Preliminary Findings on How Different Management Systems and Social Interactions Influence Fecal Glucocorticoid Metabolites in White Rhinoceros (Ceratotherium simum)
Abstract
:Simple Summary
Abstract
1. Introduction
- -
- Captive breeding (captivity)—Rhinos in small or very small areas (<10 km2), with partial or total food supplementation, a high frequency of veterinary care intervention, and a manipulated reproduction system;
- -
- Semi-wild (semi-captivity)—Rhinoceroses in areas that are generally small (<10 km2), with partial food supplementation and high management intensity, but with a natural reproduction system. The animals live in an enclosure under human care, but the conditions seek to reproduce, as closely as possible, the conditions prevailing in their natural environment;
- -
- Wild (free-ranging)—Free rhinos in large areas (>10 km2) with no food supplementation, little veterinary intervention, and natural reproductive systems.
2. Materials and Methods
2.1. Animals
- ○
- Zoo Madrid (n = 2). This group consists of two white rhinoceroses—one female (“Female 1”, 15 years old) and one male (“Male 1”, 40 years old approximately) housed together in a daily outdoor area. These animals live in captivity under the following characteristics—enclosure of size <10 km2, total food supplementation, high frequency of veterinary care intervention, and manipulated reproduction system. Regarding social interactions, as these animals live in the same enclosure together with their young, they have intraspecific interactions. However, they are not able to establish any close inter-specific relations.
- ○
- Bioparc Valencia (n = 4). This group includes two females (named “Female 2” and “Female 3”, 7 and 6 years old, respectively) and a male (“Male 2”, 20 years old) housed together in a daily outdoor area. An additional male (“Male 3”, 35 years old) is housed in a separate enclosure with olfactive, auditory, and visual contact with the other rhinos to try to stimulate natural sexual behavior in the group. This captive population lives in larger enclosures, but these are still smaller than 10 km2. The animals have total food supplementation, a high frequency of veterinary intervention, and a manipulated reproduction system after previous attempts to achieve gestation through natural copulation without success. Here the animals have wide social interactions as they establish intra- and inter-specific contact with typical animal species of the savannah (zebras and antelopes, among others).
- ○
- South African Reserve (n = 8). This group consists of 8 white rhinoceroses at reproductive age or close to it, and variable individual conditions—5 females (“Female 4, 5, 6, 7, 8”, aged 30, 30, 10, 21, and 13 years old, respectively) and 3 males (“Male 4, 5, 6”, aged 7, 7, and 9 years old, respectively). All the females were accompanied by their young. This group of animals is kept under a management system that combines features of both semi-captivity and free-ranging management conditions, but since the aforementioned description is mainly based on breeding conditions, we will consider this last group as being in a free-ranging management system with the following characteristics—the animals live in a private reserve in South Africa of over 10,000 hectares (>100 km2) with many different animal and plant species, allowing the rhinoceroses to use their space and time freely. These animals rarely receive partial food supplementation (especially during winter) and occasional management, but they have a natural reproduction system and very little veterinary intervention (only in extraordinary circumstances). Animals receive eventual human care and little tourist/vehicle pressure, with a prevailing wild environment and a natural reproduction system. Animals roam in this large, enclosed area, where they establish relations of their choice among themselves (intra-specific) and with other typical African species (inter-specific).
2.2. Sample Collection, Conservation and Lyophilization
2.3. Extraction
2.4. HPLC Analysis
2.5. Hormonal Analysis
2.6. Statistical Study
3. Results
3.1. Fecal Glucocorticoid Metabolite Identification by HPLC
3.2. Fecal Glucocorticoid Metabolite Identification and Acute Stress
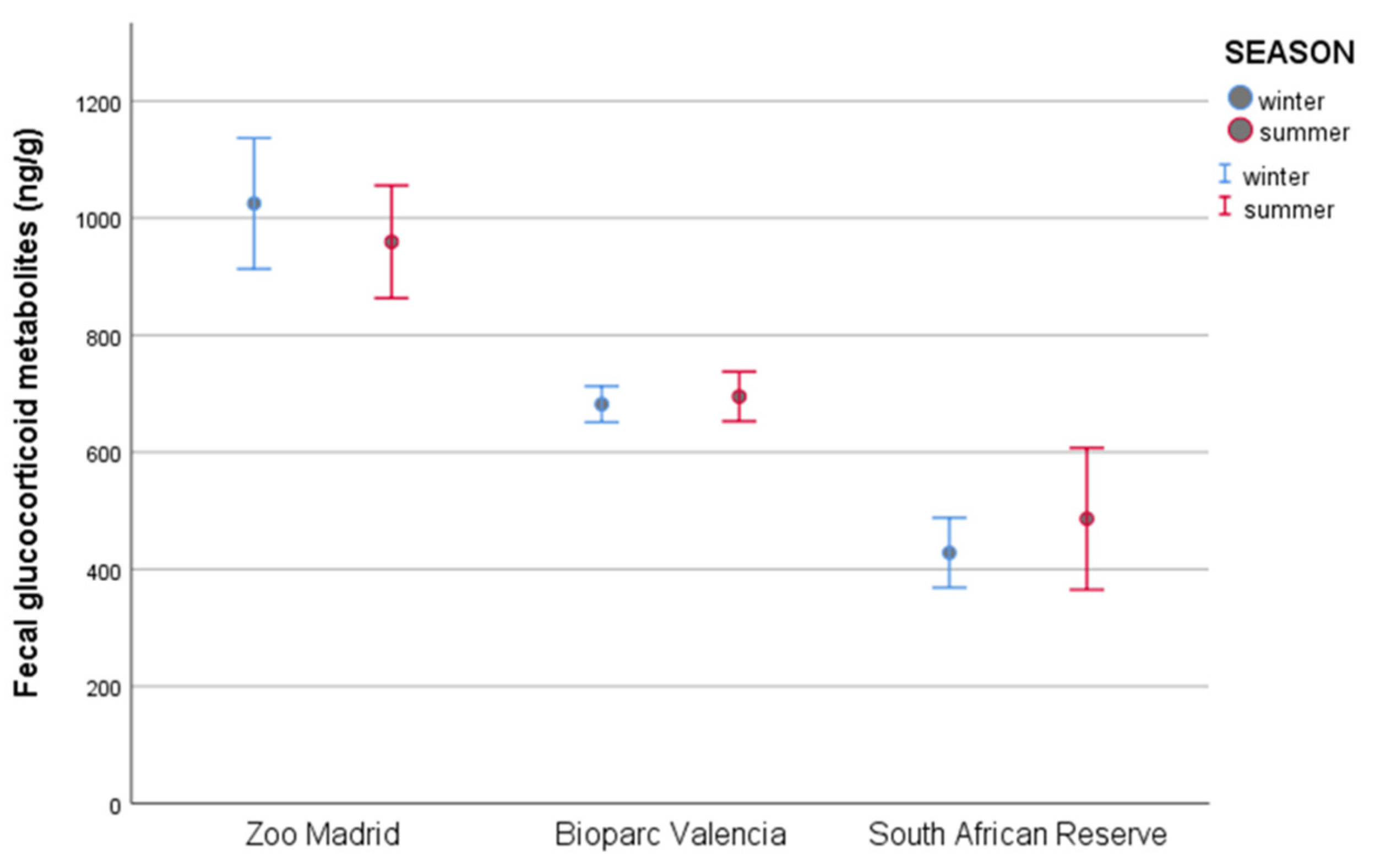
3.3. Fecal Glucocorticoid Metabolite Analysis: Analysis per Season
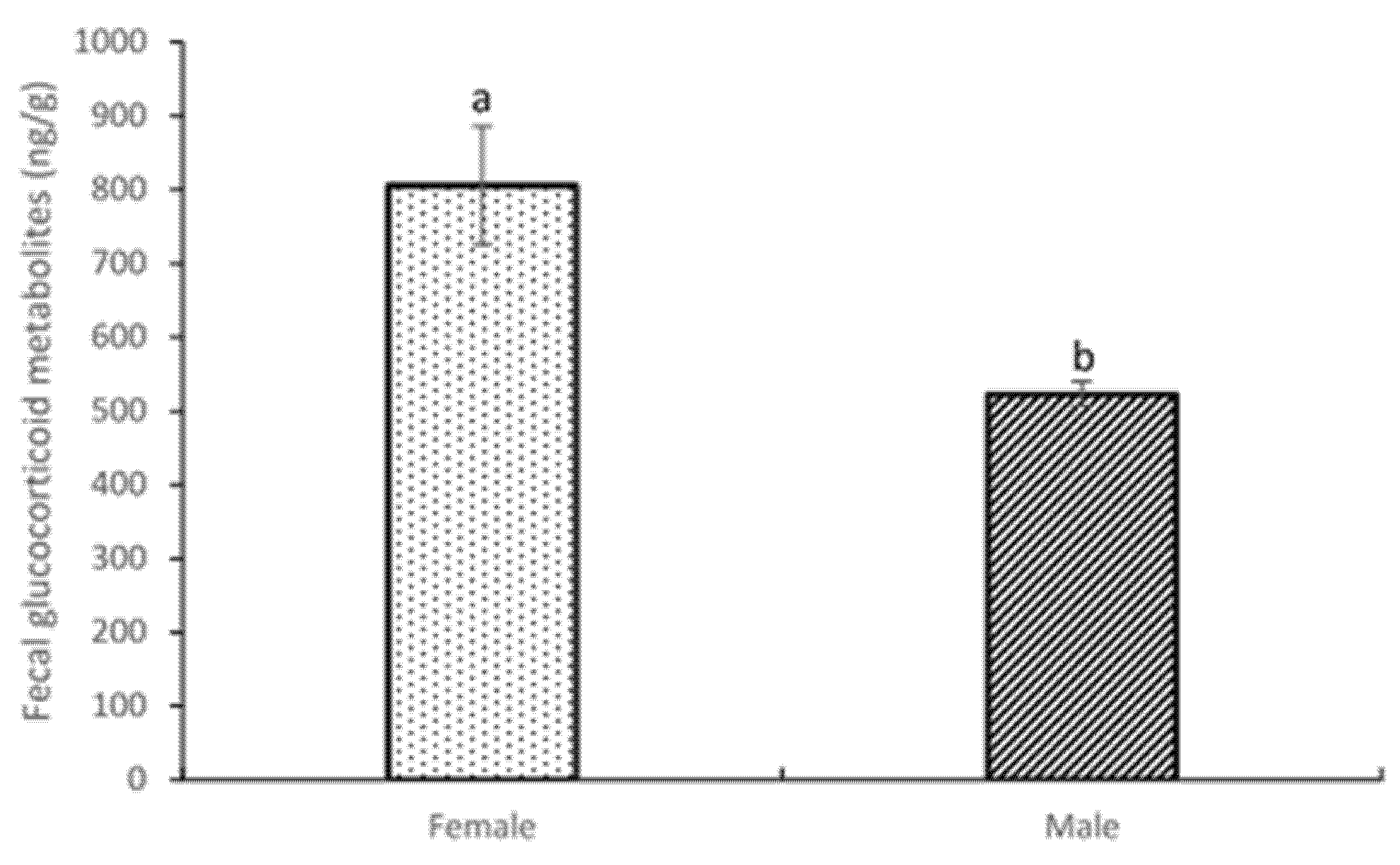
3.4. Fecal Glucocorticoid Metabolite Analysis: General Analysis by Sex
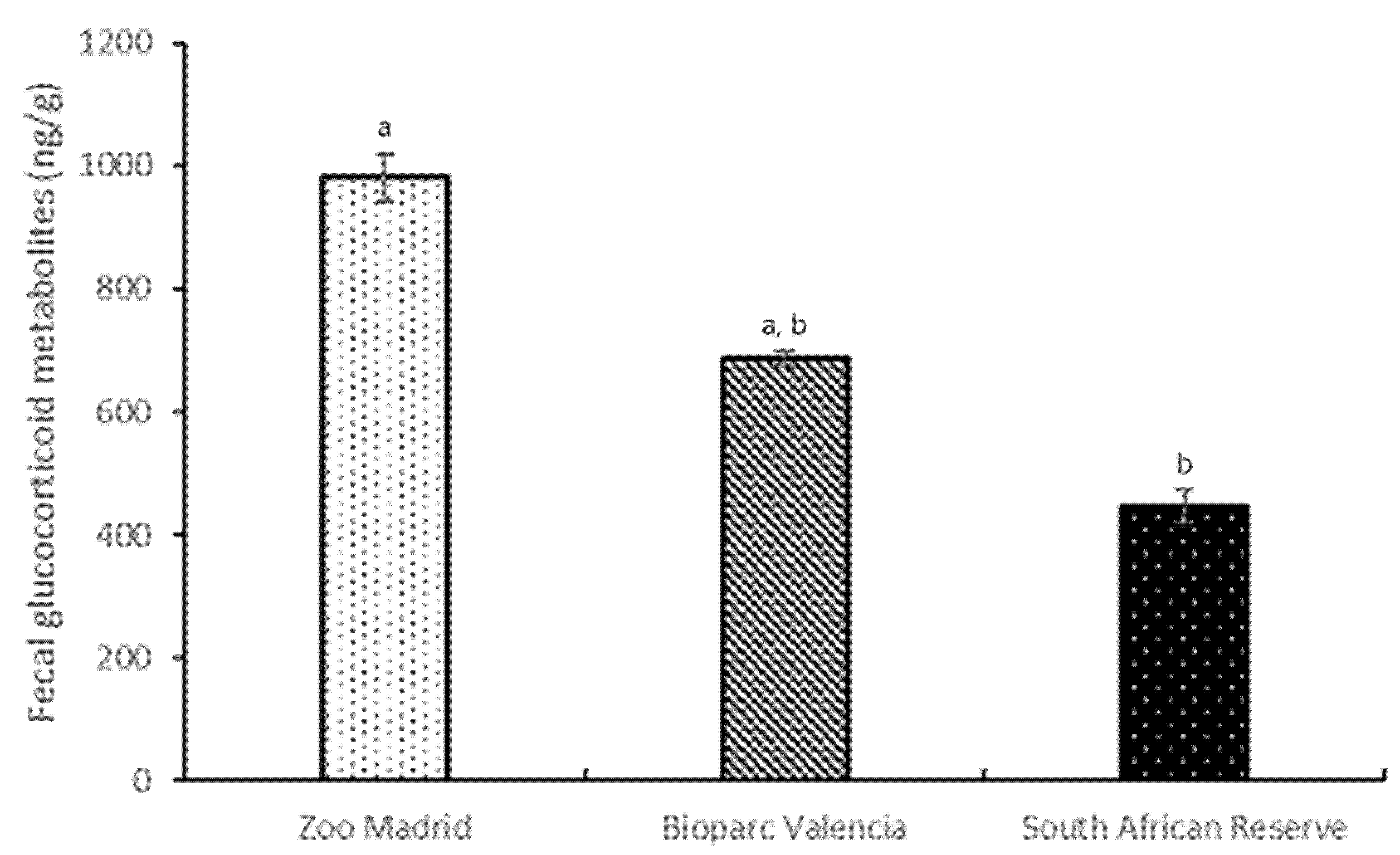
3.5. Fecal Glucocorticoid Metabolite Analysis: General Analysis per Group
Variables Sex and Group
3.6. Social and Behavioral Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emslie, R. Ceratotherium simum; The IUCN Red List of Threatened Species: Cambridge, UK, 2012; p. e.T4185A16980466. [Google Scholar]
- Cromsigt, J.P.G.M.; te Beest, M. Restoration of a megaherbivore: Landscape-level impacts of white rhinoceros in Kruger National Park, South Africa. J. Ecol. 2014, 102, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Kruger, M. Capture and Boma Stress Responses in The White Rhinoceros (Ceratotherium simum). Ph.D. Thesis, University of Witwatersrand, Johannesburg, South Africa, 2017. [Google Scholar]
- Swaisgood, R.; Dickman, D.; White, A. A captive population in crisis: Testing hypotheses for reproductive failure in captive-born southern white rhinoceros females. Biol. Conserv. 2006, 129, 468–476. [Google Scholar] [CrossRef]
- Hutchins, M.; Kreger, M. Rhinoceros behaviour: Implications for captive management and conservation. Int. Zoo Yearb. 2006, 40, 150–173. [Google Scholar] [CrossRef]
- Carlstead, K.; Brown, J.L. Relationships between patterns of Fecal corticoid excretion and behavior, reproduction, and environmental factors in captive black (Diceros bicornis) and white (Ceratotherium simum) rhinoceros. Zoo Biol. 2005, 24, 215–232. [Google Scholar] [CrossRef]
- Leader-Williams, N.; Brett, R.; Brooks, M.; Craig, I.; Toit, R.; Emslie, R.; Knight, M.; Stanley Price, M.; Stockil, O. A scheme for differentiating and defining the different situations under which rhinos are conserved. Pachyderm 1997, 23, 24–28. [Google Scholar]
- Hermes, R.; Hildebrandt, T.B.; Goritz, F. Reproductive problems directly attributable to long-term captivity—asymmetric reproductive aging. Anim. Reprod. Sci. 2004, 82–83, 49–60. [Google Scholar] [CrossRef]
- de Kloet, E.R.; Joels, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Cinková, I. Sexual, Social and Playful Behavior of White Rhinoceros (Ceratotherium simum) in Zoological Garden. Bachelor’s Thesis, University of Palacký Olomuc, Olomuc, Czech Republic, 2006. [Google Scholar]
- Schwarzenberger, F. The many uses of non-invasive fecal steroid monitoring in zoo and wildlife species. Int. Zoo Yearb. 2007, 41, 52–74. [Google Scholar] [CrossRef]
- Wasser, S.; Hunt, K.; Brown, J.; Cooper, K.; Crockett, C.; Bechert, U.; Millspaugh, J.; Larson, S.; Monfort, S. A Generalized Fecal Glucocorticoid Assay for Use in a Diverse Array of Nondomestic Mammalian and Avian Species. Gen. Comp. Endocrinol. 2000, 120, 260–275. [Google Scholar] [CrossRef]
- Monfort, S. Non-invasive endocrine measures of reproduction and stress in wild populations. In Reproductive Sciences and Integrated Conservation; Holt, W.V., Pickard, A.R., Roger, J.C., Wildt, D.E., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 147–165. [Google Scholar]
- Kersey, D.C.; Dehnhard, M. The use of noninvasive and minimally invasive methods in endocrinology for threatened mammalian species conservation. Gen. Comp. Endocrinol. 2014, 203, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.L.; Shultz, S.; Pilgrim, M.; Walker, S.L. Irregular ovarian activity, body condition and behavioural differences are associated with reproductive success in female eastern black rhinoceros (Diceros bicornis michaeli). Gen. Comp. Endocrinol. 2015, 214, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.O. Vertebrate Endocrinology, 4th ed.; Academic Press: San Diego, CA, USA, 2007; p. 560. [Google Scholar]
- Illera, J.; Illera, J.C.; Silván, G.; Moreno, A. Endocrinología de Pequeños Animales; LID Editorial Empresarial SL: Barcelona, Spain, 2011. [Google Scholar]
- Sheriff, M.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Reeder, D.; Kramer, K. Stress in Free-Ranging Mammals: Integrating Physiology, Ecology, and Natural History. J. Mammal. 2005, 86, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Cavigelli, S.A.; Caruso, M.J. Sex, social status and physiological stress in primates: The importance of social and glucocorticoid dynamics. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140103. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.W., Jr.; Tolson, P.; Hamad, N. Remote assessment of stress in white rhinoceros (Ceratotherium simum) and black rhinoceros (Diceros bicornis) by measurement of adrenal steroids in feces. J. Zoo Wildl. Med. 2002, 33, 214–221. [Google Scholar] [CrossRef]
- Linklater, W.; Macdonald, E.; Flamand, J.; Czekala, N. Declining and low fecal corticoids are associated with distress, not acclimation to stress, during the translocation of African rhinoceros. Anim. Conserv. 2010, 13, 104–111. [Google Scholar] [CrossRef]
- Capiro, J.M.; Stoops, M.A.; Freeman, E.W.; Clawson, D.; Schook, M.W. Effects of management strategies on glucocorticoids and behavior in Indian rhinoceros (Rhinoceros unicornis): Translocation and operant conditioning. Zoo Biol. 2014, 33, 131–143. [Google Scholar] [CrossRef]
- Badenhorst, M.; Otto, M.; Goot, A.C.V.D.; Ganswindt, A. Stress steroid levels and the short-term impact of routine dehorning in female southern white rhinoceroses (Ceratotherium simum simum). Afr. Zool. 2016, 51, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Pavitt, A.; Pemberton, J.; Kruuk, L.; Walling, C. Testosterone and cortisol concentrations vary with reproductive status in wild female red deer. Ecol. Evol. 2016, 6, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Tilbrook, A.J.; Turner, A.; Clarke, I. Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev. Reprod. 2000, 5, 105–113. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J. Glucocorticoids, Stress, and Fertility. Minerva Endocrinol. 2010, 35, 109–125. [Google Scholar] [PubMed]
- Flauger, B.; Krueger, K.; Gerhards, H.; Mostl, E. Simplified method to measure glucocorticoid metabolites in faeces of horses. Vet. Res. Commun. 2010, 34, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, C.A.H.; Papageorge, S.; Wasser, S.K. Noninvasive stress and reproductive measures of social and ecological pressures in free-ranging African elephants. Conserv. Biol. 2001, 15, 1134–1142. [Google Scholar] [CrossRef]
- Barja, I.; Silvan, G.; Illera, J.C. Relationships between sex and stress hormone levels in feces and marking behavior in a wild population of Iberian wolves (Canis lupus signatus). J. Chem. Ecol. 2008, 34, 697–701. [Google Scholar] [CrossRef]
- Creel, S.; Dantzer, B.; Goymann, W.; Rubenstein, D.R. The ecology of stress: Effects of the social environment. Funct. Ecol. 2013, 27, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Touma, C.; Palme, R. Measuring Fecal Glucocorticoid Metabolites in Mammals and Birds: The Importance of Validation. Ann. N. Y. Acad. Sci. 2005, 1046, 54–74. [Google Scholar] [CrossRef]
- Breuner, C.W.; Delehanty, B.; Boonstra, R. Evaluating stress in natural populations of vertebrates: Total CORT is not good enough. Funct. Ecol. 2013, 27, 24–36. [Google Scholar] [CrossRef]
- Ranglack, D.; Neuman-Lee, L.; French, S.; du Toit, J. Considerations of context and scale when using fecal glucocorticoids to indicate stress in large mammals: A study of wild American plains bison. Southwest. Nat. 2017, 62, 62–68. [Google Scholar] [CrossRef]
- Chinnadurai, S.K.; Millspaugh, J.J.; Matthews, W.S.; Canter, K.; Slotow, R.; Washburn, B.E.; Woods, R.J. Validation of fecal glucocorticoid metabolite assays for South African herbivores. J. Wildl. Manag. 2009, 73, 1014–1020. [Google Scholar] [CrossRef]
- Corlatti, L.; Palme, R.; Frey-Roos, F.; Hackländer, K. Climatic cues and glucocorticoids in a free-ranging riparian population of red deer (Cervus elaphus). Folia Zool. 2011, 60, 176–180. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Greaver, C.; Knight, G.A.; Knight, M.H.; Smit, I.P.J.; Pienaar, D. Disruption of Rhino Demography by Poachers May Lead to Population Declines in Kruger National Park, South Africa. PLoS ONE 2015, 10, e0127783. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, F.; Möstl, E.; Bamberg, E.; Pammer, J.; Schmehlik, O. Concentrations of progestagens and oestrogens in the faeces of pregnant Lipizzan, trotter and thoroughbred mares. J. Reprod. Fertil. Suppl. 1991, 44, 489–499. [Google Scholar] [PubMed]
- Schwarzenberger, F.; Möstl, E.; Palme, R.; Bamberg, E. Faecal steroid analysis for non-invasive monitoring of reproductive status in farm, wild and zoo animals. Anim. Reprod. Sci. 1996, 42, 515–526. [Google Scholar] [CrossRef]
- Palme, R.; Mostl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Int. J. Mammal. Biol. 1997, 62, 192–197. [Google Scholar]
- Menargues, A.; Urios, V.; Limiñana, R. Seasonal pattern of salivary cortisol secretion in the greater one-horned rhino (Rhinoceros unicornis). Anim. Welf. 2013, 22, 467–472. [Google Scholar] [CrossRef] [Green Version]
- de Andrés, P.J.; Cáceres, S.; Crespo, B.; Silván, G.; Illera, J.C. Non-Invasive Determination of Annual Fecal Cortisol, Androstenedione, and Testosterone Variations in a Herd of Male Asian Elephants (Elephas maximus) and Their Relation to Some Climatic Variables. Animals 2021, 11, 2723. [Google Scholar] [CrossRef]
- Cinková, I.; Bičík, V. Social and reproductive behaviour of critically endangered northern white rhinoceros in a zoological garden. Mammal. Biol. Z. Für Säugetierkd. 2013, 78, 50–54. [Google Scholar] [CrossRef]
- Patton, M.; Swaisgood, R.; Czekala, N.; White, A.; Fetter, G.A.; Montagne, J.-P.; Rieches, R.G.; Lance, V. Reproductive cycle length and pregnancy in the Southern White Rhinoceros (Ceratotherium simum simum) as determined by fecal pregnane analysis and observations of mating behavior. Zoo Biol. 1999, 18, 111–127. [Google Scholar] [CrossRef]
- Brown, J.L.; Bellem, A.C.; Fouraker, M.; Wildt, D.E.; Roth, T.L. Comparative analysis of gonadal and adrenal activity in the black and white rhinoceros in North America by noninvasive endocrine monitoring. Zoo Biol. 2001, 20, 463–486. [Google Scholar] [CrossRef]
- Wielebnowski, N.C.; Fletchall, N.; Carlstead, K.; Busso, J.M.; Brown, J.L. Noninvasive assessment of adrenal activity associated with husbandry and behavioral factors in the North American clouded leopard population. Zoo Biol. 2002, 21, 77–98. [Google Scholar] [CrossRef]
- Jensvold, M.; Sanz, C.; Fouts, R.; Fouts, D. Effect of Enclosure Size and Complexity on the Behaviors of Captive Chimpanzees (Pan troglodytes). J. Appl. Anim. Welf. Sci. 2001, 4, 53–69. [Google Scholar] [CrossRef]
- Hogan, E.S.; Houpt, K.A.; Sweeney, K. The effect of enclosure size on social interactions and daily activity patterns of the captive Asiatic wild horse (Equus przewalskii). Appl. Anim. Behav. Sci. 1988, 21, 147–168. [Google Scholar] [CrossRef]
- Breton, G.; Barrot, S. Influence of enclosure size on the distances covered and paced by captive tigers (Panthera tigris). Appl. Anim. Behav. Sci. 2014, 154, 66–75. [Google Scholar] [CrossRef]
- Fanson, K.V.; Wielebnowski, N.C.; Shenk, T.M.; Lucas, J.R. Comparative patterns of adrenal activity in captive and wild Canada lynx (Lynx canadensis). J. Comp. Physiol. B 2012, 182, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Van der Weyde, L.; Martin, G.; Paris, M.C.J. Monitoring stress in captive and free-ranging African wild dogs (Lycaon pictus) using faecal glucocorticoid metabolites. Gen. Comp. Endocrinol. 2016, 226, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Pirovino, M. Fecal glucocorticoid measurements and their relation to rearing, behaviour and environmental factors in the European pileated gibbon population (Hylobates pileatus). Int. J. Primatol. 2011, 32, 1161–1178. [Google Scholar] [CrossRef]
- Barja, I.; Silvan, G.; Rosellini, S.; Pineiro, A.; Gonzalez-Gil, A.; Camacho, L.; Illera, J.C. Stress physiological responses to tourist pressure in a wild population of European pine marten. J. Steroid Biochem. Mol. Biol. 2007, 104, 136–142. [Google Scholar] [CrossRef]
- Zwijacz-Kozica, T.; Selva, N.; Barja, I.; Silvan, G.; Martínez-Fernández, L.; Illera, J.; Jodłowski, M. Concentration of fecal cortisol metabolites in chamois in relation to tourist pressure in Tatra National Park (South Poland). Acta Theriol. 2013, 58, 227–235. [Google Scholar] [CrossRef]
- Emslie, R.; Brooks, M. African Rhino Status Survey and Conservation Action Plan; IUCN: Cambridge, UK, 1999. [Google Scholar]
- He, L.; Wang, W.-X.; Li, L.-H.; Liu, B.-Q.; Liu, G.; Liu, S.-Q.; Qi, L.; Hu, D.-F. Effects of crowding and sex on fecal cortisol levels of captive forest musk deer. Biol. Res. 2014, 47, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Perret, M.; Predine, J. Effects of long-term grouping on serum cortisol levels in Microcebus murinus (Prosimii). Horm. Behav. 1984, 18, 346–358. [Google Scholar] [CrossRef]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effect of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003, 130, 267–278. [Google Scholar] [CrossRef]
- Navarro-Castilla, Á.; Barja, I.; Olea, P.; Piñeiro, A.; Mateo-Tomás, P.; Silvan, G.; Illera, J. Are degraded habitats from agricultural crops associated with elevated faecal glucocorticoids in a wild population of common vole (Microtus arvalis)? Mammal. Biol. Z. Saugetierkd. 2014, 79, 36–43. [Google Scholar] [CrossRef]
- Handa, R.J.; McGivern, R.F. Stress Response: Sex Differences. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Oxford, UK, 2009; pp. 511–517. [Google Scholar]
- Handa, R.J.; Burgess, L.H.; Kerr, J.E.; O’Keefe, J.A. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994, 28, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, H. African Rhinoceroses in Captivity: The White Rhinoceros Ceratotherium simum (Burchell1817), the Black Rhinoceros Diceros bicornis (Linnaeus1758). Bachelor’s Thesis, University of Copenhagen, København, Denmark, 1982. [Google Scholar]
- Pienaar, D.J. Social organization and behaviour of the white rhinoceros. In Proceedings of the Symposium on Rhinos as Game Ranch Animals, Pretoria, South Africa, 9–10 September 1994; pp. 87–92. [Google Scholar]
- Creel, S. Social dominance and stress hormones. Trends Ecol. Evolut. 2001, 16, 491–497. [Google Scholar] [CrossRef]
- Metrione, L.C.; Penfold, L.M.; Waring, G.H. Social and spatial relationships in captive southern white rhinoceros (Ceratotherium simum simum). Zoo Biol. 2007, 26, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Cockrem, J.F. Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef]
- Moberg, G. The Biology of Animal Stress; CABI Publishing: New York, NY, USA, 2000. [Google Scholar]
- Mooring, M.S.; Patton, M.L.; Lance, V.A.; Hall, B.M.; Schaad, E.W.; Fetter, G.A.; Fortin, S.S.; McPeak, K.M. Glucocorticoids of bison bulls in relation to social status. Hormon. Behav. 2006, 49, 369–375. [Google Scholar] [CrossRef]



| Study N° | Group | Management Characteristics | Social Interactions | Sex | Age | Mean ± S.E.M. fGCM (ng/g Dry Feces) | |||
|---|---|---|---|---|---|---|---|---|---|
| System | Enclosure | Food Supplementation | Breeding | Inter-Specific Relations | |||||
| 1 | Zoo Madrid | Captive | Small | Total | Manipulated reproduction system | Absent | Female 1 | 15 | 1115.76 ± 34.43 * |
| 2 | Male 1 | 40 | 812.73 ± 61.65 | ||||||
| 3 | Bioparc Valencia | Captive | Medium | Total | Manipulated reproduction system | Present | Female 2 | 7 | 790.17 ± 23.13 |
| 4 | Female 3 | 6 | 790.11 ± 31.14 | ||||||
| 5 | Male 2 | 20 | 645.44 ± 18.32 | ||||||
| 6 | Male 3 | 35 | 545.45 ± 13.62 | ||||||
| 7 | South African Reserve | Free-ranging | Large | Partial | Natural reproduction system | Present | Female 4 | 30 | 989.12 ± 62.75 |
| 8 | Female 5 | 30 | 388.93 ± 37.65 | ||||||
| 9 | Female 6 | 10 | 692.50 ± 46.98 | ||||||
| 10 | Female 7 | 21 | 797.88 ± 156.05 | ||||||
| 11 | Female 8 | 13 | 314.07 ± 40.32 | ||||||
| 12 | Male 4 | 7 | 268.37 ± 19.16 | ||||||
| 13 | Male 5 | 7 | 203.25 ± 13.03 | ||||||
| 14 | Male 6 | 9 | 200.63 ± 12.57 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, L.; Silván, G.; Cáceres, S.; Caperos, J.M.; Fernández-Morán, J.; Casares, M.; Crespo, B.; de Andrés, P.J.; Illera, J.C. Preliminary Findings on How Different Management Systems and Social Interactions Influence Fecal Glucocorticoid Metabolites in White Rhinoceros (Ceratotherium simum). Animals 2022, 12, 897. https://doi.org/10.3390/ani12070897
Martínez L, Silván G, Cáceres S, Caperos JM, Fernández-Morán J, Casares M, Crespo B, de Andrés PJ, Illera JC. Preliminary Findings on How Different Management Systems and Social Interactions Influence Fecal Glucocorticoid Metabolites in White Rhinoceros (Ceratotherium simum). Animals. 2022; 12(7):897. https://doi.org/10.3390/ani12070897
Chicago/Turabian StyleMartínez, Leticia, Gema Silván, Sara Cáceres, Jose Manuel Caperos, Jesús Fernández-Morán, Miguel Casares, Belén Crespo, Paloma Jimena de Andrés, and Juan Carlos Illera. 2022. "Preliminary Findings on How Different Management Systems and Social Interactions Influence Fecal Glucocorticoid Metabolites in White Rhinoceros (Ceratotherium simum)" Animals 12, no. 7: 897. https://doi.org/10.3390/ani12070897






