Muscle Fatty Acids, Meat Flavor Compounds and Sensory Characteristics of Xiangxi Yellow Cattle in Comparison to Aberdeen Angus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Muscle Sampling
2.2. Proximate Composition Analysis
2.3. Free Amino Acid Analysis
2.4. Analysis of 5′-Nucleotides
2.5. Fatty Acid Analysis
2.6. Volatile Compounds Analysis
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
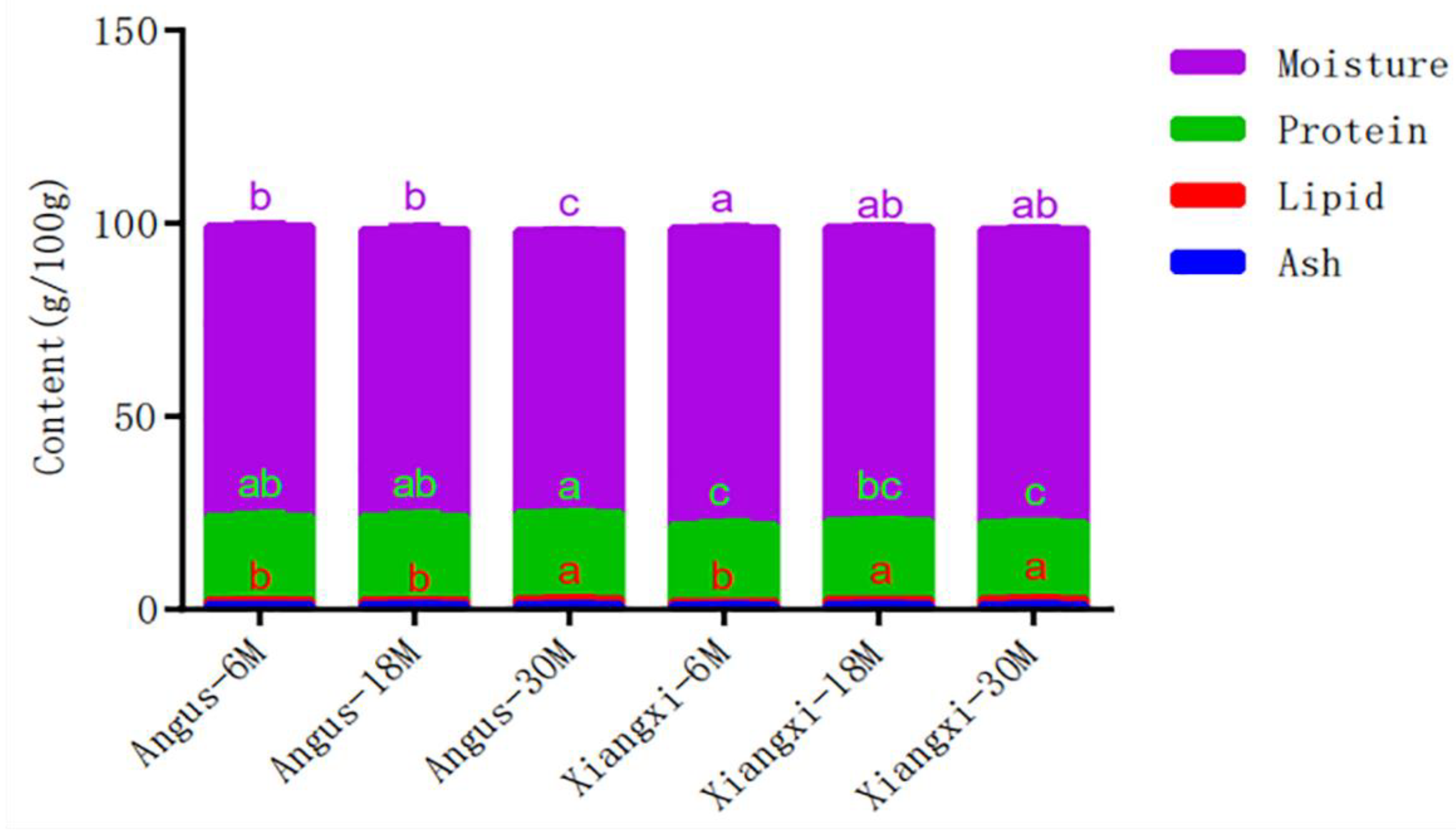
3.1. Proximate Composition
3.2. Free Amino Acids
3.3. 5’-nucleotides
3.4. Fatty Acid Content
3.5. Volatile Compounds
3.6. Sensory Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambaz, A.; Scheeder, M.L.; Kreuzer, M.; Dufey, P.A. Meat quality of Angus, Simmental, Charolais and Limousin steers compared at the same intramuscular fat content. Meat Sci. 2003, 63, 491–500. [Google Scholar] [CrossRef]
- Sami, A.S.; Augustin, C.; Schwarz, F.J. Effects of feeding intensity and time on feed on performance, carcass characteristics and meat quality of Simmental bulls. Meat Sci. 2004, 67, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.X.; Zan, L.S.; Li, L.X.; Men, J.W. Studies on the tenderization effects of lactic acid on Qin-chuan beef at different ages. J. Northwest Agric. For. Univ. 2009, 37, 30–35. [Google Scholar] [CrossRef]
- Choat, W.T.; Paterson, J.A.; Rainey, B.M.; King, M.C.; Smith, G.C.; Belk, K.E.; Lipsey, R.J. The effects of cattle sex on carcass character istics and longissimus muscle palatability. J. Anim. Sci. 2006, 84, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Huertas, S.M.; Gil, A.D.; Piaggio, J.M.; Van Eerdenburg, F.M. Transportation of beef cattle to slaughterhouses and how this relates to animal welfare and carcase bruising in an extensive production system. Anim. Welf. 2010, 19, 281–285. [Google Scholar]
- Irshad, A.; Gurunathan, K.; Kumar, S.; Kumar, A.; Kumar, A.; Vishnuraj, M.R.; Shukla, V. Factors Influencing Carcass Composition of Livestock: A Review. J. Anim. Prod. Adv. 2012, 3, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Warner, R. Sensory and Flavor Chemistry Characteristics of Australian Beef: Influence of Intramuscular Fat, Feed, and Breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef]
- Bures, D.; Barton, L. Performance, carcass traits and meat quality of Aberdeen Angus, Gascon, Holstein and Fleckvieh finishing bulls. Livest. Sci. 2018, 214, 231–237. [Google Scholar] [CrossRef]
- Li, J.B.; Sun, A.; Long, Y.; He, F.; Huang, C.; Li, S.; Luo, Y.; Li, H.B.; Hou, Z.P.; Dong, C. Effects of age on production per formance and meat quality of xiangxi cattle. Pak. J. Agric. Sci. 2020, 57, 1437–1445. [Google Scholar]
- Picard, B.; Gagaoua, M.; Al Jammas, M.; Bonnet, M. Beef tenderness and intramuscular fat proteomic biomarkers: Effect of gender and rearing practices. J. Proteom. 2019, 200, 1–10. [Google Scholar] [CrossRef]
- Long, Z.C.; Ma, W.J. Characteristics and utilization of Chinese Xiangxi cattle. Mod. Agric. Sci. Technol. 2009, 16, 274–275. [Google Scholar]
- Yang, X.L.; Peng, B.; Tian, Q.B.; Yan, P.Q.; Qin, Y.Y. Comparison of body size and weight between Xiangxi yellow cattle and cross bred with Simmental cattle. Hunan J. Anim. Sci. Vet. Med. 2007, 5, 23–24. [Google Scholar]
- Li, J.B.; Li, Z.C.; Xu, M.L.; Liu, Y.Y.; Yao, M.Q.; Yi, K.L. Fattening performance and meat quality of hybridized combinations of Xiangxi cattle. China Herbiv. Sci. 2012, 32, 20–24. [Google Scholar]
- Su, J.K.; Wang, X.; Lu, J.Q.; Qiao, Y.J.; Gao, X.Y. Determination of Amino Acids with OPA-FMOC by On-line Derivatization. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 135–138. [Google Scholar]
- Okruszek, A.; Woloszyn, J.; Haraf, G.; Orkusz, A.; Werenska, M. Chemical composition and amino acid profiles of goose muscles from native Polish breeds. Poult. Sci. 2013, 92, 1127–1133. [Google Scholar] [CrossRef]
- Guo, Q.; Kong, X.F.; Hu, C.J.; Zhou, B.; Wang, C.T.; Shen, Q.W. Fatty Acid Content, Flavor Compounds, and Sensory Quality of Pork Loin as Affected by Dietary Supplementation with l-arginine and Glutamic Acid. J. Food Sci. 2019, 84, 3445–3453. [Google Scholar] [CrossRef]
- Yu, W.J.; Hou, J.W.; Zhu, B.S. Accurate determination of fourteen fatty acids in pork by external standard-gas chromatography-mass spectrometry. Anal. Instrum. 2012, 3, 10–16. [Google Scholar]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Clinquart, A.; Ellies-Oury, M.P.; Hocquette, J.F.; Guillier, L.; Santé-Lhoutellier, V.; Prache, S. Review: On-farm and processing factors affecting bovine carcass and meat quality. Animal 2022, 16 (Suppl. 1), 100426. [Google Scholar] [CrossRef]
- Oberli, M.; Lan, A.; Khodorova, N.; Sante-Lhoutellier, V.; Walker, F.; Piedcoq, J.; Gaudichon, C. Compared with Raw Bovine Meat, Boiling but Not Grilling, Barbecuing, or Roasting Decreases Protein Digestibility without Any Major Consequences for Intestinal Mucosa in Rats, although the Daily Ingestion of Bovine Meat Induces Histologic Modifications in the Colon. J. Nutr. 2016, 146, 1506–1513. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, A.S.; Uneyama, H. Amino acid sensing in the gastrointestinal tract. Amino Acids 2013, 45, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Toshihide, N.; Mee, R.R.; Akihiro, O.; Hiromichi, K. Components Contributing to the Improvement of Meat Taste during Stor age. Biosci. Biotechnol. Biochem. 1988, 52, 2323–2330. [Google Scholar] [CrossRef]
- Zhuang, K.J.; Wu, N.; Wang, X.C.; Wu, X.G.; Wang, S.; Long, X.W.; Wei, X. Effects of 3 Feeding Modes on the Volatile and Nonvolatile Compounds in the Edible Tissues of Female Chinese Mitten Crab (Eriocheir sinensis). J. Food Sci. 2016, 81, S968–S981. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.A.; Kang, D.R.; Belal, S.A.; Cho, E.; Jung, J.; Jung, Y.; Choi, Y.; Shim, K. Meat Quality and Physicochemical Trait Assessments of Berkshire and Commercial 3-way Crossbred Pigs. Food Sci. Anim. Resour. 2016, 36, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Hollo, G.; Csapo, J.; Szucs, E.; Tozser, J.; Repa, I.; Hollo, I. Influence of breed, slaughter weight and gender on chemical composition of beef. Part 1. Amino acid profile and biological value of proteins. Anim. Biosci. 2001, 14, 1555–1559. [Google Scholar] [CrossRef]
- Hollo, G.; Nuernberg, K.; Hollo, I.; Csapo, J.; Seregi, J.; Repa, I.; Ender, K. Effect of feeding on the composition of longissmus muscle of Hungarian Grey and Holstein Friesian bulls-III. Amino acid composition and mineral content. Arch. Anim. Breed. 2007, 50, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Zainal, S.; Siti Roha, A.M.; Noriham, A. Amino acid composition of droughtmaster beef at various beef cuts. Agric. Sci. 2013, 4, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Rhue, M.R.; Tajima, T.; Kato, H. Mechanism of the increase of free amino acids during the storage of meats. In Proceedings of the 42nd International Congress of Meat Science and Technology, Lillehammer, Norway, 22–25 August 1996; pp. 412–413. [Google Scholar]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.L.; Liu, W.L.; Zhao, L.M. Novel umami ingredients: Umami peptides and their taste. J. Food Sci. 2017, 82, 16–23. [Google Scholar] [CrossRef]
- Beppu, K.; Shono, H.; Kawakami, A.; Takashi, T.; Watanabe, S.; Yoshida, A.; Kuroda, M.; Fujimoto, C.; Kanamura, R.; Ohnishi, H.; et al. Dietary supplementation with monosodium glutamate with dietary balance such as protein, salt and sugar intake with increasing T1R3 taste receptor gene expression in healthy females. J. Med. Investig. 2021, 68, 315–320. [Google Scholar] [CrossRef]
- Tikk, M.; Tikk, K.; Torngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of inosine monophosphate and its degradation products during aging of pork of different qualities in relation to basic taste and retronasal flavor perception of the meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef]
- Rotola-Pukkila, M.K.; Pihlajaviita, S.T.; Kaimainen, M.T.; Hopia, A.I. Concentration of Umami Compounds in Pork Meat and Cooking Juice with Different Cooking Times and Temperatures. J. Food Sci. 2015, 80, C2711–C2716. [Google Scholar] [CrossRef] [PubMed]
- Shizuko, Y.; Tomoko, Y.; Shingo, I.; Tsunehiko, N. Measurement of the relative taste intensity of some l-α-amino acids and 5′-nucleotides. J. Food Sci. 1971, 36, 846–849. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Mezgebo, G.B.; Monahan, F.J.; McGee, M.; O’Riordan, E.G.; Richardson, I.R.; Brunton, N.P.; Moloney, A.P. Fatty acid, volatile and sensory characteristics of beef as affected by grass silage or pasture in the bovine diet. Food Chem. 2017, 235, 86–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Legako, J.F.; Brooks, J.C.; O’Quinn, T.G.; Hagan, T.J.; Polkinghorne, R.; Farmer, L.J.; Miller, M.F. Consumer palatability scores and volatile beef flavor compounds of five USDA quality grades and four muscles. Meat Sci. 2015, 100, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Ba, H.V.; Seo, H.; Seong, P.; Cho, S.; Kang, S.; Kim, Y.; Kim, J. Live weights at slaughter significantly affect the meat quality and flavor components of pork meat. Anim. Sci. J. 2019, 90, 667–679. [Google Scholar] [CrossRef]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Insani, E.M.; Biolatto, A.; Sancho, A.M.; Garcia, P.T.; Pensel, N.A.; Josifovich, J.A. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Sci. 2005, 70, 35–44. [Google Scholar] [CrossRef]
- Elmore, J.S.; Cooper, S.L.; Enser, M.; Donald, S.; Sinclair, L.A.; Wilkinson, R.G.; Wood, J.D. Dietary manipulation of fatty acid composition in lamb meat and its effect on the volatile aroma compounds of grilled lamb. Meat Sci. 2005, 69, 233–242. [Google Scholar] [CrossRef]
- Flores, M.; Toldra, F.; Spanier, A.M.; Grimm, C.C. Correlations of sensory and volatile compounds of spanish serrano dry-cured ham as a function of two processing times. J. Agric. Food Chem. 1997, 45, 2178–2186. [Google Scholar] [CrossRef]
- Cano-Garcia, L.; Rivera-Jimenez, S.; Belloch, C.; Flores, M. Generation of aroma compounds in a fermented sausage meat model system by Debaryomyces hansenii strains. Food Chem. 2014, 151, 364–373. [Google Scholar] [CrossRef]
- Jeremiah, L.E.; Smith, G.C.; Carpenter, Z.L. Palatability of individual muscles from ovine leg steaks as related to chronological age and marbling. J. Food Sci. 1971, 36, 45–47. [Google Scholar] [CrossRef]
- Brekke, C.J.; Wellington, G.H. Effect of Animal Weight on Palatability of Veal Leg Roasts. J. Anim. Sci. 1972, 35, 937–940. [Google Scholar] [CrossRef]
- Miller, R.K.; Moeller, S.J.; Goodwin, R.N.; Lorenzen, C.L.; Savell, J.W. Consistency in meat quality. In Proceedings of the 46th International Congress of Meat Science & Technology, Buenos Aires, Argentina, 27 August–1 September 2000; pp. 566–580. [Google Scholar]

| Free Amino Acids | Threshold (mg/100 g) | Taste Attribute | Angus | Xiangxi Yellow Cattle | s.e.m. | Sig. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 Month | 18 Month | 30 Month | 6 Month | 18 Month | 30 Month | Breed | Month | B × M | ||||
| Asp | 100 | Uma (+) | 0.98 | 0.98 | 0.78 | N.D. | 1.53 | 1.43 | 0.16 | — | — | —. |
| Glu | 30 | Uma (+) | 5.45 | 5.52 | 5.88 | 5.77 | 4.58 | 3.70 | 0.32 | n.s. | n.s. | n.s. |
| Asn | 100 | Uma (+) | 3.13 abc | 3.93 ab | 4.68 a | 2.02 bc | 1.23 c | 1.69 c | 0.41 | ** | n.s. | n.s. |
| Ser | 150 | Swt (+) | 3.94 | 5.34 | 4.38 | 5.06 | 4.23 | 5.50 | 0.24 | n.s. | n.s. | n.s. |
| Gln | — | — | 30.97 | 45.04 | 51.81 | 33.56 | 38.09 | 40.03 | 2.62 | n.s. | n.s. | n.s. |
| His | 20 | Bit (−) | 1.24 | 1.80 | 0.86 | 1.60 | N.D. | N.D. | 0.22 | — | — | — |
| Gly | 130 | Swt (+) | 11.84 b | 17.21 ab | 11.50 b | 23.37 a | 18.12 ab | 19.38 a | 1.38 | ** | n.s. | n.s. |
| Thr | 260 | Swt (+) | 3.59 | 4.38 | 3.20 | 3.70 | 3.02 | 3.70 | 0.22 | n.s. | n.s. | n.s. |
| Cit | — | — | N.D. | N.D. | N.D. | 0.63 | N.D. | N.D. | 0.07 | — | — | — |
| Arg | 50 | Bit/Swt (+) | 5.39 | 5.26 | 5.03 | 4.80 | 5.09 | 7.91 | 0.43 | n.s. | n.s. | n.s. |
| Ala | 60 | Swt (+) | 148.23 ab | 156.12 a | 136.36 ab | 96.85 b | 115.83 ab | 90.65 b | 8.87 | * | n.s. | n.s. |
| Tyr | — | Bit (−) | 2.56 | 4.56 | 3.65 | 3.90 | 3.32 | 2.47 | 0.36 | n.s. | n.s. | n.s. |
| Cys | — | Bit/Swt/Sul (-) | 2.09 d | 2.74 cd | 2.27 d | 3.32 c | 4.21 b | 5.49 a | 0.37 | *** | ** | * |
| Val | 40 | Swt/Bit (−) | 6.05 a | 7.01 a | 5.72 a | 2.75 b | 2.82 b | 4.53 ab | 0.53 | ** | n.s. | n.s. |
| Met | 30 | Bit/Swt/Sul (-) | 3.45 ab | 4.70 a | 3.59 ab | 1.84 bc | 2.02 bc | 1.37 c | 0.39 | ** | n.s. | n.s. |
| Nva | — | — | 48.26 a | 47.71 a | 38.56 a | 10.43 b | 10.87 b | 9.29 b | 5.51 | *** | n.s. | n.s. |
| Trp | — | — | 10.80 | 5.29 | 8.54 | N.D. | N.D. | N.D. | 1.33 | — | — | — |
| Phe | 90 | Bit (−) | 9.70 b | 10.81 ab | 9.65 b | 12.93 a | 11.84 ab | 11.23 ab | 0.44 | * | n.s. | n.s. |
| Ile | 90 | Bit (−) | 6.60 | 7.87 | 6.85 | 6.86 | 5.95 | 6.21 | 0.36 | n.s. | n.s. | n.s. |
| Leu | 190 | Bit (−) | 8.68 a | 10.04 a | 8.82 a | 4.15 b | 3.89 b | 3.65 b | 0.85 | *** | n.s. | n.s. |
| Lys | 50 | Swt/Bit (−) | 3.69 | 4.62 | 2.75 | 2.21 | 2.39 | 2.23 | 0.35 | n.s. | n.s. | n.s. |
| Hyp | — | — | 0.84 | 1.02 | 0.82 | 1.02 | 0.56 | 1.03 | 0.08 | n.s. | n.s. | n.s. |
| Sar | — | — | 0.69 b | 0.50 b | 1.89 a | 0.56 b | 0.79 b | 1.92 a | 0.18 | n.s. | *** | n.s. |
| Pro | 300 | Swt/Bit (−) | 1.78 | 2.21 | 1.17 | 2.58 | 1.14 | 1.23 | 0.20 | n.s. | n.s. | n.s. |
| Umami AA | — | — | 9.56 b | 10.43 ab | 11.34 a | 7.79 c | 7.34 c | 6.82 c | 0.52 | *** | n.s. | n.s. |
| Sweet AA | — | — | 179.10 ab | 196.88 a | 165.07 ab | 136.51 b | 147.54 ab | 127.21 b | 8.80 | ** | n.s. | n.s. |
| Bitter AA | — | — | 39.69 | 47.77 | 40.71 | 39.38 | 36.31 | 38.32 | 1.61 | n.s. | n.s. | n.s. |
| Total | — | — | 319.90 a | 354.61 a | 318.71 a | 236.86 b | 241.47 b | 224.60 b | 16.23 | ** | n.s. | n.s. |
| Threshold (μg/g) | Angus | Xiangxi Yellow Cattle | s.e.m. | Sig. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 Month | 18 Month | 30 Month | 6 Month | 18 Month | 30 Month | Breed | Month | B × M | |||
| The concentrations of 5′-nucleotide and EUC | |||||||||||
| 5′-GMP | 125 | 56.19 b | 50.86 b | 50.46 b | 34.09 c | 60.95 b | 71.88 a | 3.57 | n.s. | ** | ** |
| 5′-IMP | 250 | 1636.27 bc | 1901.33 a | 1810.32 ab | 1155.67 d | 1930.63 a | 1566.70 c | 81.19 | ** | *** | * |
| 5′-AMP | 500 | 104.46 a | 89.50 b | 87.96 b | 77.96 b | 90.82 b | 105.08 a | 3.09 | n.s. | n.s. | ** |
| EUC | 300 | 1.20 ab | 1.39 a | 1.42 a | 0.89 b | 1.20 ab | 0.82 b | 0.09 | * | n.s. | n.s. |
| TAV of 5′-Nucleotide and EUC | |||||||||||
| 5′-GMP | 125 | 0.45 b | 0.41 b | 0.41 b | 0.28 c | 0.49 b | 0.58 a | 0.03 | * | *** | *** |
| 5′-IMP | 250 | 6.55 bc | 7.61 a | 7.24 ab | 4.62 d | 7.73 a | 6.27 c | 0.33 | ** | *** | ** |
| 5′-AMP | 500 | 0.21 a | 0.18 ab | 0.17 b | 0.16 b | 0.18 ab | 0.21 a | 0.01 | n.s. | n.s. | ** |
| EUC | 300 | 39.98 ab | 46.25 a | 47.24 a | 29.46 b | 39.87 ab | 27.26 b | 2.86 | * | n.s. | n.s. |
| Fatty Acids | Angus | Xiangxi Yellow Cattle | s.e.m. | Sig. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 Month | 18 Month | 30 Month | 6 Month | 18 Month | 30 Month | Breed | Month | B × M | ||
| C10: 0 | 1.85 bc | 2.75 a | 3.01 a | 1.58 c | 2.87 a | 2.12 b | 0.17 | ** | *** | ** |
| C12: 0 | 1.73 e | 2.94 c | 3.54 b | 2.32 d | 5.36 a | 2.55 d | 0.35 | *** | *** | *** |
| C13: 0 | 0.37 | 0.22 | 0.49 | N.D. | 0.79 | 0.38 | 0.12 | — | — | — |
| C14: 0 | 9.20 d | 21.13 c | 34.93 a | 7.67 d | 28.52 b | 34.04 a | 3.32 | n.s. | *** | ** |
| C14: 1 | 4.42 b | 6.27 b | 6.00 b | 11.23 a | 9.50 a | 8.87 a | 0.73 | *** | n.s. | n.s. |
| C15: 0 | 6.52 d | 11.67 c | 10.93 c | 13.96 b | 15.02 b | 22.26 a | 1.45 | *** | *** | *** |
| C16: 0 | 156.37 d | 193.69 bc | 215.25 ab | 166.03 cd | 166.83 cd | 225.73 a | 8.24 | n.s. | ** | n.s. |
| C16: 1 | 3.08 c | 3.42 c | 3.68 c | 4.66 bc | 8.18 a | 5.59 b | 0.54 | *** | * | * |
| C17: 0 | 24.63 c | 48.16 b | 42.83 b | 40.58 b | 43.91 b | 67.91 a | 3.91 | ** | *** | ** |
| C17: 1 | 7.51 de | 9.48 cd | 10.56 bc | 6.33 e | 11.67 b | 14.98 a | 0.87 | ** | *** | ** |
| C18: 0 | 157.92 bc | 163.89 bc | 185.35 ab | 117.63 d | 136.18 cd | 200.65 a | 8.82 | * | ** | * |
| C18: 1 n9 t | 1.24 | 0.96 | 1.13 | 1.59 | 1.45 | 1.67 | 0.10 | n.s. | n.s. | n.s. |
| C18: 1 n9 c | 313.13 c | 318.21 c | 685.23 a | 233.24 c | 497.92 b | 675.58 a | 54.41 | n.s. | *** | ** |
| C18: 2 n6 | 38.41 b | 87.66 a | 72.87 a | 53.37 b | 74.86 a | 52.12 b | 5.17 | n.s. | *** | * |
| C18: 3 n3 | 4.42 d | 5.20 d | 6.19 bc | 4.92 d | 10.64 ab | 13.63 a | 1.11 | ** | * | n.s. |
| C19: 0 | 0.93 d | 1.91 c | 4.79 a | 3.25 b | 3.89 b | 4.68 a | 0.43 | *** | *** | ** |
| C20: 0 | 1.55 d | 1.67 d | 3.52 b | 2.65 c | 5.02 a | 2.93 c | 0.36 | *** | *** | *** |
| C20: 1 | 5.02 b | 5.13 b | 5.14 b | 7.98 b | 14.71 a | 6.68 b | 1.07 | ** | ** | * |
| C20: 3 n6 | 14.46 d | 22.64 bc | 15.26 cd | 24.02 b | 33.22 a | 17.80 bcd | 1.99 | ** | ** | n.s. |
| C20: 4 n6 | 9.13 ab | 6.88 b | 9.28 ab | 12.12 a | 12.26 a | 5.81 b | 0.78 | n.s. | * | ** |
| C20: 5 n3 | 3.66 ab | 5.07 a | 2.97 ab | 4.78 a | 5.28 a | 1.63 b | 0.41 | n.s. | ** | n.s. |
| C22: 6 n3 | 13.66 | 14.48 | 14.28 | 17.03 | 16.78 | 16.29 | 0.65 | n.s. | n.s. | n.s. |
| SFA | 361.04 d | 447.98 bc | 504.62 ab | 355.64 d | 408.37 cd | 563.23 a | 23.14 | n.s. | *** | n.s. |
| MUFA | 334.39 c | 343.46 c | 711.72 a | 265.03 c | 543.41 b | 713.36 a | 55.34 | n.s. | *** | ** |
| PUFA | 83.73 d | 141.93 ab | 120.84 bc | 116.24 bc | 153.03 a | 107.27 cd | 7.02 | * | *** | * |
| PUFA/SFA | 0.23 b | 0.32 a | 0.24 b | 0.33 a | 0.37 a | 0.19 b | 0.02 | n.s. | ** | * |
| ∑n − 3 | 21.73 b | 24.75 ab | 23.44 ab | 26.73 ab | 32.70 a | 31.54 a | 1.45 | * | n.s. | n.s. |
| ∑n − 6 | 62.00 d | 117.19 a | 97.41 b | 89.51 bc | 120.34 a | 75.73 cd | 6.47 | n.s. | *** | ** |
| ∑n − 6/∑n − 3 | 2.87 cd | 4.77 a | 4.16 ab | 3.35 bcd | 3.76 abc | 2.42 d | 0.26 | * | * | * |
| Total | 779.16 d | 933.36 c | 1337.18 a | 736.90 d | 1104.81 b | 1383.85 a | 76.52 | * | *** | * |
| Volatile Compounds | LRI | I.M | Angus | Xiangxi Yellow Cattle | s.e.m. | Sig. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 M | 18 M | 30 M | 6 M | 18 M | 30 M | B | M | B × M | ||||
| Octanol | 1073 | MS + LRI | 1562.68 c | 1766.95 c | 1786.42 c | 3221.31 b | 4753.63 a | 4513.87 a | 404.14 | *** | * | n.s. |
| 1-Octen-3-ol | 980 | MS + LRI | N.D. | 563.55 | 711.82 | 1344.24 | 2042.90 | 2450.20 | 263.20 | — | — | — |
| Dodecanol | 1476 | MS + LRI | 812.98 | 914.08 | 1028.85 | 795.94 | 879.51 | 817.37 | 29.04 | n.s. | n.s. | n.s. |
| Hexadecanol | 1880 | MS + LRI | 1084.61 | 1176.22 | 1081.27 | 1052.83 | 951.78 | 1059.17 | 42.22 | n.s. | n.s. | n.s. |
| 2-Hexyl-1-decanol | 1786 | MS + LRI | 2523.30 | 2716.63 | 2967.30 | 3012.78 | 2806.27 | 2972.66 | 170.64 | n.s. | n.s. | n.s. |
| 2-Octyl-1-decanol | 1677 | MS | 3314.86 c | 3361.78 c | 3203.98 c | 4040.57 b | 4166.29 b | 5637.14 a | 258.56 | *** | * | ** |
| Alcohols | — | — | 9298.41 d | 10,499.19 d | 10,779.63 d | 13,467.67 c | 15,600.37 b | 17,450.40 a | 895.47 | *** | ** | n.s. |
| Octanal | 1003 | MS + LRI | 2156.62 | 2795.93 | 3107.31 | 2941.68 | 3198.27 | 2996.77 | 134.93 | n.s. | n.s. | n.s. |
| Nonanal | 1105 | MS + LRI | 12,437.41 | 11,939.67 | 13,864.11 | 12,062.94 | 11,796.93 | 11,435.95 | 308.25 | n.s. | n.s. | n.s. |
| Decanal | 1207 | MS + LRI | 3936.42 b | 3883.30 b | 5383.10 a | 2001.75 c | 2050.26 c | 2198.34 c | 393.47 | *** | n.s. | n.s. |
| Undecanal | 1308 | MS + LRI | 1827.03 | 1800.41 | 2048.18 | 1650.41 | 1915.99 | 1697.08 | 49.63 | n.s. | n.s. | n.s. |
| Dodecanal | 1409 | MS + LRI | 1828.89 c | 1869.27 c | 2108.36 b | 2141.69 b | 2625.82 a | 2651.77 a | 100.07 | *** | ** | * |
| Tridecenal | 1511 | MS + LRI | 643.17 b | 671.93 b | 743.96 b | 1561.11 a | 1487.35 a | 1435.51 a | 124.41 | *** | n.s. | n.s. |
| Tetradecanal | 1612 | MS + LRI | 2080.45 d | 2065.69 d | 2541.90 c | 4969.85 b | 5527.77 a | 5495.24 a | 474.36 | *** | * | n.s. |
| Pentadecanal | 1714 | MS + LRI | 7904.48 b | 9965.40 b | 9098.07 b | 15162.01 a | 16267.62 a | 15499.82 a | 1046.87 | *** | n.s. | n.s. |
| Hexadecanal | 1816 | MS + LRI | 5292.71 e | 7460.42 d | 7317.41 d | 11584.75 c | 13660.54 b | 15454.13 a | 1113.10 | *** | *** | * |
| Heptadecanal | 1918 | MS + LRI | 3312.56 c | 3633.17 c | 3367.36 c | 6422.51 b | 7699.21 a | 7502.23 a | 584.24 | *** | * | n.s. |
| 4,8,12-Tetradecatrienal,5,9,13-trimethyl- | 1839 | MS + RI | 1376.66 c | 2742.84 b | 3778.69 a | 1528.07 c | 1882.98 c | 1514.37 c | 267.11 | ** | ** | ** |
| Octadecanal | 2020 | MS + LRI | 952.16 c | 1403.26 b | 1520.57 b | 4167.96 a | 4336.31 a | 4398.71 a | 458.13 | *** | * | n.s. |
| Aldehydes | — | — | 43,748.53 d | 50,231.27 c | 54,879.00 c | 66,194.69 b | 72,511.41 a | 72,217.51 a | 3356.49 | *** | ** | n.s. |
| 2-Undecanone | 1295 | MS + LRI | 891.76 c | 863.95 c | 988.44 b | 816.63 c | 1208.38 a | 1313.74 a | 58.43 | ** | ** | * |
| 6,10-Ddimethylundeca-5,9-Dien-2-One | 1454 | MS + LRI | 1922.12 d | 2470.47 c | 3477.13 a | 1718.02 d | 2891.99 b | 2751.12 bc | 181.04 | n.s. | *** | ** |
| ketones | — | — | 2813.87 c | 3334.42 b | 4465.57 a | 2534.65 c | 4100.37 a | 4064.86 a | 218.43 | n.s. | *** | * |
| Hexanoic acid | 996 | MS + LRI | N.D. | N.D. | N.D. | 2856.90 | 3306.70 | 3200.74 | 489.59 | — | — | — |
| Nonanoic acid | 1281 | MS + LRI | 359.76 c | 316.16 c | 1129.82 b | 2400.57 a | 1975.30 a | 2589.58 a | 283.29 | *** | * | n.s. |
| Tetradecanoic acid | 1767 | MS + LRI | 2597.96 | 2532.56 | 3003.73 | 2787.84 | 2239.76 | 1958.00 | 126.26 | n.s. | n.s. | n.s. |
| Hexadecanoic acid | 1967 | MS + LRI | 15,178.13 b | 13,546.74 b | 25,978.42 a | 16,443.53 b | 14,910.24 b | 13,761.61 b | 1351.42 | * | * | ** |
| Organic acids | — | — | 18,135.84 cd | 16,395.46 d | 30,111.97 a | 24,488.84 b | 22,432.00 bc | 21,509.92 bcd | 1410.95 | n.s. | * | ** |
| Sulfurous acid, 2-ethylhexyl isohexyl ester | 1488 | MS | 1133.18 c | 1606.11 b | 1154.14 c | 2029.43 a | 2063.72 a | 2228.23 a | 134.32 | *** | n.s. | * |
| Dibutyl phthalate phthalate | 1869 | MS + LRI | 730.74 b | 858.64 a | 921.87 a | 585.54 c | 546.07 c | 729.26 b | 41.52 | *** | ** | n.s. |
| Esters | — | — | 1863.93 c | 2464.75 b | 2076.00 c | 2614.97 ab | 2609.78 ab | 2957.49 a | 114.39 | ** | n.s. | * |
| Dodecane | 1199 | MS + LRI | 1462.54 | 1455.51 | 1546.10 | 1578.45 | 1878.32 | 1553.50 | 64.35 | n.s. | n.s. | n.s. |
| Dodecane, 2-methyl- | 1263 | MS + LRI | 2844.87 | 2918.08 | 3156.25 | 3056.76 | 3835.46 | 4111.04 | 189.53 | n.s. | n.s. | n.s. |
| 2-Bromo dodecane | 1420 | MS + LRI | 1738.91 | 1666.24 | 1713.57 | 1805.80 | 2027.01 | 1899.76 | 42.48 | n.s. | n.s. | n.s. |
| Tridecane | 1299 | MS + LRI | 2820.92 | 2803.98 | 2633.76 | 3187.22 | 3063.63 | 3273.30 | 129.82 | n.s. | n.s. | n.s. |
| Tetradecane | 1399 | MS + LRI | 3039.50 | 2765.86 | 2616.46 | 2795.86 | 2825.50 | 2818.13 | 120.74 | n.s. | n.s. | n.s. |
| Tetradecane, 5-methyl- | 1463 | MS + LRI | 700.51 c | 921.07 b | 616.93 c | 1148.52 a | 1186.09 a | 1249.20 a | 74.25 | *** | * | ** |
| Dodecane, 4,6-dimethyl- | 1279 | MS + LRI | 3328.02 bc | 4555.31 ab | 3053.44 c | 5227.35 a | 4862.95 a | 5211.14 a | 286.16 | ** | n.s. | n.s. |
| Nonane,5-(2-methylpropyl)- | 1243 | MS + LRI | 516.49 c | 749.95 bc | 618.19 c | 1029.52 ab | 1152.55 a | 1163.55 a | 81.89 | ** | n.s. | n.s. |
| Pentadecane | 1498 | MS + LRI | 3655.60 | 2630.71 | 2738.06 | 4339.99 | 3848.17 | 3771.00 | 250.12 | n.s. | n.s. | n.s. |
| Hexadecane | 1508 | MS + LRI | 3638.01 | 2954.00 | 3153.00 | 4458.47 | 4167.34 | 3553.81 | 208.23 | n.s. | n.s. | n.s. |
| Heptadecane | 1707 | MS + LRI | 3988.82 d | 4467.90 c | 3806.79 d | 6592.67 b | 8476.24 a | 8313.08 a | 606.22 | *** | * | * |
| Octadecane | 1798 | MS + LRI | 900.44 | 897.78 | 813.70 | 1183.77 | 1303.83 | 1310.13 | 63.77 | n.s. | n.s. | n.s. |
| Hexadecane, 2,6,10,14-tetramethyl- | 1751 | MS + LRI | 1219.59 d | 1914.40 c | 981.28 d | 1825.97 c | 3014.26 a | 2434.92 b | 209.84 | *** | *** | * |
| 2-Hexadecene, 3,7,11,15-tetramethyl- | 1844 | MS + LRI | 1687.12 d | 1771.59 d | 1727.33 d | 2014.26 bc | 2966.85 ab | 3849.04 a | 260.56 | ** | n.s. | n.s. |
| Hydrocarbons | — | — | 31,541.30 b | 32,472.35 b | 29,175.13 b | 40,244.58 a | 44,608.17 a | 44,511.47 a | 1976.78 | *** | n.s. | n.s. |
| 1,3-Dioxolane, 2-heptyl- | 1159 | MS + LRI | 3031.31 | 2717.41 | 1944.82 | 2379.02 | 2786.93 | 2115.00 | 153.26 | n.s. | n.s. | n.s. |
| 2(3 H)-Furanone, 5-hexyldihydro- | 1364 | MS + LRI | 637.09 c | 745.65 c | 752.07 c | 1384.04 b | 1701.92 a | 1714.51 a | 138.97 | *** | ** | n.s. |
| Heterocyclic compounds | — | — | 3668.40 | 3463.06 | 2696.89 | 3763.06 | 4488.85 | 3829.51 | 103.03 | n.s. | n.s. | n.s. |
| 2, 4-Ditert-Butyl Phenol | 1517 | MS + LRI | 3970.92 c | 4646.68 c | 4531.10 c | 12,622.93 b | 15,771.76 a | 16,589.06 a | 1644.51 | *** | ** | ** |
| Dioctyl ether | 1684 | MS + LRI | 1425.05 e | 1653.03 d | 1703.15 d | 2841.83 c | 3156.70 b | 3639.71 a | 255.93 | *** | *** | * |
| Others | — | — | 5395.97 d | 6299.71 d | 6234.25 d | 15,464.76 c | 18,928.46 b | 20,228.77 a | 1897.79 | *** | *** | ** |
| Sensory Test | Angus | Xiangxi Yellow Cattle | s.e.m. | Sig. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 Month | 18 Month | 30 Month | 6 Month | 18 Month | 30 Month | Breed | Month | B × M | ||
| Tenderness | 4.50 a | 4.42 a | 3.79 b | 4.38 a | 4.25 a | 3.58 b | 0.11 | n.s. | *** | n.s. |
| Juiciness | 3.59 b | 3.85 a | 3.88 a | 3.41 b | 3.94 a | 3.86 a | 0.06 | n.s. | ** | n.s. |
| Beefy flavor | 3.80 c | 4.06 b | 4.08 b | 4.07 b | 4.47 a | 4.57 a | 0.08 | *** | ** | n.s. |
| Abnormal flavor | 2.59 c | 2.94 b | 2.83 bc | 3.64 a | 3.44 a | 3.42 a | 0.12 | *** | n.s. | * |
| Flavor liking | 3.73 | 3.88 | 4.12 | 3.74 | 3.92 | 3.93 | 0.05 | n.s. | n.s. | n.s. |
| Overall liking | 4.21 | 4.25 | 4.21 | 3.99 | 4.23 | 4.10 | 0.04 | n.s. | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Wang, X.; Guo, Q.; Deng, H.; Luo, J.; Yi, K.; Sun, A.; Chen, K.; Shen, Q. Muscle Fatty Acids, Meat Flavor Compounds and Sensory Characteristics of Xiangxi Yellow Cattle in Comparison to Aberdeen Angus. Animals 2022, 12, 1161. https://doi.org/10.3390/ani12091161
Chen D, Wang X, Guo Q, Deng H, Luo J, Yi K, Sun A, Chen K, Shen Q. Muscle Fatty Acids, Meat Flavor Compounds and Sensory Characteristics of Xiangxi Yellow Cattle in Comparison to Aberdeen Angus. Animals. 2022; 12(9):1161. https://doi.org/10.3390/ani12091161
Chicago/Turabian StyleChen, Dong, Xiaoyan Wang, Qian Guo, Huifen Deng, Jie Luo, Kangle Yi, Ao Sun, Kun Chen, and Qingwu Shen. 2022. "Muscle Fatty Acids, Meat Flavor Compounds and Sensory Characteristics of Xiangxi Yellow Cattle in Comparison to Aberdeen Angus" Animals 12, no. 9: 1161. https://doi.org/10.3390/ani12091161






