Goats Naturally Infected with the Spanish Goat Encephalitis Virus (SGEV): Pathological Features and An Outbreak
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Goat Herd History
2.2. Pathological Examination
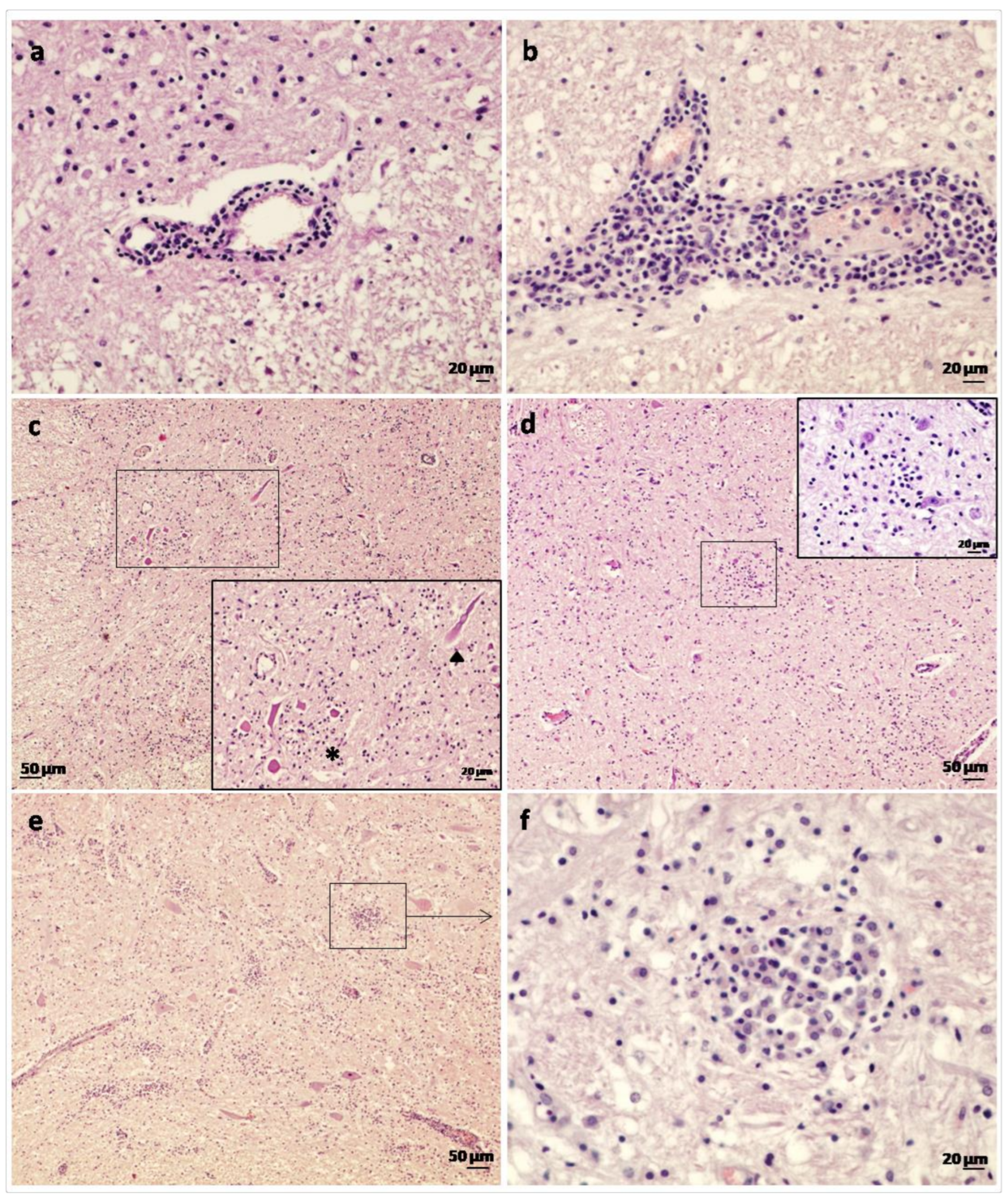
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansfield, K.L.; Jizhou, L.; Phipps, L.P.; Johnson, N. Emerging tick-borne viruses in the twenty-first century. Front. Cell. Infect. Microbiol. 2017, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses, 1st ed.; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Reid, H.W. Epidemiology of louping-ill. In Vectors in Virus Biology, 1st ed.; Mayo, M.A., Harrap, K.H., Eds.; Academic Press: London, UK; Orlando, FL, USA, 1984; pp. 161–178. [Google Scholar]
- Jeffries, C.L.; Mansfield, K.L.; Phipps, L.P.; Wakeley, P.R.; Mearns, R.; Schock, A.; Bell, S.; Breed, A.C.; Fooks, A.R.; Johnson, N. Louping ill virus: An endemic tick-borne disease of Great Britain. J. Gen. Virol. 2014, 95, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Dagleish, M.P.; Clark, J.J.; Robson, C.; Tucker, M.; Orton, R.J.; Rocchi, M.S. A fatal case of louping ill in a dog: Immunolocation and full genome sequencing of the virus. J. Comp. Pathol. 2018, 165, 23–32. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Reid, H.W.; Pow, I.; Gilmour, J.S. A disease resembling louping-ill in sheep in the Basque region of Spain. Vet. Rec. 1987, 121, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Balseiro, A.; Royo, L.J.; Pérez-Martínez, C.; de Mera, I.F.; Höfle, U.; Polledo, L.; Marreros, N.; Casais, R.; Marín, J.F. Louping ill in goats, Spain, 2011. Emerg. Infect. Dis. 2012, 18, 976–978. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Balseiro, A.; Johnson, N.; Ayllón, N.; Höfle, U.; Alberdi, P.; de Mera, I.G.F.; García Marín, J.F.; Gortázar, C.; de la Fuente, J.; et al. Identification and characterization of a novel tick-borne flavivirus subtype in goats (Capra hircus) in Spain. J. Gen. Virol. 2015, 96, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.W.; Buxton, D.; Pow, I.; Finlayson, J. Transmission of louping-ill virus in goat milk. Vet. Rec. 1984, 114, 163–165. [Google Scholar] [CrossRef]
- Salinas, L.M.; Casais, R.; Marín, J.F.G.; Dalton, K.P.; Royo, L.J.; del Cerro, A.; Gayo, E.; Dagleish, M.P.; Alberdi, P.; Juste, R.A.; et al. Vaccination against louping ill virus protects goats from experimental challenge with Spanish goat encephalitis virus. J. Comp. Pathol. 2017, 156, 409–418. [Google Scholar] [CrossRef]
- Papadopoulos, O.; Paschaleri-Papadopoulou, E.; Deligaris, N.; Doukas, G. Isolation of tick-borne encephalitis virus from a flock of goats with abortions and fatal disease (preliminary report). Vet. News Greece 1971, 3, 112–114. [Google Scholar]
- Gray, D.; Webster, K.; Berry, J.E. Evidence of louping ill and tick-borne fever in goats. Vet. Rec. 1988, 122, 66. [Google Scholar] [CrossRef]
- Dagleish, M.P.; Benavides, J.; Chianini, F. Immunohistochemical diagnosis of infectious diseases of sheep. Small Ruminant Res. 2010, 92, 19–35. [Google Scholar] [CrossRef]
- Salinas, L.M.; Casais, R.; Marín, J.F.G.; Dalton, K.P.; Royo, L.J.; del Cerro, A.; Gayo, E.; Dagleish, M.P.; Juste, R.A.; Balseiro, A. Lambs are susceptible to experimental challenge with Spanish goat encephalitis virus. J. Comp. Pathol. 2017, 156, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Balseiro, A.; Royo, L.J.; Pérez-Martínez, C.; Copano, M.F.; Rubio, T.; Höfle, U.; Prieto, J.M.; Marín, J.F.G. Louping ill in goat in Spain: More than a forgotten disease. In Proceedings of the 30th Meeting of the European Society of Veterinary Pathology, León, Spain, 5–8 September 2012; p. 74. [Google Scholar]
- Ruiz-Fons, F.; Balseiro, A.; Willoughby, K.; Oleaga, A.; Dagleish, M.P.; Pérez-Ramírez, E.; Havlíková, S.; Klempa, B.; Llorente, F.; Martín-Hernando, M.P. Clinical infection of Cantabrian chamois (Rupicapra pyrenaica parva) by louping ill virus: New concern for mountain ungulate conservation? Eur. J. Wild. Dis. 2014, 60, 691–694. [Google Scholar] [CrossRef]
- Doherty, P.C.; Vantsis, J.T. Louping-ill encephalomyelitis in the sheep. VII. Influence of immune status on neuropathology. J. Comp. Pathol. 1973, 83, 481–490. [Google Scholar] [CrossRef]
- Reid, H.W.; Buxton, D.; Brodie, T.A.; Holmes, P.H.; Urquhart, G.M. Response of sheep to experimental concurrent infection with tick-borne fever (Cytoecetes ohagocytophila) and louping-ill virus. Res. Vet. Sci. 1986, 41, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.; Reid, H.W. Immune responses of sheep to louping-ill virus vaccine. Vet. Rec. 1981, 109, 529–531. [Google Scholar] [PubMed]
- Hartley, W.J.; Martin, W.B.; Hakiolu, F.; Chifney, S.T.E. A viral encephalitis of sheep in Turkey. Pendik Inst. J. 1969, 2, 89–100. [Google Scholar]
- Doherty, P.C.; Reid, H.W. Experimental louping-ill in sheep and lamb. II. Neuropathology. J. Comp. Pathol. 1971, 81, 331–337. [Google Scholar] [CrossRef]
- Sheahan, B.J.; Moore, M.; Atkins, G.J. The pathogenicity of Louping ill virus for mice and lambs. J. Comp. Pathol. 2002, 126, 137–146. [Google Scholar] [CrossRef]
- Doherty, P.C.; Reid, H.W. Louping-ill encephalomyelitis in the sheep. II. Distribution of virus and lesions in nervous tissue. J. Comp. Pathol. 1971, 81, 531–536. [Google Scholar] [CrossRef]
- Macaldowie, C.; Patterson, I.A.; Nettleton, P.F.; Low, H.; Buxton, D. Louping ill in llamas (Lama glama) in the Hebrides. Vet. Rec. 2005, 156, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Krueger, N.; Reid, H.W. Detection of louping ill virus in formalin-fixed, paraffin wax-embedded tissues of mice, sheep and a pig by the avidin–biotin–complex immunoperoxidase technique. Vet. Rec. 1994, 135, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.Y.; Guzman, H.; Zhang, H.; da Rosa, A.P.T.; Tesh, R.B. West Nile virus infection in the golden hamster (Mesocricetus auratus): A model for West Nile encephalitis. Emerg. Infect. Dis. 2001, 7, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Laurenson, M.K.; McKEndrick, I.J.; Reid, H.W.; Challenor, R.; Mathewson, G.K. Prevalence, spatial distribution and the effect of control measures on louping-ill virus in the Forest of Bowland, Lancashire. Epidemiol. Infect. 2007, 135, 963–973. [Google Scholar] [CrossRef] [PubMed]


| Goat No. | Anatomical Location | |||||||
|---|---|---|---|---|---|---|---|---|
| Spinalcord | Medulla Oblongata | Pons | Cerebellum | Thalamus/ Hypothalamus | Midbrain | Cerebral Cortex | ||
| Peduncles | Cortex | |||||||
| 1 Lesions IHC | ++ - | ++ + | ++ + | ++ - | +++ - | ++ - | ++ - | + - |
| 2 Lesions IHC | +++ + | +++ + | +++ + | ++ + | +++ + | +++ + | ++ + | + - |
| 3 Lesions IHC | ++ - | ++ - | +++ - | ++++ - | +++ - | ++ - | ++ - | + - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balseiro, A.; Pérez-Martínez, C.; Dagleish, M.P.; Royo, L.J.; Polledo, L.; García Marín, J.F. Goats Naturally Infected with the Spanish Goat Encephalitis Virus (SGEV): Pathological Features and An Outbreak. Animals 2023, 13, 72. https://doi.org/10.3390/ani13010072
Balseiro A, Pérez-Martínez C, Dagleish MP, Royo LJ, Polledo L, García Marín JF. Goats Naturally Infected with the Spanish Goat Encephalitis Virus (SGEV): Pathological Features and An Outbreak. Animals. 2023; 13(1):72. https://doi.org/10.3390/ani13010072
Chicago/Turabian StyleBalseiro, Ana, Claudia Pérez-Martínez, Mark P. Dagleish, Luis J. Royo, Laura Polledo, and Juan F. García Marín. 2023. "Goats Naturally Infected with the Spanish Goat Encephalitis Virus (SGEV): Pathological Features and An Outbreak" Animals 13, no. 1: 72. https://doi.org/10.3390/ani13010072
APA StyleBalseiro, A., Pérez-Martínez, C., Dagleish, M. P., Royo, L. J., Polledo, L., & García Marín, J. F. (2023). Goats Naturally Infected with the Spanish Goat Encephalitis Virus (SGEV): Pathological Features and An Outbreak. Animals, 13(1), 72. https://doi.org/10.3390/ani13010072








