Male African Elephant (Loxodonta africana) Behavioral Responses to Estrous Call Playbacks May Inform Conservation Management Tools
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
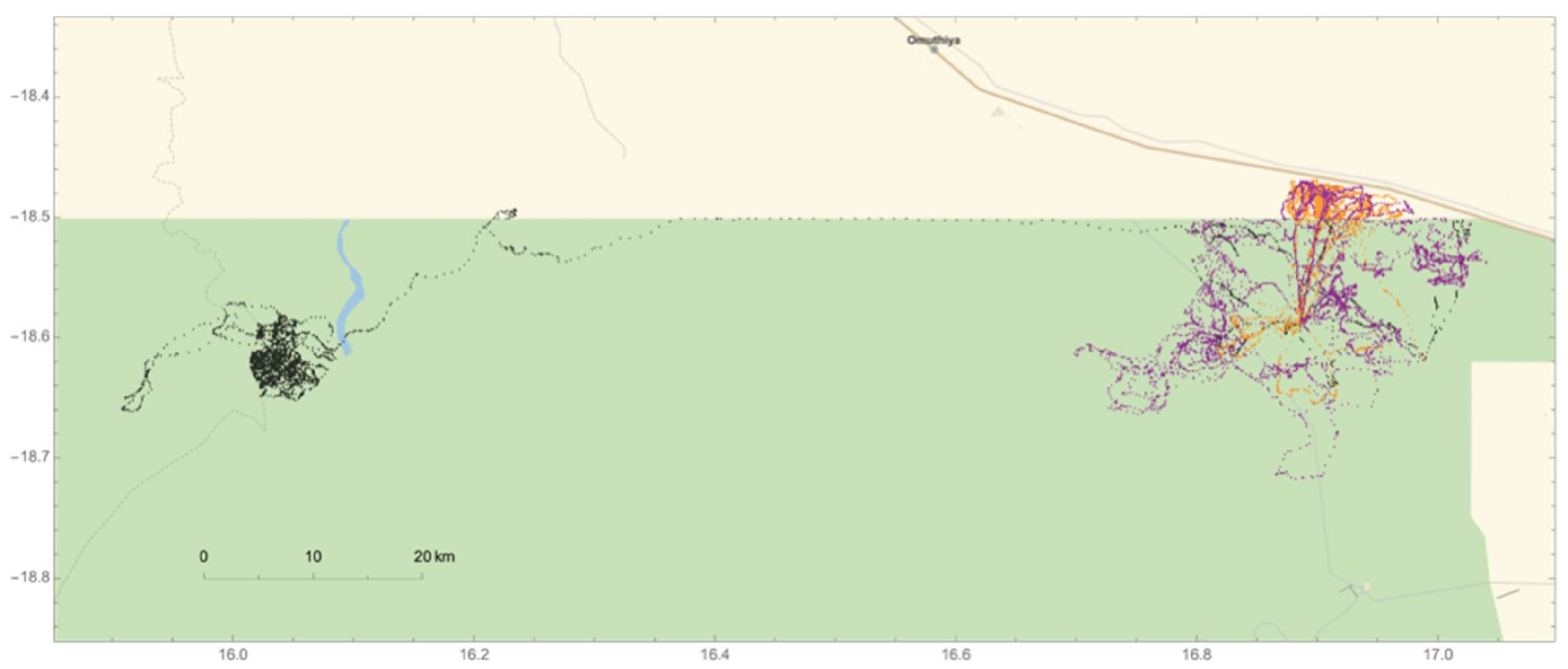
2.1. Study Site
2.2. Elephant Identification, Age Classification, and Musth Status
2.3. Estrous Call Playback Protocols
2.4. Response Scores and Behavioral Data Collection
2.5. Male Elephant Movement
2.6. Statistical Analyses
3. Results
3.1. Response Scores and the Duration of Behavioral Responses
3.2. Comparing Rates of Defecation and Musth Behaviors across Time Periods
3.3. Male Elephant Movement Patterns across Seasons and Reproductive States
4. Discussion
4.1. Factors of Decision-Making in Mature Adult Male Elephants
4.2. Young Adult Responses and the Potential Influence of Social and Environmental Conditions
4.3. Divergent Responses over Time in One Musth Male
4.4. Behaviors before and after Estrous Playbacks
4.5. The Spatial Intersection between Male Elephants and Human-Modified Landscapes
4.6. Considerations for Future Estrous Playback Studies and Applications
5. Conclusions
Future Directions of HEC Mitigation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emlen, S.T.; Oring, L.W. Ecology, Sexual Selection, and the Evolution of Mating Systems. Science 1977, 197, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Poole, J.H.; Lee, P.C.; Njiraini, N.; Moss, C.J. Longevity, Competition, and Musth: A Long-Term Perspective on Male Reproductive Strategies. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Moss, C.J., Croze, H.J., Lee, P.C., Eds.; University of Chicago Press: Chicago, IL, USA, 2011; pp. 272–288. ISBN 978-0-226-54226-3. [Google Scholar]
- Sukumar, R.; Gadgil, M. Male-Female Differences in Foraging on Crops by Asian Elephants. Anim. Behav. 1988, 36, 1233–1235. [Google Scholar] [CrossRef]
- Candolin, U. Reproduction under Predation Risk and the Trade-off between Current and Future Reproduction in the Threespine Stickleback. Proc. R. Soc. B Boil. Sci. 1998, 265, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Grignolio, S.; Rossi, I.; Bassano, B.; Apollonio, M. Predation Risk as a Factor Affecting Sexual Segregation in Alpine Ibex. J. Mammal. 2007, 88, 1488–1497. [Google Scholar] [CrossRef]
- Chiyo, P.I.; Lee, P.C.; Moss, C.J.; Archie, E.A.; Hollister-Smith, J.A.; Alberts, S.C. No Risk, No Gain: Effects of Crop Raiding and Genetic Diversity on Body Size in Male Elephants. Behav. Ecol. 2011, 22, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Poole, J. Musth and Male-Male Competition in the African Elephant. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1982. [Google Scholar]
- Clutton-Brock, T.H.; Albon, S.D.; Guinness, F.E. Great Expectations: Dominance, Breeding Success and Offspring Sex Ratios in Red Deer. Anim. Behav. 1986, 34, 460–471. [Google Scholar] [CrossRef]
- Meyer, J. Sexual Dimorphic Social Development and Female Intrasexual Chemical Signaling of African Elephants (Loxodonta africana). In Electronic Theses and Dissertations; Georgia Southern University: Statesboro, GA, USA, 2006. [Google Scholar]
- Poole, J.H.; Moss, C.J. Musth in the African Elephant, Loxodonta africana. Nature 1981, 292, 830–831. [Google Scholar] [CrossRef]
- Hall-Martin, A.J. Role of Musth in the Reproductive Strategy of the African Elephant (Loxodonta africana). S. Afr. J. Sci. 1987, 83, 616. [Google Scholar] [CrossRef]
- Poole, J.H. Rutting Behavior in African Elephants: The Phenomenon of Musth. Behaviour 1987, 102, 283–316. [Google Scholar] [CrossRef]
- Ganswindt, A.; Rasmussen, H.B.; Heistermann, M.; Hodges, J.K. The Sexually Active States of Free-Ranging Male African Elephants (Loxodonta africana): Defining Musth and Non-Musth Using Endocrinology, Physical Signals, and Behavior. Horm. Behav. 2005, 47, 83–91. [Google Scholar] [CrossRef]
- Schulte, B.A.; Rasmussen, L.E.L. Musth, Sexual Selection, Testosterone, and Metabolites. In Advances in Chemical Signals in Vertebrates; Johnston, R.E., Müller-Schwarze, D., Sorensen, P.W., Eds.; Springer: Boston, MA, USA, 1999; pp. 383–397. ISBN 978-1-4615-4733-4. [Google Scholar]
- Moss, C.; Poole, J. Relationships and Social Structure in African Elephants. In Primate Social Relationships: An Integrated Approach; Hinde, R.A., Ed.; Blackwell Scientific Publications: Oxford, UK, 1983; pp. 315–325. [Google Scholar]
- Croze, H.J.; Moss, C.J. Patterns of Occupancy in Time and Space. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Moss, C.J., Croze, H.J., Lee, P.C., Eds.; University of Chicago Press: Chicago, IL, USA, 2011; pp. 89–106. ISBN 978-0-226-54226-3. [Google Scholar]
- Wittemyer, G.; Getz, W.M.; Vollrath, F.; Douglas-Hamilton, I. Social Dominance, Seasonal Movements, and Spatial Segregation in African Elephants: A Contribution to Conservation Behavior. Behav. Ecol. Sociobiol. 2007, 61, 1919–1931. [Google Scholar] [CrossRef]
- Harris, G.M.; Russell, G.J.; van Aarde, R.I.; Pimm, S.L. Rules of Habitat Use by Elephants Loxodonta africana in Southern Africa: Insights for Regional Management. Oryx 2008, 42, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Hahn, N.R.; Wall, J.; Denninger-Snyder, K.; Goss, M.; Sairowua, W.; Mbise, N.; Estes, A.B.; Ndambuki, S.; Mjingo, E.E.; Douglas-Hamiliton, I.; et al. Risk Perception and Tolerance Shape Variation in Agricultural Use for a Transboundary Elephant Population. J. Anim. Ecol. 2022, 91, 112–123. [Google Scholar] [CrossRef]
- O’Connell-Rodwell, C.E.; Rodwell, T.; Rice, M.; Hart, L.A. Living with the Modern Conservation Paradigm: Can Agricultural Communities Co-Exist with Elephants? A Five-Year Case Study in East Caprivi, Namibia. Biol. Conserv. 2000, 93, 381–391. [Google Scholar] [CrossRef]
- Douglas-Hamilton, I.; Krink, T.; Vollrath, F. Movements and Corridors of African Elephants in Relation to Protected Areas. Naturwissenschaften 2005, 92, 158–163. [Google Scholar] [CrossRef]
- Galanti, V.; Preatoni, D.; Martinoli, A.; Wauters, L.A.; Tosi, G. Space and Habitat Use of the African Elephant in the Tarangire–Manyara Ecosystem, Tanzania: Implications for Conservation. Mamm. Biol. 2006, 71, 99–114. [Google Scholar] [CrossRef]
- Ngene, S.M.; Van Gils, H.; Van Wieren, S.E.; Rasmussen, H.; Skidmore, A.K.; Prins, H.H.T.; Toxopeus, A.G.; Omondi, P.; Douglas-Hamilton, I. The Ranging Patterns of Elephants in Marsabit Protected Area, Kenya: The Use of Satellite-Linked GPS Collars. Afr. J. Ecol. 2010, 48, 386–400. [Google Scholar] [CrossRef]
- Osborn, F.; Parker, G. Linking Two Elephant Refuges with a Corridor in the Communal Lands of Zimbabwe. Afr. J. Ecol. 2003, 41, 68–74. [Google Scholar] [CrossRef]
- Graham, M.D.; Douglas-Hamilton, I.; Adams, W.M.; Lee, P.C. The Movement of African Elephants in a Human-Dominated Land-Use Mosaic. Anim. Conserv. 2009, 12, 445–455. [Google Scholar] [CrossRef]
- Barnes, R.F.W. Elephant Behaviour in a Semi-Arid Environment. Afr. J. Ecol. 1983, 21, 185–196. [Google Scholar] [CrossRef]
- Poole, J.H. Mate Guarding, Reproductive Success and Female Choice in African Elephants. Anim. Behav. 1989, 37, 842–849. [Google Scholar] [CrossRef]
- Whitehouse, A.M.; Schoeman, D.S. Ranging Behaviour of Elephants within a Small, Fenced Area in Addo Elephant National Park, South Africa. Afr. Zool. 2003, 38, 95–108. [Google Scholar] [CrossRef]
- Leggett, K.E.A. Home Range and Seasonal Movement of Elephants in the Kunene Region, Northwestern Namibia. Afr. Zool. 2006, 41, 17–36. [Google Scholar] [CrossRef]
- Poole, J.; Moss, C. Elephant Mate Searching: Group Dynamics and Vocal and Olfactory Communication. Proc. Symp. Zool. Soc. Lond. 1989, 61, 111–125. [Google Scholar]
- Hoare, R. African Elephants and Humans in Conflict: The Outlook for Co-Existence. Oryx 2000, 34, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, L.J.; Khadka, K.K.; Van Den Hoek, J.; Naithani, K.J. Human-Elephant Conflict: A Review of Current Management Strategies and Future Directions. Front. Ecol. Evol. 2019, 6, 235. [Google Scholar] [CrossRef] [Green Version]
- Mumby, H.S.; Plotnik, J.M. Taking the Elephants’ Perspective: Remembering Elephant Behavior, Cognition and Ecology in Human-Elephant Conflict Mitigation. Front. Ecol. Evol. 2018, 6, 122. [Google Scholar] [CrossRef] [Green Version]
- Chiyo, P.I.; Moss, C.J.; Alberts, S.C. The Influence of Life History Milestones and Association Networks on Crop-Raiding Behavior in Male African Elephants. PLoS ONE 2012, 7, e31382. [Google Scholar] [CrossRef]
- Hoare, R.E. Determinants of Human–Elephant Conflict in a Land-Use Mosaic. J. Appl. Ecol. 1999, 36, 689–700. [Google Scholar] [CrossRef]
- Hoare, R. Lessons from 20 Years of Human–Elephant Conflict Mitigation in Africa. Hum. Dimens. Wildl. 2015, 20, 289–295. [Google Scholar] [CrossRef]
- Sukumar, R. The Management of Large Mammals in Relation to Male Strategies and Conflict with People. Biol. Conserv. 1991, 55, 93–102. [Google Scholar] [CrossRef]
- Mutinda, M.; Chenge, G.; Gakuya, F.; Otiende, M.; Omondi, P.; Kasiki, S.; Soriguer, R.C.; Alasaad, S. Detusking Fence-Breaker Elephants as an Approach in Human-Elephant Conflict Mitigation. PLoS ONE 2014, 9, e91749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, R.M.; Henley, M.D.; Parrini, F. Elephant Movement Patterns in Relation to Human Inhabitants in and around the Great Limpopo Transfrontier Park. Koedoe 2015, 57, 7. [Google Scholar] [CrossRef] [Green Version]
- Nyirenda, V.R.; Lindsey, P.A.; Phiri, E.; Stevenson, I.; Chomba, C.; Namukonde, N.; Myburgh, W.J.; Reilly, B.K. Trends in Illegal Killing of African Elephants (Loxodonta africana) in the Luangwa and Zambezi Ecosystems of Zambia. Environ. Nat. Resour. Res. 2015, 5, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Okello, M.M.; Njumbi, S.J.; Kiringe, J.W.; Isiiche, J. Prevalence and Severity of Current Human-Elephant Conflicts in Amboseli Ecosystem, Kenya: Insights from the Field and Key Informants. Nat. Resour. 2014, 5, 462–477. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, N.; Williams, C.; Dhakal, M. Current Status of Asian Elephant in Nepal. Gajha 2011, 35, 87–92. [Google Scholar]
- Sukumar, R. Ecology of the Asian Elephant in Southern India. II. Feeding Habits and Crop Raiding Patterns. J. Trop. Ecol. 1990, 6, 33–53. [Google Scholar] [CrossRef]
- Palita, S.K.; Purohit, K. Human-Elephant Conflict: Case Studies from Orissa and Suggested Measures for Mitigation. 2008. Available online: https://www.researchgate.net/publication/202290784 (accessed on 2 September 2021).
- Mijele, D.; Obanda, V.; Omondi, P.; Soriguer, R.C.; Gakuya, F.; Otiende, M.; Hongo, P.; Alasaad, S. Spatio-Temporal Distribution of Injured Elephants in Masai Mara and the Putative Negative and Positive Roles of the Local Community. PLoS ONE 2013, 8, e71179. [Google Scholar] [CrossRef] [Green Version]
- Osborn, F.V.; Parker, G.E. Towards an Integrated Approach for Reducing the Conflict between Elephants and People: A Review of Current Research. Oryx 2003, 37, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Buchholtz, E.; Fitzgerald, L.; Songhurst, A.; McCulloch, G.; Stronza, A. Overlapping Landscape Utilization by Elephants and People in the Western Okavango Panhandle: Implications for Conflict and Conservation. Landsc. Ecol. 2019, 34, 1411–1423. [Google Scholar] [CrossRef]
- Hoare, R.E.; Toit, J.T.D. Coexistence between People and Elephants in African Savannas. Conserv. Biol. 1999, 13, 633–639. [Google Scholar] [CrossRef]
- Lee, P.C.; Graham, M.D. African Elephants and Human-Elephant Interactions: Implications for Conservation. Int. Zoo Yearb. 2006, 40, 9–19. [Google Scholar] [CrossRef]
- Soltis, J.; King, L.E.; Douglas-Hamilton, I.; Vollrath, F.; Savage, A. African Elephant Alarm Calls Distinguish between Threats from Humans and Bees. PLoS ONE 2014, 9, e89403. [Google Scholar] [CrossRef] [PubMed]
- Wijayagunawardane, M.P.B.; Short, R.V.; Samarakone, T.S.; Nishany, K.B.M.; Harrington, H.; Perera, B.V.P.; Rassool, R.; Bittner, E.P. The Use of Audio Playback to Deter Crop-Raiding Asian Elephants. Wildl. Soc. Bull. 2016, 40, 375–379. [Google Scholar] [CrossRef]
- Poole, J. The Behavioral Context of African Elephant Acoustic Communication. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Moss, C.J., Croze, H.J., Lee, P.C., Eds.; University of Chicago Press: Chicago, USA, 2011. [Google Scholar]
- King, L.E.; Douglas-Hamilton, I.; Vollrath, F. African Elephants Run from the Sound of Disturbed Bees. Curr. Biol. 2007, 17, R832–R833. [Google Scholar] [CrossRef] [Green Version]
- King, L.E. The Interaction between the African Elephant (Loxodonta africana africana) and the African Honey Bee (Apis mellifera scutellata) and Its Potential Application as an Elephant Deterrent. Ph.D. Thesis, University of Oxford, Oxford, UK, 2010. [Google Scholar]
- McComb, K.; Shannon, G.; Sayialel, K.N.; Moss, C. Elephants Can Determine Ethnicity, Gender, and Age from Acoustic Cues in Human Voices. Proc. Natl. Acad. Sci. USA 2014, 111, 5433–5438. [Google Scholar] [CrossRef] [Green Version]
- Langbauer, W.R.; Payne, K.B.; Charif, R.A.; Rapaport, L.; Osborn, F.V. African Elephants Respond to Distant Playbacks of Low-Frequency Conspecific Calls. J. Exp. Biol. 1991, 157, 35–46. [Google Scholar] [CrossRef]
- Thuppil, V.; Coss, R.G. Playback of Felid Growls Mitigates Crop-Raiding by Elephants Elephas maximus in Southern India. Oryx 2016, 50, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Moss, C.J. Oestrous Behaviour and Female Choice in the African Elephant. Behaviour 1983, 86, 167–195. [Google Scholar] [CrossRef]
- Allen, W.R. Ovulation, Pregnancy, Placentation and Husbandry in the African Elephant (Loxodonta africana). Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 821–834. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, T.B.; Göritz, F.; Hermes, R.; Reid, C.; Dehnhard, M.; Brown, J.L. Aspects of the Reproductive Biology and Breeding Management of Asian and African Elephants Elephas maximus and Loxodonta africana. Int. Zoo Yearb. 2006, 40, 20–40. [Google Scholar] [CrossRef]
- Poole, J.; Granli, P. The Elephant Ethogram: A Library of African Elephant Behaviour. Pachyderm 2021, 62, 105–111. [Google Scholar]
- Poole, J.H.; Payne, K.; Langbauer, W.R.; Moss, C.J. The Social Contexts of Some Very Low Frequency Calls of African Elephants. Behav. Ecol. Sociobiol. 1988, 22, 385–392. [Google Scholar] [CrossRef]
- Poole, J.H. Signals and Assessment in African Elephants: Evidence from Playback Experiments. Anim. Behav. 1999, 58, 185–193. [Google Scholar] [CrossRef]
- Thouless, C.R.; Dublin, H.T.; Blanc, J.J.; Skinner, D.P.; Daniels, T.E.; Taylor, R.D.; Maisels, F.; Fredrick, H.L.; Bouché, P. African Elephant Status Report 2016: An Update from the African Elephant Database; IUCN/SSC African Elephant Specialist Group: Gland, Switzerland, 2016. [Google Scholar]
- de Beer, Y.; Kilian, W.; Versfeld, W.; van Aarde, R.J. Elephants and Low Rainfall Alter Woody Vegetation in Etosha National Park, Namibia. J. Arid Environ. 2006, 64, 412–421. [Google Scholar] [CrossRef]
- Polansky, L.; Kilian, W.; Wittemyer, G. Elucidating the Significance of Spatial Memory on Movement Decisions by African Savannah Elephants Using State–Space Models. Proc. R. Soc. B Boil. Sci. 2015, 282, 20143042. [Google Scholar] [CrossRef] [Green Version]
- Craig, G.C.; Gibson, D.S.C.; Uiseb, K.H. Namibia’s Elephants—Population, Distribution and Trends. Pachyderm 2021, 62, 35–52. [Google Scholar]
- Thurber, M.I.; O’Connell-Rodwell, C.E.; Turner, W.C.; Nambandi, K.; Kinzley, C.; Rodwell, T.C.; Faulkner, C.T.; Felt, S.A.; Bouley, D.M. Effects of Rainfall, Host Demography, and Musth on Strongyle Fecal Egg Counts in African Elephants (Loxodonta africana) in Namibia. J. Wildl. Dis. 2011, 47, 172–181. [Google Scholar] [CrossRef]
- O’Connell-Rodwell, C.E.; Wood, J.D.; Kinzley, C.; Rodwell, T.C.; Alarcon, C.; Wasser, S.K.; Sapolsky, R. Male African Elephants (Loxodonta africana) Queue When the Stakes Are High. Ethol. Ecol. Evol. 2011, 23, 388–397. [Google Scholar] [CrossRef]
- O’Connell-Rodwell, C.E.; Wood, J.D.; Kinzley, C.; Rodwell, T.C.; Poole, J.H.; Puria, S. Wild African Elephants (Loxodonta africana) Discriminate between Familiar and Unfamiliar Conspecific Seismic Alarm Calls. J. Acoust. Soc. Am. 2007, 122, 823–830. [Google Scholar] [CrossRef]
- O’Connell-Rodwell, C.E.; Freeman, P.T.; Kinzley, C.; Sandri, M.N.; Berezin, J.L.; Wiśniewska, M.; Jessup, K.; Rodwell, T.C. A Novel Technique for Aging Male African Elephants (Loxodonta africana) Using Craniofacial Photogrammetry and Geometric Morphometrics. Mamm. Biol. 2022, in press. [Google Scholar]
- Kaul, M.P.; Armstrong, B.D. Visual Displays of Wild African Elephants during Musth. Mammalia 2002, 66, 159–172. [Google Scholar] [CrossRef]
- Poole, J.; Granli, P. Signals, Gestures and Behaviors of African Elephants. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Moss, C.J., Croze, H.J., Lee, P.C., Eds.; University of Chicago Press: Chicago, IL, USA, 2011. [Google Scholar]
- O’Connell-Rodwell, C.E.; Wood, J.D.; Rodwell, T.C.; Puria, S.; Partan, S.R.; Keefe, R.; Shriver, D.; Arnason, B.T.; Hart, L.A. Wild Elephant (Loxodonta africana) Breeding Herds Respond to Artificially Transmitted Seismic Stimuli. Behav. Ecol. Sociobiol. 2006, 59, 842–850. [Google Scholar] [CrossRef]
- Noldus, L.P.J.J. The Observer: A Software System for Collection and Analysis of Observational Data. Behav. Res. Methods Instrum. Amp Comput. 1991, 23, 415–429. [Google Scholar] [CrossRef]
- Grubb, T.G.; Eakle, W.L. Recording Wildlife Locations with the Universal Transverse Mercator (UTM) Grid System; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Ft. Collins, CO, USA, 1988; p. RM-RN-483. [Google Scholar]
- Chiyo, P.I.; Archie, E.A.; Hollister-Smith, J.A.; Lee, P.C.; Poole, J.H.; Moss, C.J.; Alberts, S.C. Association Patterns of African Elephants in All-Male Groups: The Role of Age and Genetic Relatedness. Anim. Behav. 2011, 81, 1093–1099. [Google Scholar] [CrossRef]
- Lee, P.C.; Poole, J.H.; Njiraini, N.; Sayialel, C.N.; Moss, C.J. Male Social Dynamics: Independence and Beyond. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; University of Chicago Press: Chicago, IL, USA, 2011; ISBN 978-0-226-54223-2. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Gamer, M.; Lemon, J.; Singh, I.F.P. Irr: Various Coefficients of Interrater Reliability and Agreement; 2019.
- Shrout, P.E.; Fleiss, J.L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Kumar, R. Comparative Analysis of Parametric and Non-Parametric Tests. J. Comput. Math. Sci. 2015, 6, 336–342. [Google Scholar]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Kitchen, C.M.R. Nonparametric versus Parametric Tests of Location in Biomedical Research. Am. J. Ophthalmol. 2009, 147, 571–572. [Google Scholar] [CrossRef] [Green Version]
- Poole, J.H. Announcing Intent: The Aggressive State of Musth in African Elephants. Anim. Behav. 1989, 37, 140–152. [Google Scholar] [CrossRef]
- Webber, C.E.; Sereivathana, T.; Maltby, M.P.; Lee, P.C. Elephant Crop-Raiding and Human–Elephant Conflict in Cambodia: Crop Selection and Seasonal Timings of Raids. Oryx 2011, 45, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Hollister-Smith, J.A.; Poole, J.H.; Archie, E.A.; Vance, E.A.; Georgiadis, N.J.; Moss, C.J.; Alberts, S.C. Age, Musth and Paternity Success in Wild Male African Elephants, Loxodonta africana. Anim. Behav. 2007, 74, 287–296. [Google Scholar] [CrossRef]
- Rasmussen, H.B.; Okello, J.B.A.; Wittemyer, G.; Siegismund, H.R.; Arctander, P.; Vollrath, F.; Douglas-Hamilton, I. Age- and Tactic-Related Paternity Success in Male African Elephants. Behav. Ecol. 2008, 19, 9–15. [Google Scholar] [CrossRef]
- Lee, P.C.; Lindsay, W.K.; Moss, C.J. Ecological Patterns of Variability in Demographic Rates. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; University of Chicago Press: Chicago, IL, USA, 2011; pp. 74–88. ISBN 978-0-226-54226-3. [Google Scholar]
- Parker, G.A. Assessment Strategy and the Evolution of Fighting Behaviour. J. Theor. Biol. 1974, 47, 223–243. [Google Scholar] [CrossRef]
- Slotow, R.; van Dyk, G.; Poole, J.; Page, B.; Klocke, A. Older Bull Elephants Control Young Males. Nature 2000, 408, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.H.; Kasman, L.H.; Ramsay, E.C.; Lasley, B.L. Musth and Urinary Testosterone Concentrations in the African Elephant (Loxodonta africana). Reproduction 1984, 70, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, L.E.L.; Hall-Martin, A.J.; Hess, D.L. Chemical Profiles of Male African Elephants, Loxodonta africana: Physiological and Ecological Implications. J. Mammal. 1996, 77, 422–439. [Google Scholar] [CrossRef] [Green Version]
- Moss, C. Elephant Memories: Thirteen Years in the Life of an Elephant Family; University of Chicago Press: Chicago, IL, USA, 1988; ISBN 978-0-226-14853-3. [Google Scholar]
- Poole, J.H. Elephants in Musth, Lust. Nat. Hist. 1987, 96, 46–55. [Google Scholar]
- Osborn, F.; Parker, G.E. Community-Based Methods to Reduce Crop Loss to Elephants: Experiments in the Communal Lands of Zimbabwe. Pachyderm 2002, 33, 32–38. [Google Scholar]
- King, L.E.; Lala, F.; Nzumu, H.; Mwambingu, E.; Douglas-Hamilton, I. Beehive fences as a multidimensional conflict-mitigation tool for farmers coexisting with elephants. Conserv. Biol. 2017, 31, 743–752. [Google Scholar] [CrossRef]
- Karidozo, M.; Osborn, F. Can Bees Deter Elephants from Raiding Crops? An Experiment in the Communal Lands of Zimbabwe. Pachyderm 2005, 39, 26–32. [Google Scholar]
- Ngama, S.; Korte, L.; Bindelle, J.; Vermeulen, C.; Poulsen, J.R. How Bees Deter Elephants: Beehive Trials with Forest Elephants (Loxodonta africana cyclotis) in Gabon. PLoS ONE 2016, 11, e0155690. [Google Scholar] [CrossRef] [PubMed]
- Bagley, K.R.; Goodwin, T.E.; Rasmussen, L.E.L.; Schulte, B.A. Male African Elephants, Loxodonta africana, Can Distinguish Oestrous Status via Urinary Signals. Anim. Behav. 2006, 71, 1439–1445. [Google Scholar] [CrossRef]
- Bagley, K.R. Chemosensory Behavior and Development of African Male Elephants (Loxodonta africana). In Electronic Theses and Dissertations; Georgia Southern University: Statesboro, GA, USA, 2004. [Google Scholar]
- Schulte, B.A.; Freeman, E.W.; Goodwin, T.E.; Hollister-Smith, J.; Rasmussen, L.E.L. Honest Signalling through Chemicals by Elephants with Applications for Care and Conservation. Appl. Anim. Behav. Sci. 2007, 102, 344–363. [Google Scholar] [CrossRef]
- Karimi, R.R. An Assessment of Perceived Crop Damage in a Tanzanian Village Impacted by Human-Elephant Conflict and an Investigation of Deterrent Properties of African Elephant (Loxodonta africana) Exudates Using Bioassays. In Electronic Theses and Dissertations; Georgia Southern University: Statesboro, GA, USA, 2009. [Google Scholar]
- Schulte, B.A.; LaDue, C.A. The Chemical Ecology of Elephants: 21st Century Additions to Our Understanding and Future Outlooks. Animals 2021, 11, 2860. [Google Scholar] [CrossRef] [PubMed]







| Age Class 1 | 1Q | 2Q | 3Q | 4Q |
|---|---|---|---|---|
| Relative Age (years) | 10–14 | 15–24 | 25–34 | ≥35 |
| Musth | 0 | 0 | 0 | 5 |
| Non-musth | 2 | 1 | 5 | 6 |
| Context | Focal Behaviors | Description |
|---|---|---|
| Attentiveness, Vigilance | Freeze | Upon presentation of estrous playbacks, elephant immediately stops all movement, spreads ears, leans toward, and appears to listen to the stimulus. |
| Over the Shoulder | Without orienting his body, the elephant glances back over his shoulder in the direction of the hidden speaker. This is often accompanied by the elephant discretely smelling in the direction of the acoustic source with his trunk hovering above the ground. | |
| Ears Held Out | Elephant spreads ears and appears alert while listening. | |
| Smell in Direction of Speaker | Elephant smells in the direction of the speaker, either by discretely smelling with trunk hovering above the ground, or using ‘Periscope Trunk,’ with trunk extended high or above their head toward the acoustic stimulus source. | |
| Orientation | Elephant turns his entire body toward the hidden speaker broadcasting estrous calls in the distance. This behavior is followed by returning to their original position and/or approaching the sound source. | |
| Attraction | Approach | Elephant walks eagerly and intentionally in the direction of the speaker. |
| Track | Individual searches for the source of the estrous call, often with a purposeful walk. Elephant is also observed smelling in multiple directions while sweeping his trunk across the ground with ears held out. This behavior is also characterized by frequent repositioning of the elephant’s body in relation to the sound source, including perpendicular and parallel to the object of interest, potentially in attempt to localize the signal. | |
| Musth Display | Elephant advertises his reproductive status via temporal gland secretions, urine dribbling, ear waving, trunk curling, trunk dragging, musth walking, and tusking the ground. | |
| Social Cohesion | Follow | Elephant moves behind and in the same direction as a conspecific that is often older, higher ranking, and/or socially bonded with him. The individual leading the movement may solicit following behavior through vocal and tactile cues and may also be observed waiting for the follower. |
| Avoidance | Retreat | Elephant moves quickly in the opposite direction of a perceived threat. |
| Predictor | Sum of Squares | Df | Mean Squares | F | Pr (>F) |
|---|---|---|---|---|---|
| Male Group | 0.957 | 2 | 0.478 | 7.43 | 0.005 ** |
| Residuals | 1.030 | 16 | 0.064 | ||
| Post Hoc Comparisons | Mean Difference | 95% Confidence Interval | Adjusted p-value | ||
| Lower | Upper | ||||
| Musth 4Q – Non-musth 4Q | 0.523 | 0.126 | 0.919 | 0.009 ** | |
| Musth 4Q – Non-musth 1Q–3Q | −0.072 | −0.445 | 0.301 | 0.874 | |
| Non-musth 4Q – Non-musth 1Q–3Q | 0.451 | 0.097 | 0.804 | 0.012 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connell-Rodwell, C.E.; Sandri, M.N.; Berezin, J.L.; Munevar, J.M.; Kinzley, C.; Wood, J.D.; Wiśniewska, M.; Kilian, J.W. Male African Elephant (Loxodonta africana) Behavioral Responses to Estrous Call Playbacks May Inform Conservation Management Tools. Animals 2022, 12, 1162. https://doi.org/10.3390/ani12091162
O’Connell-Rodwell CE, Sandri MN, Berezin JL, Munevar JM, Kinzley C, Wood JD, Wiśniewska M, Kilian JW. Male African Elephant (Loxodonta africana) Behavioral Responses to Estrous Call Playbacks May Inform Conservation Management Tools. Animals. 2022; 12(9):1162. https://doi.org/10.3390/ani12091162
Chicago/Turabian StyleO’Connell-Rodwell, Caitlin E., Monica N. Sandri, Jodie L. Berezin, Jaquelyn M. Munevar, Colleen Kinzley, Jason D. Wood, Maggie Wiśniewska, and J. Werner Kilian. 2022. "Male African Elephant (Loxodonta africana) Behavioral Responses to Estrous Call Playbacks May Inform Conservation Management Tools" Animals 12, no. 9: 1162. https://doi.org/10.3390/ani12091162
APA StyleO’Connell-Rodwell, C. E., Sandri, M. N., Berezin, J. L., Munevar, J. M., Kinzley, C., Wood, J. D., Wiśniewska, M., & Kilian, J. W. (2022). Male African Elephant (Loxodonta africana) Behavioral Responses to Estrous Call Playbacks May Inform Conservation Management Tools. Animals, 12(9), 1162. https://doi.org/10.3390/ani12091162







