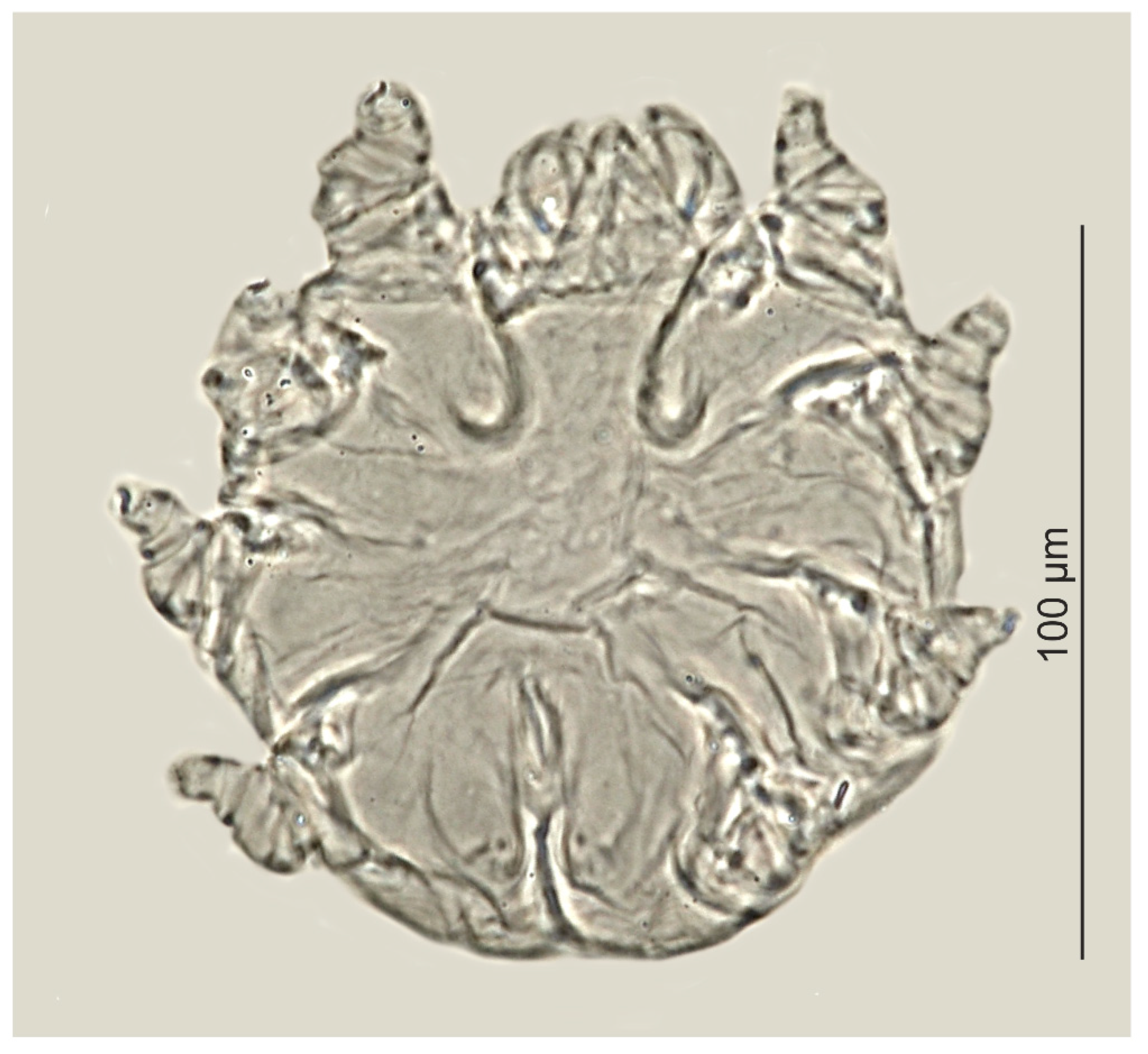
Psorergatoides kerivoluae was found in one out of the six examined brown long-eared bats (Table 3, Figure 3). Overall prevalence was 16.7%, with two individuals of P. kerivoluae (females) being found in the forehead region and in the ear canal. No skin lesions were observed in the infested bat.

Table 3.
Body (mean, range and SD, in μm) size for Psorergatoides kerivoluae.
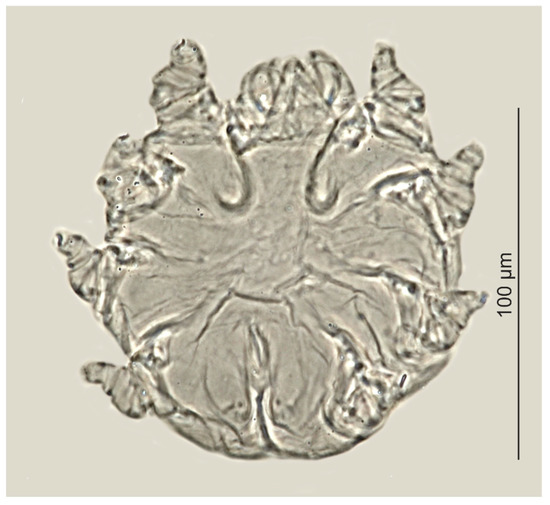
Figure 3.
Psorergatoides kerivoluae, female.
The voucher specimens were deposited in the scientific collections within the UGDIZP scientific collection.
3.4. Biodiversity of Demodecidae and Psorergatidae in Chiroptera
In the 55 studied bat species from 11 families, 45 skin mites from Prostigmata were found, including 28 Demodecidae and 18 Psorergatidae. The highest number (12 species) was found in bats classified from the Vespertilionidae family (Table 4).

Table 4.
A checklist of skin mites in the Demodecidae and Psorergatidae families reported in bats.
3.5. Co-Occurrence of Demodecidae and Psorergatidae
All examined bats were found to have skin mites. Among N. noctula, six individuals were infested with D. pusillus sp. nov.; Psorergatoides nyctali had previously been recorded in two of these individuals (retrospective study, [43]). The infestation level was low (only single individuals were found); no skin lesions caused by the presence of mites could be observed. In turn, out of the six P. auritus examined in the present study, one was found to have P. kerivoluae. Earlier, the same bat individual was found to harbor D. chiropteralis [23] and D. plecoti [6].
Mites from both families have been found in seven bat species. In addition, six bat species featured at least two Demodecidae species, with the highest number found in Carollia perspicillata: four species from three genera. Only one or two Psorergatidae species were observed. Mites from individual species exhibited clear topographic and topical preferences, with a high diversity of microhabitats: the parasites inhabited the head region (eyelids, including Meibomian glands, eye, including the corneal surface, eyelid vault and hairy skin of the head), hairy areas of the body, wing membranes and non-hairy (membranous) skin regions (Table 4).
4. Discussion
Little is known on the co-occurrence of related and ecologically-similar skin mite families from the Demodecidae and Psorergatidae in the same host, as evidenced by the lack of studies in the global literature. Analysis of host records (Table 4) indicates that these mites demonstrated synhospital occurrence in seven chiropteran species, with representatives of both families being present in each individual. In the present study, these findings are supplemented with findings in Nyctalus noctula, which were found to harbor both the previously known Psorergatoides nyctali, and a new species, D. pusillus sp. nov.
In addition, individuals of D. chiropteralis were found next to D. plecoti and P. kerivoluae in Plecotus auritus, confirming that mites from both families can co-occur in the same host. These mites occupied both distant and adjacent microhabitats within their hosts, exhibiting low density in the skin (low infestation intensity). Thus, balanced host–parasite relationships developed, without burdening the host, not causing disease symptoms and thus not manifesting their presence. These mites could hence only be detected by means of a labor-intensive digestion and decanting method, consisting of searching subsequent fragments of the entire skin surface.
Occurrence of host specific (monoxenic) parasitic mites, inhabiting different microhabitats within their hosts, comes as a rule for Demodecidae [5]. Although they most likely demonstrate a common occurrence within host populations, and their geographic distribution corresponds to the distribution of host species, their difficult detection results in their presence being sporadically recorded and described, particularly in wild, rare and protected animals [46]. The majority of demodecid mites species are known solely from individual records [47]. For example, D. chiropteralis, first described from the United Kingdom, was only found for the second time after one hundred years in Poland. In addition, despite a number of studies, only one species from the Psorergatidae, P. nyctali, has been found in N. noctula, known from only two records [42,43]. The present study brings new data on the occurrence of a Demodecidae representative in this bat species, which constitutes a new host record for the genus Nyctalus. The individuals found differ from the known Demodecidae and are described as a new species, D. pusillus sp. nov. The mite is associated with various regions of the hairy skin of the body; as such, it is likely to be the predominant species of this group in the common noctule.
The Demodecidae populate different microhabitats within their hosts, the distance/extensiveness of which determines the possibility for reproduction and spread of the mites. In many mammal species, one Demodecidae species is usually found in greater numbers than others, inhabiting more limited microhabitats. For instance, in the house mouse Mus musculus, seven specific Demodecidae taxa are known, with the most common and numerous being D. musculi, inhabiting the hairy skin of the body, whereas other demodecid mite species are restricted to narrow microhabitats (e.g., vibrissae follicles, ear canals, tongue) and are rarer and less numerous [48]. It is likely that the demodecid mite described in the common noctule in the present study may be the predominant species from this group; however, it does not complete the list of potential future discoveries.
An interesting observation was the record of P. kerivoluae in P. auritus, which was previously described on the basis of individuals obtained from Kerivoula cuprosa and K. lanosa from Congo [17]. Subsequently, P. kerivoluae was recorded from P. auritus in Belgium and Poland. Moreover, it has been recorded in five other vespertilionid bat species: Myotis muricola (Borneo), M. bocagii (Republic of Côte d’Ivoire), M. myotis (Poland), M. mystacinus (Malaysia–questionable host) and M. macropus (Australia) (Table 4). The Psorergatidae are characterized by high host specificity, i.e., they are mono- or oligoxenic. One parasite species is usually noted in typically one host species or in several, closely related hosts (typically of the same genus) [4,49]. Therefore, P. kerivoluae, which thus far has been recorded in bats from three genera (although belonging solely to one family, Vespertilionidae), has a unique, wider range of host specificity compared to the rest of the Psorergatidae. This parasite has been found within the wing membranes, where it sometimes causes skin lesions in the form of several millimeters of white dots, scabs and convex, desquamating cysts, which facilitate its detection [39,40,41]. In such cases, only few individuals have typically been found; however, because they were only obtained in these studies from superficial scrapings, often collected from live individuals, the actual infestation state is difficult to ascertain. The wing membranes [17,36,37,39,42] are also the most commonly recorded location for other Psorergatoides, but these parasites have also been recorded in the pinnaes, on the outer side of ears, in the nasal membrane, on tail and limbs [17,36,39]. An astonishingly vast geographical range of that mite (covering Palaearctic, Afrotropic, Indomalayan and Australasian regions) and partially non-overlapping geographical ranges of the particular host species suggest that P. kerivoluae may, in fact, consist of several taxa, and needs revision.
The vast majority of these observations are related to the occurrence of skin changes. Similar observations have been made for most of the described Demodecidae taxa, whose presence is known to cause nodules, cysts, eyelid swelling or blepharitis, and which have enabled detection of these mites [19,26,28]. However, it should be kept in mind that through evolution, skin mites have adapted to functioning in hosts by creating stable host–parasite relationships with the lightest possible effect on host functioning. As such, parasitoses (demodecosis, psorergatosis) are very rare, and their development is typically determined by reduced immunity or the poor condition of their hosts [5]. Therefore, detection and discovery of these parasite species, their biology and aspects of their parasitism is of a random nature, often based on singular observations.
Bats constitute the second most species-rich order within mammals (after rodents) [50,51], and their characteristic capability for active flight enables a relatively easy spread of their geographic distribution. Their particular species specializes in the utilization of different food (insects, vertebrates, blood, fruits, nectar and pollen), roosts (caves, trees, buildings and other anthropogenic structures) and strategies for survival during harsh seasons (hibernation and seasonal migrations). Even in our material, the two studied species, although both are insectivorous, represent different ecological adaptations. Nyctalus noctula is an open-space aerial hawker and long-distance seasonal migrant, hibernating mostly in hollow trees and parts of buildings above the ground, while P. auritus is a close-space foliage gleaner and sedentary species, hibernating mostly in underground roosts (caves, fortifications and cellars) [52]. It is hence only to be expected that the evolutionary success, ecological diversity and complicated body topography (membranes, ears, tragi and nose-leaves) of this group should be reflected in their equally high diversity of skin parasites, particularly when they occur asymptomatically and do not cause a burden for the host, not exceeding its tolerance threshold in terms of numbers. However, bats constitute an ecologically-separated group, compared to other mammals. The parasite transfer may occur on a significant scale solely within a given roost (between different, co-occurring bat species or genera) or within populations (between individuals of the same species), although bats may switch roosts regularly and change social behavior during their seasonal life cycle (spending time with different individuals during pregnancy and lactation, mating and hibernation). Bats are often present at high population densities within relatively small spaces due to their common roosting and tendency to form large groups in summer (nursery colonies), autumn (mating groups) and winter, even if their population densities in larger, landscape scales are unusually small for such small mammals. The development of social, even altruistic behavior in bats, would better enable skin parasites to colonize new hosts and, for some groups of mites, to become more specialized, especially those associated with bats for a longer period of time [53].
Such skin parasites include several genera found only in bats, such as Pterodex, and Psorergatoides and those known mainly from these mammals (Ophthalmodex, Stomatodex) [4,5]. The systematic diversity of skin mites appears to be greater among bats than for other mammals; however, this is not reflected by the number of species described, and this is undoubtedly a result of the generally poorer research status of skin mites in these hosts. Interestingly, the majority of the data come from Africa, South America and Asia, where local research on bats has typically addressed the acarofauna. In contrast, only a handful of studies have been devoted to the occurrence of these parasites in bats from Europe (Table 4). Despite the high interest in chiropterology, only six studies published in the 21st century have contained original data on skin mites in bats [6,23,25,41,42,43]. The explanation of that pattern may lie in the conservation status of Chiroptera that are not only legally protected but considered charismatic taxa, thus the majority of recent studies do not include deliberate collection of any specimen. Most material of arthropods parasiting on bats is, therefore, restricted to taxa collected from the body surface of living, captured-and-released individuals (Diptera: Nycteribiidae, Streblidae; Siphonaptera, Heteroptera, Acari: Spinturnicidae, Macronyssidae, Trombiculidae), while those living inside the integument (Demodecidae and Psorergatidae) are collected almost exclusively from randomly found dead individuals.
5. Conclusions
Considering the state of research on the occurrence of skin mites from Demodecidae and Psorergatidae families in other mammal orders, it is highly likely that the true number of these parasites in bats is much greater, and that their host circle among Chiroptera is more extensive. Only the recognition of the species diversity of these mites in bats will allow for a more complete analysis of the parasite–host systems and clarification of the issue of coexistence.
Author Contributions
Conceptualization, J.N.I., K.C., L.R. and M.C.; sampling, K.C., J.N.I., L.R. and M.C.; data analysis, K.C., J.N.I., L.R. and M.C.; writing—original draft preparation, J.N.I., K.C., L.R. and M.C.; writing, review and editing, K.C., J.N.I., L.R. and M.C; supervision, J.N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the use of only deceased animals.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boczek, J.; Błaszak, C. Roztocze (Acari): Znaczenie w Życiu i Gospodarce Człowieka; Wydawnictwo SGGW: Warsaw, Poland, 2005. [Google Scholar]
- Izdebska, J.N.; Krawczyk, M. Skin mites of mammals—The occurrence, significance and research prospects in Poland. In Arthropods: The Medical and Economic Importance; Buczek, A., Błaszak, C., Eds.; Akapit: Lublin, Poland, 2012; pp. 123–131. [Google Scholar]
- Bochkov, A.V. New observations on phylogeny of cheyletoid mites (Acari: Prostigmata: Cheyletoidea). Proc. Zool. Inst. RAS 2008, 312, 54–73. [Google Scholar]
- Giesen, K.M.T. A review of the parasitic mites of the family Psorergatidae (Cheyletoidea: Prostigmata: Acari) with hypotheses on the phylogenetic relationships of the species and species groups. Zool. Verh. 1990, 259, 1–69. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. The biodiversity of demodecid mites (Acariformes: Prostigmata), specific parasites of mammals with a global checklist and a new finding for Demodex sciurinus. Diversity 2020, 12, 261. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Mierzyński, Ł.; Bidziński, K. Morphological and ontogenetic characteristics of Demodex plecoti sp. nov. (Acariformes: Demodecidae) from the brown long-eared bat Plecotus auritus (Chiroptera: Vespertilionidae), with comments on parasitism. Syst. Appl. Acarol. 2019, 24, 377–388. [Google Scholar] [CrossRef]
- Desch, C.E.; Lukoschus, F.S.; Nadchatram, M. Two new species of Demodex (Acari: Demodicidae) from the Meibomian glands of the tropical old world bat, Eonycteris spelaea (Chiroptera). Int. J. Acarol. 1986, 12, 13–25. [Google Scholar] [CrossRef]
- Desch, C.E. Two new species of Demodex (Acari: Demodicidae) from the New Zealand short-tailed bat, Mystacina tuberculata Gray, 1843 (Chiroptera: Mystacinidae). N. Z. J. Zool. 1989, 16, 221–229. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. New species of Demodex (Acari: Demodecidae) with data on parasitism and occurrence of other demodecids of Rattus norvegicus (Rodentia, Muridae). Ann. Entomol. Soc. Am. 2014, 107, 740–747. [Google Scholar] [CrossRef]
- Izdebska, J.N. Demodex spp. (Acari: Demodecidae) in brown rat (Rodentia: Muridae) in Poland. Wiad. Parazytol. 2004, 50, 333–335. [Google Scholar] [PubMed]
- Zhang, Z.-Q. Repositories for mite and tick specimens: Acronyms and their nomenclature. Syst. Appl. Acarol. 2018, 23, 2432–2446. [Google Scholar] [CrossRef]
- Nutting, W.B. Hair follicle mites (Demodex spp.) of medical and veterinary concern. Cornell Vet. 1976, 66, 214–231. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. A new genus and species of demodecid mites from the tongue of a house mouse Mus musculus: Description of adult and immature stages with data on parasitism. Med. Vet. Entomol. 2016, 30, 135–143. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.E.; Reeder, D.M. (Eds.) Mammals Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2005; Available online: http://www.departments.bucknell.edu/biology/resources/msw3/ (accessed on 28 January 2022).
- Taxonomic Information System (ITIS). Available online: http://www.itis.gov (accessed on 28 January 2022).
- Fain, A. Les acariens psoriques parasites des Chauves-souris III: Le genre Psorergates Tyrell (Trombidiformes—Psorergatidae). Bull. Ann. Soc. R. Belg. Entomol. 1959, 95, 54–69. [Google Scholar]
- Fain, A. Les acariens psoriques parasites des chauves-souris. XIII: La famille Demodicidae Nicolet. Acarologia 1960, 2, 80–87. [Google Scholar]
- Vargas, M.; Bassols, I.B.; Desch, C.E.; Quintero, M.T.; Polaco, O.J. Description of two new species of the genus Demodex Owen, 1843 (Acari: Demodecidae) associated with mexican bats. Int. J. Acarol. 1995, 95, 75–82. [Google Scholar] [CrossRef]
- Kniest, F.M.; Lukoschus, F.S. Parasites of Western Australia XIII: Demodex bicaudatus new species of demodicid mite from the Meibomian glands of the bat Macroglossus minumus. Rec. West. Aust. Mus. 1981, 9, 111–118. [Google Scholar]
- Desch, C.; Lebel, R.R.; Nutting, W.B.; Lukoschus, F. Parasitic mites of Surinam: I. Demodex carolliae sp. nov. (Acari: Demodicidae) from the fruit bat Carollia perspicillata. Parasitology 1971, 62, 303–308. [Google Scholar]
- Hirst, S. On some new or little-known Acari, mostly parasitic in habit. Proc. Zool. Soc. Lond. 1921, 91, 357–378. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Mierzyński, Ł.; Bidziński, K. Demodecid mites (Acariformes, Demodecidae) in brown long-eared bat Plecotus auritus (Chiroptera, Vespertilionidae)—Second record in the world and systematic status of Demodex chiropteralis Hirst, 1921. Ann. Parasitol. 2018, 64, 109–113. [Google Scholar] [PubMed]
- Desch, C. A new species of Demodex Owen, 1843 (Acari: Demodecidae) from the meibomian glands of the vampire bat Desmodus rotundus (E. Geoffroy, 1810) (Chiroptera: Phyllostomidae: Desmodontinae) from Surinam. Int. J. Acarol. 1994, 20, 39–43. [Google Scholar] [CrossRef]
- Desch, C.; Nutting, W.B.; Lukoschus, F.S. Parasitic mites of Surinam VII: Demodex longissimus n. sp. from Carollia perspicillata and D. molossi n. sp. from Molossus molossus (Demodicidae: Trombidiformes); meibomian complex inhabitants of neotropical bats (Chiroptera). Acarologia 1972, 14, 35–53. [Google Scholar]
- Desch, C.E. A new species of demodicid mite (Acari: Prostigmata) from Western Australia parasitic on Macroglossus minimus (Chiroptera: Pteropodidae). Rec. West. Aust. Mus. 1981, 9, 41–47. [Google Scholar]
- Lukoschus, F.S.; Jongman, R.H.G.; Nutting, W.B. Parasitic mites of Surinam XII: Demodex melanopteri sp. n. (Demodicidae: Trombidiformes) from the Meibomian glands of the neotropical bat Eptesicus melanopterus. Acarologia 1972, 14, 54–58. [Google Scholar]
- Desch, C.E. Demodex biciceii: A new species of hair follicle mite (Acari: Demodecidae) from the evening bat, Nycticeius humeralis (Chiroptera: Vespertilionidae). Int. J. Acarol. 1996, 22, 187–191. [Google Scholar] [CrossRef]
- Leydig, F. Ueber Haarsackmilben und Krätzmilben. Arch. Naturgesch. 1859, 25, 338–354. [Google Scholar]
- Lukoschus, F.S.; Nutting, W.B. Parasitic mites of Surinam XIII: Ophthalmodex artibei gen. nov., spec. nov. (Prostigmata: Demodicidae) from Artibeus lituratus with notes on pathogenesis. Int. J. Acarol. 1979, 5, 299–304. [Google Scholar] [CrossRef]
- Woeltjes, A.G.W.; Lukoschus, F.S. Parasites of Western Australia XIV two new species of Ophtalmodex Lukoschus and Nutting (Acarina: Prostigmata: Demodecidae) from the eyes of bats. Rec. West. Aust. Mus. 1981, 9, 307313. [Google Scholar]
- Lukoschus, F.S.; Woeltjes, A.G.; Desch, C.E.; Nutting, W.B. Parasitic mites of Surinam XXXV: Two new Ophthalmodex spp. (O. carolliae, O. molossi: Demodicidae) from the bats Carollia perspicillata and Molossus molossus. Int. J. Acarol. 1980, 6, 45–50. [Google Scholar] [CrossRef]
- Veal, R.A.; Giesen, K.M.T.; Whitaker, J.O. A new species of the genus Ophtalmodex Lukoschus & Nutting 1979 (Prostigmata: Demodicidae) from Myotis lucifugus (Chiroptera: Vespertilionidae). Acarologia 1984, 25, 347–350. [Google Scholar]
- Lukoschus, F.S.; Woeltjes, A.G.W.; Desch, C.E.; Nutting, W.B. Parasitic mites of Surinam XX: Pterodex carolliae gen. nov., spec. nov. (Demodicidae) from the fruit bat, Carollia perspicillata. Int. J. Acarol. 1980, 6, 9–14. [Google Scholar] [CrossRef]
- Baker, A.S.; Craven, J.C. Checklist of the mites (Arachnida: Acari) associated with bats (Mammalia: Chiroptera) in the British Isles. Syst. Appl. Acarol. 2003, 14, 1–20. [Google Scholar] [CrossRef]
- Lukoschus, F.S.; Rosmalen, P.G.; Fain, A. Parasitic mites of Surinam XI. Four new species of the genus Psorergatoides Fain, 1959, (Psorergatidae: Trombidiformes). Tijdschr. Entomol. 1973, 116, 63–81. [Google Scholar]
- Giesen, K.M.T.; Lukoschus, F.S.; Fain, A. Parasites of Western Australia XV. A new species of Psorergatoides (Acarina: Psorergatidae) from Australian Bats. Rec. West. Aust. Mus. 1982, 9, 315–323. [Google Scholar]
- Lukoschus, F.S.; Louppen, J.M.W.; Fauran, P. Parasitic mites of Surinam: XIV—New observations on the genus Psorergatoides Fain, 1959 (Psorergatidae: Trombidiformes), with a key to the known species. Int. J. Acarol. 1979, 5, 311–324. [Google Scholar] [CrossRef]
- Fain, A. Les acariens psoriques parasites des Chauves-souris IX: Nouvelles observations sur le genre Psorergates Tyrell. Bull. Ann. Soc. R. Belg. Entomol. 1959, 95, 232–248. [Google Scholar]
- Haitlinger, R. External parasites of the Lower Silesian bats: V—Trombidiformes, Sarcoptiformes (Acarina). Wiad. Parazytol. 1979, 25, 105–117. [Google Scholar]
- Nelson, L.J.; Seeman, O.D.; Shinwari, M.W. Psorergatoides cf. kerivoulae (Acari: Psorergatidae) induces cutaneous lesions on the wings of Myotis macropus (Chiroptera: Vespertilionidae). Syst. Appl. Acarol. 2017, 22, 446–448. [Google Scholar]
- Baker, A.S. Psorergatoides nyctali (Prostigmata: Psorergatidae), a new mite species parasitizing the bat Nyctalus noctula (Mammalia: Chiroptera) in the British Isles. Syst. Appl. Acarol. 2005, 10, 67–74. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Fryderyk, S.; Ciechanowski, M. Spinturnix acuminatus (C.L. Koch, 1836), against the parasitofauna of the noctule bat Nyctalus noctula (Schreber, 1774). In Arthropods: Invasions and Their Control; Buczek, A., Błaszak, C., Eds.; Akapit: Lublin, Poland, 2009; pp. 23–30. [Google Scholar]
- Lukoschus, F.S. Krätzmilben an spanischen Kleinsaugern (Psorergatidae: Trombidiformes). Rev. Iber. Parasitol. 1967, 27, 203–228. [Google Scholar]
- Giesen, K.M.T.; Lukoschus, F.S.; Nadchatram, M. Three new itch mites of the family Psorergatidae (Acari, Prostigmata) from Malaysian small mammals. Malay. Nat. J. 1982, 35, 315–328. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L.; Bielecki, W. Demodex bialoviensis sp. nov. (Acariformes, Demodecidae) a new, specific parasite of the European bison Bison bonasus (Artiodactyla, Bovidae). Int. J. Parasitol. Parasites Wildl. 2022, 17, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Cierocka, K.; Izdebska, J.N.; Rolbiecki, L. Demodex crocidurae, a new demodecid mite (Acariformes: Prostigmata) parasitizing the lesser white-toothed shrew and a redescription of Demodex talpae from european mole with data on parasitism in Soricomorpha. Animals 2021, 11, 2712. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S. A new species of Demodex (Acari: Demodecidae) from the skin of the vibrissal area of the house mouse Mus musculus (Rodentia: Muridae), with data on parasitism. Syst. Appl. Acarol. 2016, 21, 1031–1039. [Google Scholar] [CrossRef]
- Walter, D.E.; Lindquist, E.E.; Smith, I.M.; Cook, D.R.; Krantz, G.W. Order Trombidiformes. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 233–420. [Google Scholar]
- Wilson, D.E.; Mittermeier, R.A. Handbook of the Mammals of the World; Lynx Editions: Barcelona, Spain, 2019; Volume 9. [Google Scholar]
- Ciechanowski, M. Nietoperze—Chiroptera. In Zoologia, Ssaki; PWN: Warsaw, Poland, 2020; Volume 3, pp. 236–304. [Google Scholar]
- Dietz, C.; von Helversen, O.; Nill, D. Bats of Britain, Europe and Northwest Africa; A. & C. Black: London, UK, 2009. [Google Scholar]
- Altringham, J.D. Bats: From Evolution to Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).