Phagocytic Activity, Oxygen Metabolism and Serum Amyloid a Concentration in Peripheral Blood of Mink with Subclinical Aleutian Virus Infection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Serological Diagnostics of AMDV
2.3. Molecular Diagnostics of AMDV
2.4. Polymerase Chain Reaction (PCR)
2.5. Determination of the Phagocytic Activity of Granulocytes and Monocytes
2.6. Determination of Oxygen Metabolism in Granulocytes and Monocytes
2.7. Determination of the Serum SAA Protein Level
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, T.H.; Hammer, A.S.; Chriél, M. Monitoring chronic infection with a field strain of Aleutian mink disease virus. Vet. Microbiol. 2014, 31, 420–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, M.; Kostro, K. Effect of persistent infection of mink with Aleutian mink disease virus on reproductive failure. Bull. Vet. Inst. Pulawy. 2014, 58, 369–373. [Google Scholar] [CrossRef]
- Kaaden, O.R.; Bartel, E.; Haas, L.; Kierek-Jaczszuk, D.; Löchelt, M.; Müller, F.; Neth, R.; Roth, S.; Stolze, B.; Van Dawen, S.; et al. Mechanisms contributing to the virus persistence in Aleutian disease. DTW. Dtsch. Tierarztl. Wochenschr. 1990, 97, 96–99. [Google Scholar]
- Farid, A.H.; Hussain, I. Dose response of black American mink to Aleutian mink disease virus. Immun. Inflamm. Dis. 2020, 8, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Alexandersen, S.; Bloom, M.E.; Perryman, S. Detailed transcription map of Aleutian mink disease parvovirus. J. Virol. 1988, 62, 3684–3694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aasted, B.; Leslie, R.G. Virus-specific B-lymphocytes are probably the primary targets for Aleutian disease virus. Vet. Immunol. Immunopathol. 1991, 28, 127–141. [Google Scholar] [CrossRef]
- Kanno, H.; Wolfinbarger, J.B.; Bloom, M.E. Identification of Aleutian mink disease parvovirus transcripts in macrophages of infected adult mink. J. Virol. 1992, 66, 5305–5312. [Google Scholar] [CrossRef] [Green Version]
- Kanno, H.; Wolfinbarger, J.B.; Bloom, M.E. Aleutian mink disease parvovirus infection of mink peritoneal macrophages and human macrophage cell lines. J. Virol. 1993, 67, 2075–2082. [Google Scholar] [CrossRef] [Green Version]
- Kanno, H.; Wolfinbarger, J.B.; Bloom, M.E. Aleutian mink disease parvovirus infection of mink macrophages and human macrophage cell line U937: Demonstration of antibody-dependent enhancement of infection. J. Virol. 1993, 67, 7017–7024. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Wolfinbarger, J.B.; Miyazawa, M.; Bloom, M.E. Replication of Aleutian mink disease parvovirus in lymphoid tissues of adult mink: Involvement of follicular dendritic cells and macrophages. J. Virol. 1991, 65, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Lodmell, D.L.; Bergman, R.K.; Bloom, M.E.; Ewalt, L.C.; Hadlow, W.J.; Race, R.E. Impaired phagocytosis by the mononuclear phagocytic system in sapphire mink affected with Aleutian disease. Proc. Soc. Exp. Biol. Med. 1990, 195, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Huntoon, K.M.; Wang, Y.; Eppolito, C.A.; Barbour, K.W.; Berger, F.G.; Shrikant, P.A.; Baumann, H. The acute phase protein haptoglobin regulates host immunity. J. Leukoc. Biol. 2008, 84, 170–181. [Google Scholar] [CrossRef]
- Lago, F.; Dieguez, C.; Gómez-Reino, J.; Gualillo, O. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 2007, 3, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role during Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Carrillo, J.L.M.; García, F.P.C.; Coronado, O.; García, M.A.M.; Cordero, J.C. Physiology and Pathology of Innate Immune Response against Pathogens. In Physiology and Pathology of Immunology; Rezaei, N., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Eckersall, P.D. Acute phase proteins as markers of inflammatory lesions. Comp. Haematol. Int. 2004, 5, 93–97. [Google Scholar] [CrossRef]
- Eckersall, P.D. Acute phase proteins as markers of infection and inflammation: Monitoring animal health, animal welfare and food safety. Ir. Vet. J. 2000, 53, 307–311. [Google Scholar]
- Eckersall, P.D.; Bell, R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet. J. 2010, 185, 23–27. [Google Scholar] [CrossRef]
- Marhaug, G.; Hackett, B.; Dowton, S.B. Serum amyloid A gene expression in rabbit, mink and mouse. Clin. Exp. Immunol. 1997, 107, 425–434. [Google Scholar] [CrossRef]
- Huang, J.H.; Liao, W.S. Synergistic induction of mouse serum amyloid A3 promoter by the inflammatory mediators IL-1 and IL-6. J. Interferon Cytokine Res. 1999, 19, 1403–1411. [Google Scholar] [CrossRef]
- Ye, R.D.; Sun, L. Emerging functions of serum amyloid A in inflammation. J. Leukoc. Biol. 2015, 98, 923–929. [Google Scholar] [CrossRef] [PubMed]


| Group I | Group II | |||
|---|---|---|---|---|
| No. | Age (Months) | Body Weight (kg) | Age (Months) | Body Weight (kg) |
| 1 | 24 | 1.8 | 12 | 1.9 |
| 2 | 24 | 1.9 | 12 | 1.8 |
| 3 | 12 | 1.8 | 24 | 2.0 |
| 4 | 12 | 1.7 | 24 | 1.8 |
| 5 | 24 | 1.9 | 12 | 1.7 |
| 6 | 24 | 1.8 | 24 | 1.9 |
| 7 | 12 | 1.7 | 12 | 2.0 |
| 8 | 24 | 1.9 | 24 | 1.7 |
| 9 | 12 | 1.8 | 12 | 1.9 |
| 10 | 12 | 1.7 | 12 | 1.9 |
| 11 | 24 | 1.8 | 24 | 1.8 |
| 12 | 24 | 1.8 | 24 | 2.1 |
| Descriptive Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Health Status | Mean | MED | SD | SE | Min | Max | p-Value |
| SAA concentration | AMDV-infected | 203.2 | 191.7 | 91.5 | 26.4 | 70.3 | 335.9 | <0.00001 |
| healthy | 14.2 | 13.5 | 4.6 | 1.4 | 8.7 | 22.4 | ||
| % phagocytic granulocytes AMDV | AMDV-infected | 50.5 | 52.7 | 12.2 | 3.2 | 29.6 | 68.4 | <0.00001 |
| healthy | 84.8 | 83.1 | 7.2 | 2.1 | 74.2 | 95.6 | ||
| % phagocytic monocytes AMDV | AMDV-infected | 70.6 | 72.2 | 10.2 | 2.9 | 48.6 | 84.2 | =0.955206 |
| healthy | 70.8 | 72.5 | 11.3 | 3.3 | 52.5 | 89.7 | ||
| MFI phagocytic granulocytes AMDV | AMDV-infected | 71.9 | 69.5 | 21.8 | 6.3 | 38.7 | 121.8 | <0.00001 |
| healthy | 565.2 | 559.2 | 73.8 | 21.3 | 456.8 | 687.7 | ||
| MFI phagocytic monocytes AMDV | AMDV-infected | 299.7 | 313.8 | 67.9 | 19.6 | 159.4 | 389.6 | =0.00003 |
| healthy | 469.3 | 485.4 | 89.5 | 25.8 | 264 | 612.8 | ||
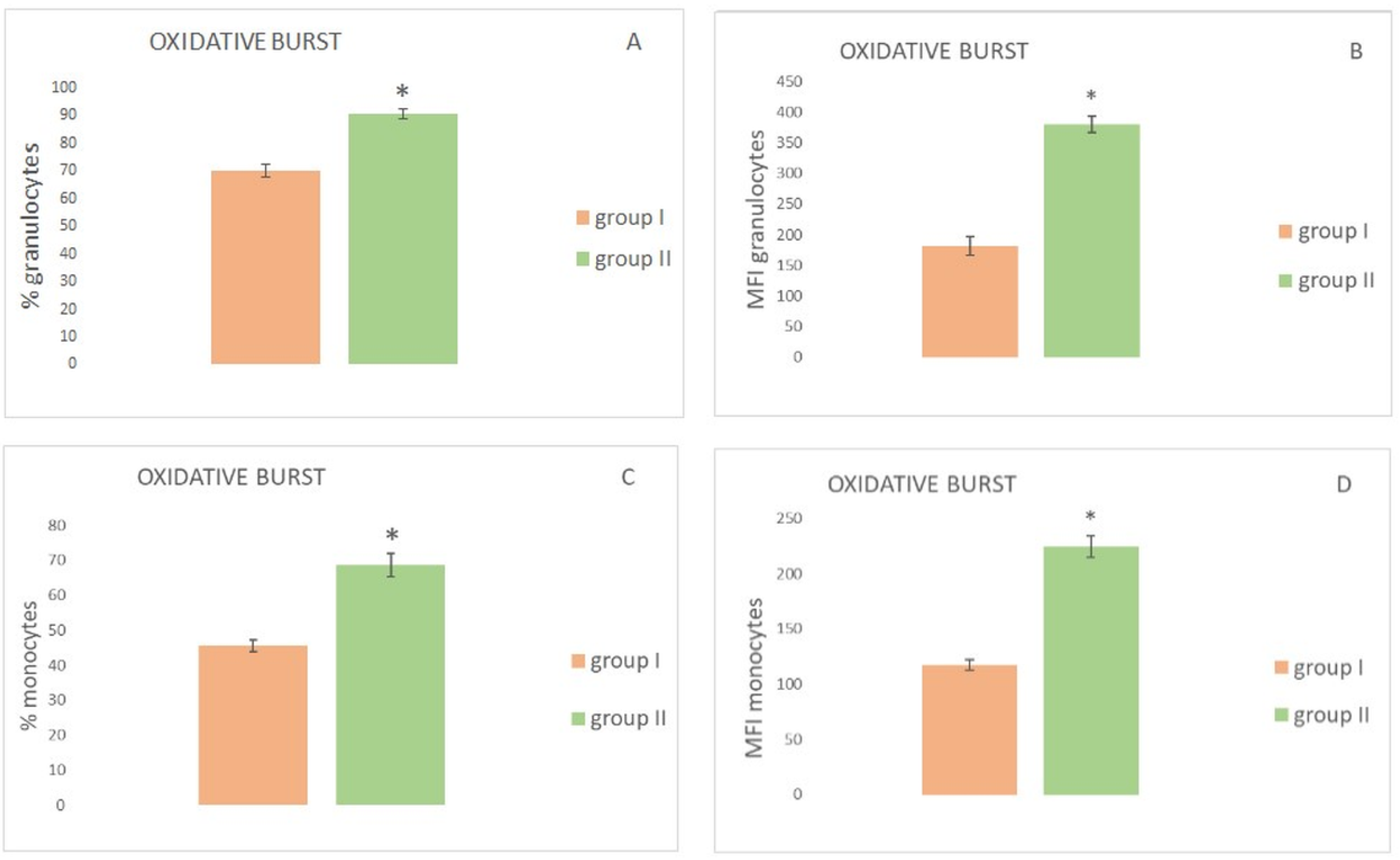
| % granulocytes performing oxidative burst AMDV minks | AMDV-infected | 70.11 | 71.55 | 7.77 | 2.24 | 56.9 | 79.2 | <0.00001 |
| healthy | 90.6 | 91.2 | 6.1 | 1.7 | 78.9 | 98.2 | ||
| % monocytes performing oxidative burst AMDV minks | AMDV-infected | 45.6 | 45.4 | 5.5 | 1.5 | 38.1 | 55.4 | <0.00001 |
| healthy | 68.6 | 70.8 | 11.3 | 3.3 | 54.3 | 82.8 | ||
| MFI granulocytes performing oxidative burst AMDV minks | AMDV-infected | 181.7 | 195.2 | 52.3 | 15.1 | 89.4 | 252.5 | <0.00001 |
| healthy | 379.4 | 391.9 | 44.4 | 12.8 | 300.1 | 435.6 | ||
| MFI monocytes performing oxidative burst AMDV minks | AMDV-infected | 117.2 | 112.4 | 16.6 | 4.8 | 93.1 | 144.1 | <0.00001 |
| healthy | 224.4 | 217.2 | 33.8 | 9.7 | 189.6 | 287.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żmuda, A.; Lisiecka, U.; Dudek, K.; Dąbrowski, R.; Gąsiorek, B.; Winiarczyk, S.; Kostro, K. Phagocytic Activity, Oxygen Metabolism and Serum Amyloid a Concentration in Peripheral Blood of Mink with Subclinical Aleutian Virus Infection. Animals 2022, 12, 987. https://doi.org/10.3390/ani12080987
Żmuda A, Lisiecka U, Dudek K, Dąbrowski R, Gąsiorek B, Winiarczyk S, Kostro K. Phagocytic Activity, Oxygen Metabolism and Serum Amyloid a Concentration in Peripheral Blood of Mink with Subclinical Aleutian Virus Infection. Animals. 2022; 12(8):987. https://doi.org/10.3390/ani12080987
Chicago/Turabian StyleŻmuda, Andrzej, Urszula Lisiecka, Katarzyna Dudek, Roman Dąbrowski, Bolesław Gąsiorek, Stanisław Winiarczyk, and Krzysztof Kostro. 2022. "Phagocytic Activity, Oxygen Metabolism and Serum Amyloid a Concentration in Peripheral Blood of Mink with Subclinical Aleutian Virus Infection" Animals 12, no. 8: 987. https://doi.org/10.3390/ani12080987
APA StyleŻmuda, A., Lisiecka, U., Dudek, K., Dąbrowski, R., Gąsiorek, B., Winiarczyk, S., & Kostro, K. (2022). Phagocytic Activity, Oxygen Metabolism and Serum Amyloid a Concentration in Peripheral Blood of Mink with Subclinical Aleutian Virus Infection. Animals, 12(8), 987. https://doi.org/10.3390/ani12080987







