The Lipidomics of Spermatozoa and Red Blood Cells Membrane Profile of Martina Franca Donkey: Preliminary Evaluation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria and Sample Collection
2.2. Ethical Statement
2.3. Sperm Assay/Spermiogram (Analysis of Seminal Fluid)
2.4. Lipidomic Profile
2.5. GC Analysis of FAMEs
2.6. Evaluation of the Fatty Acid Cluster, Corresponding Families and Homeostasis Indexes
2.7. Statistical Methods
3. Results
3.1. Semen Analysis of Healthy Donkeys
3.2. The Spermatozoa Membrane Lipidome in Healthy Donkeys
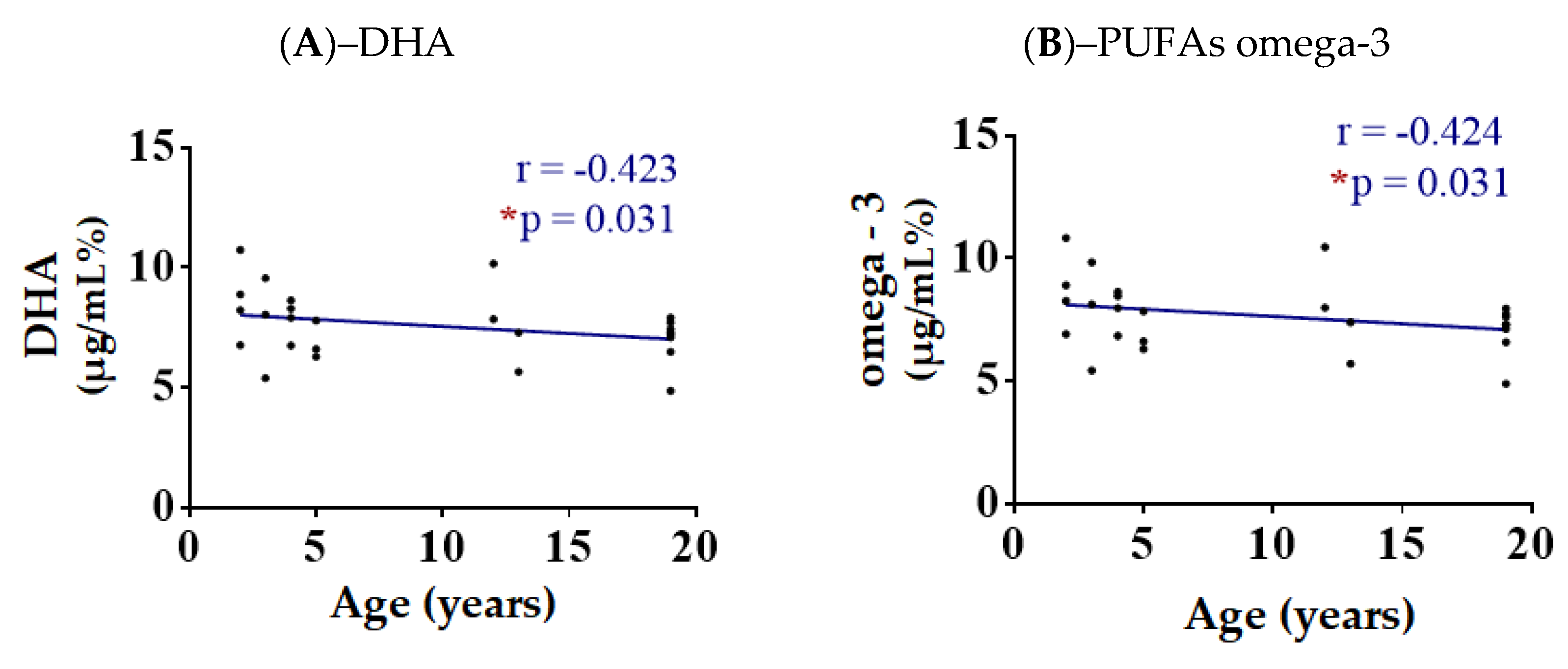
3.3. Correlations of Spermatozoa FA with the Donkeys’ Characteristics
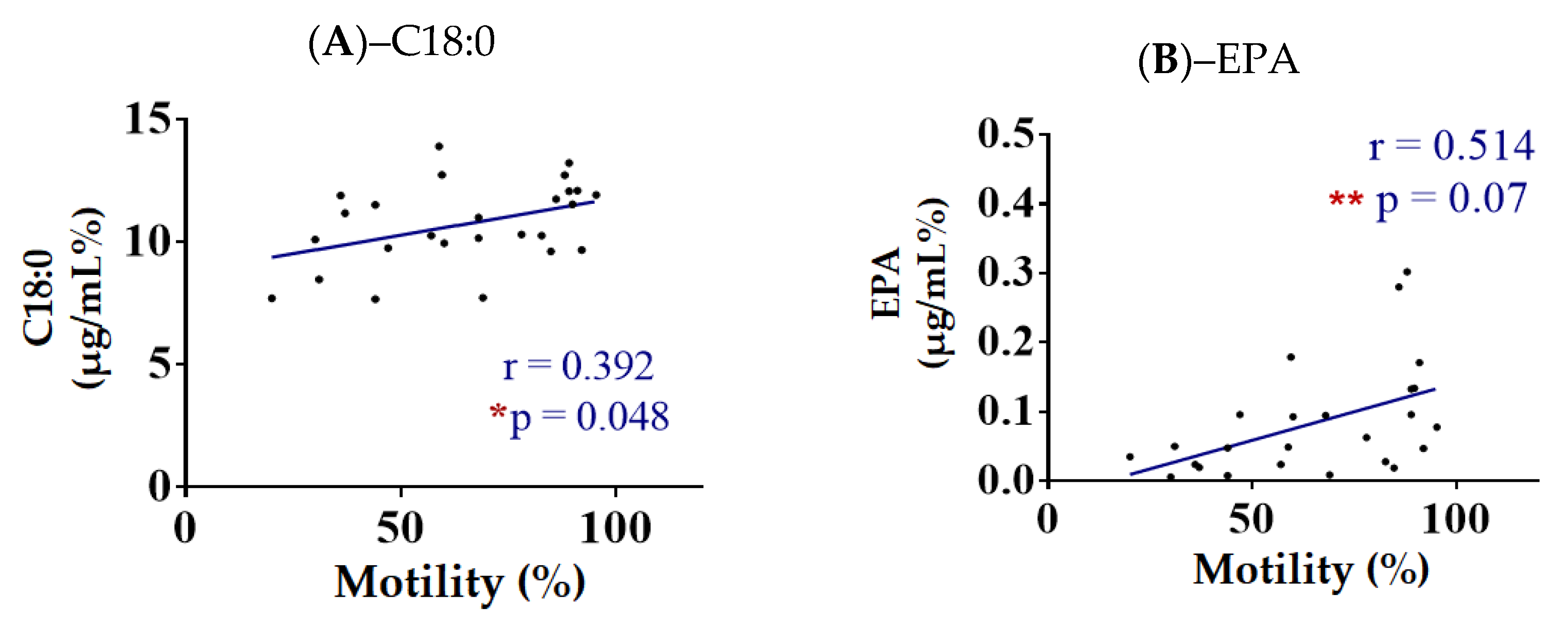
3.4. Correlations of Spermatozoa FA with Sperm Parameters
3.4.1. Correlation of Spermatozoa FA with Motility (%)
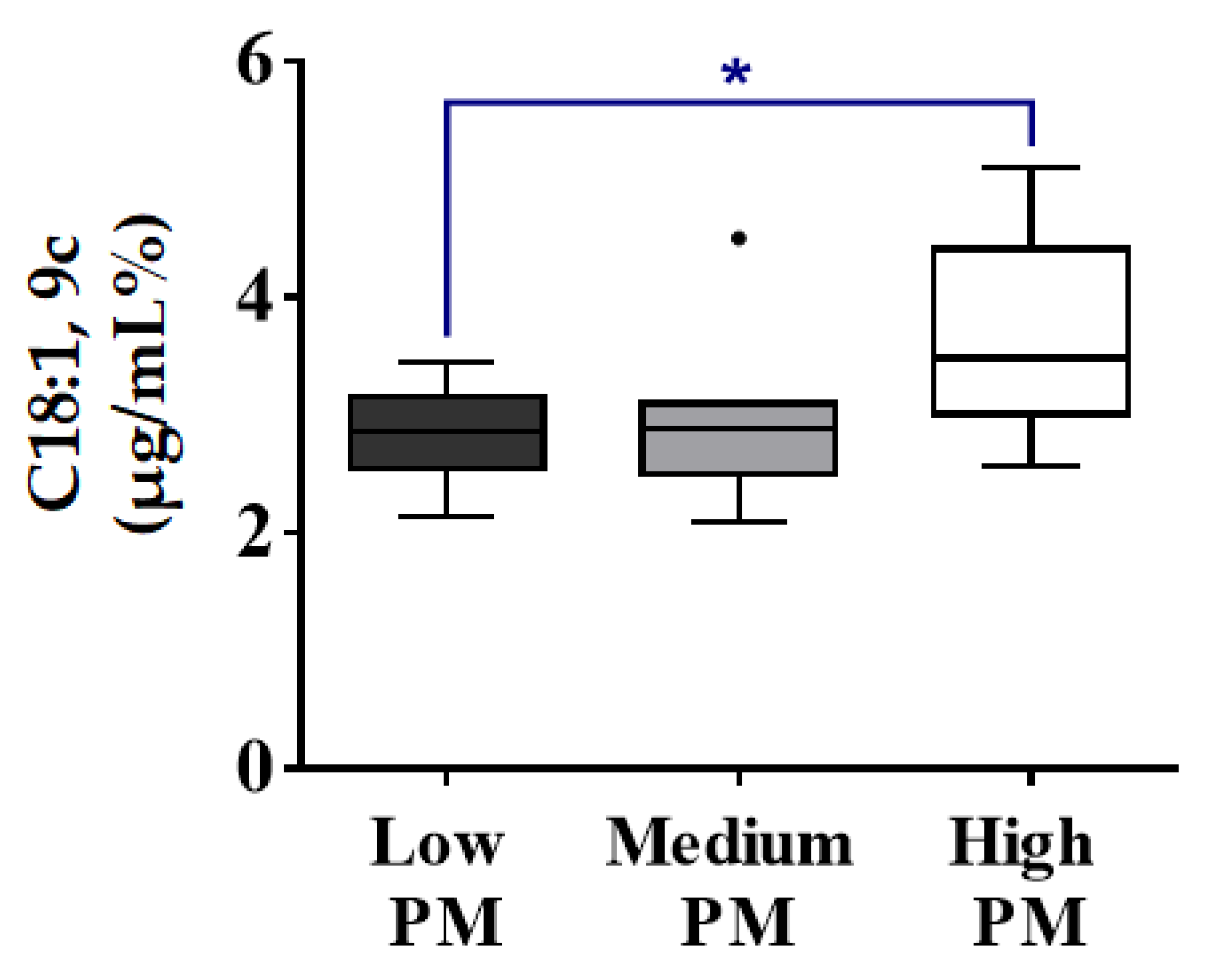
3.4.2. Correlations of Spermatozoa FA with Progressive Motility (%) (PM)
3.5. The Erythrocyte Membrane Lipidome in Healthy Donkeys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreri, C.; Chatgilialoglu, C. Role of fatty acid-based functional lipidomics in the development of molecular diagnostic tools. Expert Rev. Mol. Diagn. 2012, 12, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdoman in organization, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papachova, Z.; Cahova, M. Fatty acid signaling: The new role of intracellular lipase. Int. J. Mol. Sci. 2015, 16, 3831–3855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.-Y.; Cheng, X.-l.; Lin, R.-C. Chapter One—Lipidomics Applications for Discovering Biomarkers of Diseases in Clinical Chemistry. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 313, pp. 1–26. ISBN 9780128001776. [Google Scholar] [CrossRef]
- Abbott, S.K.; Else, P.A.; Atkins, T.A.; Hulbert, A.J. Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta 2012, 1818, 1309–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, S.; Mostyn, A.; Rutland, C.S. Fatty Acids in Veterinary Medicine and Research. In Fatty Acids; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Prasinou, P.; Crisi, P.E.; Chatgilialoglu, C.; Di Tommaso, M.; Sansone, A.; Gramenzi, A.; Belà, B.; De Santis, F.; Boari, A.; Ferreri, C. The Erythrocyte Membrane Lipidome of Healthy Dogs: Creating a Benchmark of Fatty Acid Distribution and Interval Values. Front. Vet. Sci. 2020, 7, 502. [Google Scholar] [CrossRef]
- Crisi, P.E.; Luciani, A.; Di Tommaso, M.; Prasinou, P.; De Santis, F.; Chatgilialoglu, C.; Pietra, M.; Procoli, F.; Sansone, A.; Giordano, M.V.; et al. The Fatty Acid-Based Erythrocyte Membrane Lipidome in Dogs with Chronic Enteropathy. Animals 2021, 11, 2604. [Google Scholar] [CrossRef]
- Goodrich, E.L.; Behling-Kelly, E. Particle Size Distribution of Plasma Lipoproteins in Donkeys from Death Valley Compared to a Sampling of Horses. Animals 2022, 12, 2746. [Google Scholar] [CrossRef]
- De Santis, M.; Seganfreddo, S.; Galardi, M.; Mutinelli, F.; Normando, S.; Contalbrigo, L. Donkey behaviour and cognition: A literature review. Appl. Anim. Behav. Sci. 2021, 244, 105485. [Google Scholar] [CrossRef]
- Argov, N.; Sklan, D.; Zeron, Y.; Roth, Z. Association between seasonal changes in fatty-acid composition, expression of VLDL receptor and bovine sperm quality. Theriogenology 2007, 67, 878–885. [Google Scholar] [CrossRef]
- Aurich, C.; Ortega Ferrusola, C.; Peña Vega, F.J.; Schrammel, N.; Morcuende, D.; Aurich, J. Seasonal changes in the sperm fatty acid composition of Shetland pony stallions. Theriogenology 2018, 107, 149–153. [Google Scholar] [CrossRef]
- Nolazco Sassot, L.; Villarino, N.F.; Dasgupta, N.; Morrison, J.J.; Bayly, W.M.; Gang, D.; Sanz, M.G. The lipidome of Thoroughbred racehorses before and after supramaximal exercise. Equine Vet. J. 2019, 51, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Cappai, M.G.; Taras, A.; Cossu, I.; Cherchi, R.; Dimauro, C.; Accioni, F.; Boatto, G.; Deroma, M.; Spanu, E.; Gatta, D.; et al. Effects of Dietary Zn/Se and α-Tocopherol Supplementation on Metabolic Milieu, Haemogram and Semen Traits of Breeding Stallions. Biol. Trace Elem. Res. 2021, 199, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- García, B.M.; Fernández, L.G.; Ferrusola, C.O.; Salazar-Sandoval, C.; Rodríguez, A.M.; Martinez, H.R.; Tapia, J.A.; Morcuende, D.; Peña, F.J. Membrane lipids of the stallion spermatozoon in relation to sperm quality and susceptibility to lipid peroxidation. Reprod. Domest. Anim. 2011, 46, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Picardo, M.; Gandini, L.; Dondero, F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 1996, 2, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tran, L.; Malla, B.A.; Kumar, S.; Tyagi, A.K. Polyunsaturated Fatty Acids in Male Ruminant Reproduction—A Review. Asian-Australas. J. Anim. Sci. 2017, 30, 622–637. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.Y.; Huang, Y.; Du, Q.Y.; Yao, G.D.; Sun, Y.P. Body mass index effects sperm quality: A retrospective study in Northern China. Asian J. Androl. 2017, 19, 234–237. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Mannina, L.; Paventi, G.; Rosato, M.P.; Cerolini, S.; Sobolev, A.P. Age-dependent changes in metabolic profile of turkey spermatozoa as assessed by NMR analysis. PLoS ONE 2018, 13, e0194219. [Google Scholar] [CrossRef] [Green Version]
- Nago, M.; Arichi, A.; Omura, N.; Iwashita, Y.; Kawamura, T.; Yumura, Y. Aging increases oxidative stress in semen. Investig. Clin. Urol. 2021, 62, 233–238. [Google Scholar] [CrossRef]
- Perrett, J.; Harris, I.T.; Maddock, C.; Farnworth, M.; Pyatt, A.Z.; Sumner, R.N. Systematic Analysis of Breed, Methodological, and Geographical Impact on Equine Sperm Progressive Motility. Animals 2021, 11, 3088. [Google Scholar] [CrossRef]
- Colli, L.; Perrotta, G.; Negrini, R.; Bomba, L.; Bigi, D.; Zambonelli, P.; Verini Supplizi, A.; Liotta, L.; Ajmone-Marsan, P. Detecting population structure and recent demographic history in endangered livestock breeds: The case of the Italian autochthonous donkeys. Anim. Genet. 2013, 44, 69–78. [Google Scholar] [CrossRef]
- Navas, F.J.; Jordana, J.; León, J.M.; Barba, C.; Delgado, J.V. A model to infer the demographic structure evolution of endangered donkey populations. Animal 2017, 11, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Mazzatenta, A.; Vignoli, M.; Caputo, M.; Vignola, G.; Tamburro, R.; De Sanctis, F.; Roig, J.M.; Bucci, R.; Robbe, D.; Carluccio, A. Maternal Phylogenetic Relationships and Genetic Variation among Rare, Phenotypically Similar Donkey Breeds. Genes 2021, 12, 1109. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.L.; Huntington, P.J. Body condition scoring and weight estimation of horses. Equine Vet. J. 1988, 20, 41–45. [Google Scholar] [CrossRef]
- Carter, R.A.; Geor, R.J.; Burton Staniar, W.; Cubitt, T.A.; Harris, P.A. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet. J. 2009, 179, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, D.; Penazzi, L.; Valle, E.; Raspa, F.; Bergero, D.; Formigoni, A.; Fusaro, I. When Changing the Hay Makes a Difference: A Series of Case Reports. J. Equine Vet. Sci. 2022, 113, 103940. [Google Scholar] [CrossRef] [PubMed]
- Vinassa, M.; Cavallini, D.; Galaverna, D.; Baragli, P.; Raspa, F.; Nery, J.; Valle, E. Palatability assessment in horses in relation to lateralization and temperament. Appl. Anim. Behav. Sci. 2020, 232, 105110. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Fuchs, B.; Süss, R.; Teuber, K.; Eibisch, M.; Schiller, J. Lipid analysis by thinlayer chromatography—A review of the current state. Chromatogr. A 2011, 1218, 2754–2774. [Google Scholar] [CrossRef]
- Sansone, A.; Melchiorre, M.; Chatgilialoglu, C.; Ferreri, C. Hexadecenoic fatty acid isomers: A chemical biology approach for human plasma biomarker development. Chem. Res. Toxicol. 2013, 26, 1703–1709. [Google Scholar] [CrossRef]
- Ferreri, C.; Masi, A.; Sansone, A.; Giacometti, G.; Larocca, A.V.; Menounou, G.; Scanferlato, R.; Tortorella, S.; Rota, D.; Conti, M.; et al. Fatty Acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansone, A.; Tolika, E.; Louka, M.; Sunda, V.; Deplano, S.; Melchiorre, M.; Anagnostopoulos, D.; Chatgilialoglu, C.; Formisano, C.; Di Micco, R.; et al. Hexadecenoic Fatty Acid Isomers in Human Blood Lipids and Their Relevance for the Interpretation of Lipidomic Profiles. PLoS ONE 2016, 11, e0152378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogan, T.; Grossman Dahan, D.; Laor, R.; Argov-Argaman, N.; Zeron, Y.; Komsky-Elbaz, A.; Kalo, D.; Roth, Z. Association between Fatty Acid Composition, Cryotolerance and Fertility Competence of Progressively Motile Bovine Spermatozoa. Animals 2021, 11, 2948. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C. Membrane Lipidomics for Personalized Health; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Carluccio, A.; Panzani, S.; Contri, A.; Bronzo, V.; Robbe, D.; Veronesi, M.C. Influence of season on testicular morphometry and semen characteristics in Martina Franca jackasses. Theriogenology 2013, 79, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Gandini, L.; Maresca, V.; Rago, R.; Sgrò, P.; Dondero, F.; Picardo, M. Fatty acid composition of spermatozoa and immature germ cells. Mol. Hum. Reprod. 2000, 6, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Gulaya, N.M.; Margitich, V.M.; Govseeva, N.M.; Klimashevsky, V.M.; Gorpynchenko, I.I.; Boyko, M.I. Phospholipid composition of human sperm and seminal plasma in relation to sperm fertility. Arch. Androl. 2001, 46, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Tavilani, H.; Doosti, M.; Abdi, K.; Vaisiraygani, A.; Joshaghani, H.R. Decreased polyunsaturated and increased saturated fatty acid concentration in spermatozoa from asthenozoospermic males as compared with normozoospermic males. Andrologia 2006, 38, 173–178. [Google Scholar] [CrossRef]
- Esmaeili, V.; Shahverdi, A.H.; Moghadasian, M.H.; Alizadeh, A.R. Dietary fatty acids affect semen quality: A review. Andrology 2015, 3, 450–461. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Hosseini, S.Y.; Dadkhah, F.; Asgari, M.A. Relationship of omega-3 and omega-6 fatty acids with semen characteristics, and anti-oxidant status of seminal plasma: A comparison between fertile and infertile men. Clin. Nutr. 2010, 29, 100–105. [Google Scholar] [CrossRef]
- Retterstøl, K.; Tran, T.N.; Haugen, T.B.; Christophersen, B.O. Metabolism of very long chain polyunsaturated fatty acids in isolated rat germ cells. Lipids 2001, 36, 601–606. [Google Scholar] [CrossRef]
- Tavilani, H.; Vatannejad, A.; Akbarzadeh, M.; Atabakhash, M.; Khosropou, S.; Mohaghgeghi, A. Correlation Between Lipid Profile of Sperm Cells and Seminal Plasma with Lipid Profile of Serum in Infertile Men. Avicenna J. Med. Biochem. 2014, 2, e19607. [Google Scholar] [CrossRef] [Green Version]
- Collodel, G.; Castellini, C.; Lee, J.C.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxidative Med. Cell. Longev. 2020, 2020, 7038124. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Moretti, E.; Noto, D.; Iacoponi, F.; Signorini, C. Fatty Acid Profile and Metabolism Are Related to Human Sperm Parameters and Are Relevant in Idiopathic Infertility and Varicocele. Mediat. Inflamm. 2020, 2020, 3640450. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Baumann, S.; Rolle-Kampczyk, U.; Schiller, J.; von Bergen, M.; Grunewald, S. Metabolomic profiling reveals correlations between spermiogram parameters and the metabolites present in human spermatozoa and seminal plasma. PLoS ONE 2019, 14, e0211679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimptsch, A.; Pyttel, S.; Paasch, U.; Mohr, C.; Heinrich, J.M.; Schiller, J. A MALDI MS investigation of the lysophosphatidylcholine/phosphatidylcholine ratio in human spermatozoa and erythrocytes as a useful fertility marker. Lipids 2014, 49, 287–293. [Google Scholar] [CrossRef]
- Andersen, J.M.; Rønning, P.O.; Herning, H.; Bekken, S.D.; Haugen, T.B.; Witczak, O. Fatty acid composition of spermatozoa is associated with BMI and with semen quality. Andrology 2016, 4, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Aksoy, Y.; Aksoy, H.; Altinkaynak, K.; Aydin, H.R.; Ozkan, A. Sperm fatty acid composition in subfertile men. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 75–79. [Google Scholar] [CrossRef]
- Rikans, L.E.; Hornbrook, K.R. Lipid peroxidation, antioxidant protection and aging. Biochim. Biophys. Acta 1997, 1362, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Love, C.C.; Noble, J.K.; Standridge, S.A.; Bearden, C.T.; Blanchard, T.L.; Varner, D.D.; Cavinder, C.A. The relationship between sperm quality in cool-shipped semen and embryo recovery rate in horses. Theriogenology 2015, 84, 1587–1593. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Feng, C.; Liu, R.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Lu, H.; Zhang, T.; Zeng, W. Exogenous Oleic Acid and Palmitic Acid Improve Boar Sperm Motility via Enhancing Mitochondrial Β-Oxidation for ATP Generation. Animals 2020, 10, 591. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C. (Ed.) Chapter 2—Polyunsaturated Fatty Acid Biosynthesis and Metabolism in Adult Mammals. In Polyunsaturated Fatty Acid Metabolism; AOCS Press: Urbana, IL, USA, 2018; pp. 15–30. ISBN 9780128112304. [Google Scholar] [CrossRef]
- Islam, M.M.; Umehara, T.; Tsujita, N.; Shimada, M. Saturated fatty acids accelerate linear motility through mitochondrial ATP production in bull sperm. Reprod. Med. Biol. 2021, 20, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Contri, A.; De Amicis, I.; Robbe, D.; Carluccio, A. Differences between epididymal and ejaculated sperm characteristics in donkey. Anim. Reprod. Sci. 2011, 128, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Argov-Argaman, N.; Mahgrefthe, K.; Zeron, Y.; Roth, Z. Season-induced variation in lipid composition is associated with semen quality in Holstein bulls. Reproduction 2013, 145, 479–489. [Google Scholar] [CrossRef] [PubMed]
| Chemical Composition | Concentrate | Hay |
|---|---|---|
| DM (%) | 89.73 | 91.47 |
| CP (%) | 18.25 | 13.90 |
| NDF (%) | 25.84 | 55.10 |
| ADF (%) | 9.45 | 41.16 |
| ADL (%) | 2.75 | 9.18 |
| ASH (%) | 7.40 | 7.40 |
| Item | Median | Min | Max | Mean | SD |
|---|---|---|---|---|---|
| Reaction time (min) | 10.0 | 4.0 | 45.0 | 12.6 | 10.1 |
| Total volume (mL) | 50.0 | 20.0 | 70.0 | 51.5 | 12.9 |
| Gel-free volume (mL) | 43.8 | 10.0 | 60.0 | 41.4 | 13.7 |
| Concentration (mln/mL) | 440.0 | 120.0 | 1600.0 | 517.6 | 379.8 |
| Dead (mln/mL) | 85.5 | 19.0 | 196.8 | 88.4 | 41.5 |
| Motility (%) | 68.0 | 20.0 | 95.3 | 65.2 | 23.1 |
| Progressive Motility (%) | 40.2 | 4.0 | 59.9 | 37.8 | 16.8 |
| FAME (%μg/mL) * | Median | Min | Max | Mean | SD |
|---|---|---|---|---|---|
| C14:0 | 4.3 | 2.1 | 5.5 | 4.1 | 1.0 |
| C16:0 | 30.3 | 24.5 | 35.5 | 30.3 | 2.9 |
| C16:1 | 0.08 | 0.04 | 0.34 | 0.1 | 0.1 |
| C18:0 | 10.7 | 7.7 | 13.9 | 10.8 | 1.7 |
| 9c,C18:1 | 3 | 2.1 | 5.1 | 3.2 | 0.8 |
| 11c,C18:1 | 3.3 | 1.5 | 6.6 | 3.5 | 0.9 |
| C18:2 | 4.5 | 3.4 | 7.4 | 4.7 | 1.0 |
| C20:3 | 1.95 | 0.8 | 3.1 | 2.0 | 0.5 |
| C20:4 | 6.3 | 3.1 | 9.9 | 6.6 | 1.4 |
| C20:5 | 0.06 | 0.01 | 0.3 | 0.1 | 0.1 |
| C22:5 | 27.8 | 21.5 | 31 | 27.6 | 2.6 |
| C22:6 | 7.6 | 4.8 | 10.7 | 7.6 | 1.4 |
| SFA 1 | 45 | 37.9 | 52.4 | 45.1 | 3.8 |
| MUFA 2 | 6.8 | 5.5 | 9.1 | 6.8 | 0.8 |
| PUFA omega-3 3 | 7.7 | 4.8 | 10.8 | 7.7 | 1.4 |
| PUFA omega-6 4 | 41 | 47 | 28.9 | 40.4 | 3.8 |
| PUFA 5 | 48.4 | 41 | 54.4 | 48.0 | 3.7 |
| SFA/MUFA | 6.6 | 4.6 | 8.4 | 6.7 | 1.1 |
| Omega-6/Omega-3 | 5.4 | 3.3 | 8.7 | 5.5 | 1.3 |
| PUFA balance 8 | 15.76 | 10.3 | 23.1 | 16.0 | 3.0 |
| UI 9 | 233.8 | 190 | 266 | 229.9 | 17.7 |
| PI 10 | 261.7 | 209 | 304 | 258.9 | 20.9 |
| FAME (%μg/mL) | Min | Max | Mean | SD |
|---|---|---|---|---|
| C14:0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C16:0 | 8.3 | 12 | 10.3 | 1.2 |
| C16:1 | 0.6 | 1.6 | 1.1 | 0.3 |
| C18:0 | 7.4 | 12.5 | 9.4 | 1.3 |
| 9c,C18:1 | 27.6 | 33.9 | 30.3 | 1.6 |
| 11c,C18:1 | 0.39 | 1.4 | 0.9 | 0.3 |
| C18:2 | 40.3 | 49.3 | 46.3 | 2,1 |
| C20:3 | 0.09 | 0.17 | 0.1 | 0.02 |
| C20:4 | 0.9 | 1.9 | 1.4 | 0.3 |
| C20:5 | 0.17 | 0.19 | 0.2 | 0.01 |
| C22:5 | 0.0 | 0.0 | 0.0 | 0.0 |
| C22:6 | 0.01 | 0.09 | 0.03 | 0.02 |
| SFA 1 | 17.9 | 22.5 | 19.7 | 1.0 |
| MUFA 2 | 30 | 35.5 | 32.3 | 1.3 |
| PUFA omega-3 3 | 0.19 | 0.27 | 0.2 | 0.02 |
| PUFA omega-6 4 | 41.7 | 50.6 | 47.8 | 2.0 |
| PUFA 5 | 42 | 50.9 | 48.0 | 2.0 |
| SFA/MUFA 6 | 0.57 | 0.68 | 0.6 | 0.03 |
| Omega-6/omega-3 7 | 185.8 | 257.5 | 222.6 | 21.2 |
| PUFA balance 8 | 0.39 | 0.54 | 0.5 | 0.04 |
| (UI) 9 | 122 | 137 | 131.9 | 3.1 |
| (PI) 10 | 48 | 58.2 | 54.2 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasinou, P.; De Amicis, I.; Fusaro, I.; Bucci, R.; Cavallini, D.; Parrillo, S.; Caputo, M.; Gramenzi, A.; Carluccio, A. The Lipidomics of Spermatozoa and Red Blood Cells Membrane Profile of Martina Franca Donkey: Preliminary Evaluation. Animals 2023, 13, 8. https://doi.org/10.3390/ani13010008
Prasinou P, De Amicis I, Fusaro I, Bucci R, Cavallini D, Parrillo S, Caputo M, Gramenzi A, Carluccio A. The Lipidomics of Spermatozoa and Red Blood Cells Membrane Profile of Martina Franca Donkey: Preliminary Evaluation. Animals. 2023; 13(1):8. https://doi.org/10.3390/ani13010008
Chicago/Turabian StylePrasinou, Paraskevi, Ippolito De Amicis, Isa Fusaro, Roberta Bucci, Damiano Cavallini, Salvatore Parrillo, Maurizio Caputo, Alessandro Gramenzi, and Augusto Carluccio. 2023. "The Lipidomics of Spermatozoa and Red Blood Cells Membrane Profile of Martina Franca Donkey: Preliminary Evaluation" Animals 13, no. 1: 8. https://doi.org/10.3390/ani13010008





