Simple Summary
25-hydroxyvitamin D3 [25(OH)VD3] plays an important role in regulating calcium and phosphorus metabolism via upregulating the expression of calcium ion channel proteins, resulting in improved bone quality. Many publications have reported that dietary supplementation of 25(OH)VD3 could improve the antioxidase activity and immune function of weaned pigs. However, evidence has proven that the biological response of 25(OH)VD3 on pig nutrition depends on the inclusion doses in the diets. This study was conducted to explore the roles of 25(OH)VD3 with different inclusion doses in growing-finishing pigs.
Abstract
The study was conducted to evaluate the effects of 25(OH)VD3 with different inclusion levels of 0, 25, 50 and 75 μg/kg in the diet on growth performance, nutrient digestibility, bone properties and pork quality in growing-finishing pigs. The results showed that the average daily gain (p < 0.05) and body weight (p < 0.10) of pigs showed a trend of increasing quadratically as inclusion levels of 25(OH)VD3 increased. Dietary supplementation of 50 μg/kg 25(OH)VD3 increased calcium digestibility compared with the 0 μg/kg group (p < 0.05), and calcium and phosphorus digestibility increased quadratically as inclusion levels of 25(OH)VD3 increased (p < 0.05). Dietary supplementation of 50 μg/kg 25(OH)VD3 increased concentrations of polyunsaturated fatty acids, and decreased contents of saturated and monounsaturated fatty acids in the longissimus dorsi of pigs (p < 0.05). The addition of 25, 50 and 75 μg/kg 25(OH)VD3 to the diet increased breaking strength and bone stiffness in the tibia compared with the 0 μg/kg group (p < 0.05). Dietary supplementation of 50 μg/kg 25(OH)VD3 improved the activities of superoxide dismutase (SOD) and catalase (CAT), and increased the messenger RNA (mRNA) expression of Cu/Zn SOD in the longissimus dorsi compared with the 0 μg/kg group (p < 0.05). Supplementing 50 μg/kg 25(OH)VD3 improved the mRNA expression of calcium-binding protein D9k (CaBP-D9k) and D28k (CaBP-D28K) in the liver compared with the 0 μg/kg 25(OH)D3 group (p < 0.05). In conclusion, a diet with an added dose of 50 μg/kg 25(OH)VD3 showed a greatest growth performance of growing-finishing pigs, and 25(OH)VD3 enhanced calcium deposition and antioxidant capacity in the longissimus dorsi, which may be associated with improved expression of calcium ion channel proteins.
1. Introduction
Vitamin D is one of the most important nutrients for maintaining the normal growth performance of pigs. A deficiency or metabolic abnormalities of vitamin D are known to be associated with myopathies such as muscle weakness, hypotonia, and skeletal muscle atrophy [1]. In practice, the common forms of vitamin D supplied in pig feed are vitamin D3 (VD3) and 25-hydroxyvitamin D3 [25(OH)VD3]. Vitamin D3 is a fat-soluble vitamin with a cholecalciferol-like biological activity, and 25(OH)D3 can be synthesized by VD3 in the liver [2]. However, the primary active form of vitamin D is 1,25-hydroxyvitamin D3 [1,25(OH)VD3], which is converted from VD3 and 25(OH)VD3 in the liver and kidney [3]. 25-hydroxyvitamin D3 has stronger physiological activity and can promote animal bone development, improve the feed conversion ratio and enhance disorder resistance [4]. Dietary supplementation of 25(OH)VD3 can not only shorten the metabolic process of VD3 in the body, but also avoid the effects of intestinal injury and liver and kidney dysfunction in pigs [5]. In addition, 25(OH)VD3 plays an important role in regulating calcium and phosphorus metabolism via upregulating the expression of calcium ion channel proteins, resulting in improved bone quality [6,7]. Many publications have reported that dietary supplementation of 25(OH)VD3 could improve the antioxidase activity and immune function of weaned pigs [8]. However, evidence has proven that the biological response of 25(OH)VD3 on pig nutrition depends on the inclusion doses in the diets [9]. A suitable inclusion level of 25(OH)VD3 could elicit osteoblast proliferation and improve bone formation and mineralization via activating the signaling pathway of vitamin D receptors [10], but excessive supplementation of 25(OH)VD3 caused bone catabolism and inhibited bone mineralization [11]. Therefore, the relationship between different inclusion levels of 25(OH)VD3 and pig nutrition should be further explored.
In our previous publication, the effects of different inclusion levels of 25(OH)VD3 on weanling pigs were explored, and the results indicated that a dietary addition of 50 μg/kg 25(OH)VD3 supplementation partly improved the growth performance, immune function, antioxidant status, intestinal morphology and bone properties of weaned piglets compared with dietary treatments of 25 μg/kg and 75 μg/kg 25(OH)VD3 [8]. However, the roles of 25(OH)VD3 with different dietary inclusion doses of 25(OH)VD3 on the nutrition of growing-finishing pigs have been unclear. Therefore, we hypothesized that the beneficial effects of 25(OH)VD3 on the growth performance and antioxidase activity of growing-finishing pigs, and the responses of 25(OH)VD3 on the pigs, are associated with the inclusion doses of 25(OH)VD3. In the present study, the effects of different inclusion levels of 25(OH)VD3 on growth performance, pork quality, antioxidant capacity, and nutrient digestibility in growing-finishing pigs were explored.
2. Materials and Methods
2.1. Animals and Diets
A total of 192 growing-finishing pigs (Duroc × Landrace × Yorkshire) with an initial body weight of 46.5 ± 2.7 kg were randomly allocated into 4 dietary treatments with 6 replicates per treatment, and each replicate included 8 pigs (4 boars and 4 gilts). The diets were formulated to provide adequate nutrition in three phases, mixed, and then supplemented with 0, 25, 50 and 75 μg/kg 25(OH)VD3 (Table 1). For each diet within each phase, the 25(OH)VD3 was first mixed with dicalcium phosphate and limestone, and then added to wheat bran to help ensure the distribution before the diets were completed with the corn and soybean meal. Subsamples of the diets were collected using multipoint sampling. All diets were supplemented with 25 μg/kg vitamin D3 from the premix, and the analyzed concentrations of vitamin D were 30.5, 55.1, 80.9 and 105.3 μg/kg in the diets supplemented with 0, 25, 50 and 75 μg/kg 25(OH)VD3, respectively. The feeding trial lasted 84 days and was conducted at the Swine Research Unit of China Agricultural University (Hebei, China). The addition of 0.3% chromic oxide as an indigestible marker was used to determine nutrient digestibility in the last 14 days of the feeding trial. The ingredient composition and nutritive levels of the diets with an inclusion level of 0 μg/kg 25(OH)D3 in the different phases of the pigs are shown in Table 1. Vitamin and mineral premixes were supplemented to meet the nutrient requirements of growing-finishing pigs according to the NRC (2012) [12]. During the whole feeding trial, all the pigs were provided ad libitum access to water and rations. The piglets in each replicate were housed in pens (1.5 × 1.2 × 1.0 m3), and the humidity and temperature of the feeding room were controlled automatically at 45–55% and 18 °C–22 °C. All pigs were dewormed according to the routine procedures of the pig farm. At days 28, 56 and 84, the piglets were weighed and the feed consumption was recorded to calculate the average daily gain (ADG) and average daily feed intake (ADFI). A ratio of ADG to ADFI (G: F) for the pigs was calculated.

Table 1.
Dietary formulation and analyzed or calculated nutrient levels (%) for each phase.
2.2. Sample Collection
At the end of the experiment, 300 g of fresh feces were collected from each replicate (pen) and then dried in the oven at 65 °C for 72 h. Considering the best growth performance was observed in the 50 μg/kg 25(OH)VD3 group compared with the other dietary treatments with different inclusion doses of 25(OH)VD3 at the end of the feeding trial, one barrow close to the average body weight of each replicate in the 0 μg/kg and 50 μg/kg 25(OH)VD3 groups was slaughtered to collect samples of longissimus dorsi, liver and tibia using an electric shock slaughter method. The carcass traits were measured immediately after slaughter, and the longissimus dorsi at the left 10th to 11th ribs were taken for determining pH, meat color and drip loss at 45 min and 24 h after the slaughter. In addition, samples of longissimus dorsi were frozen at −20 °C to analyze compositions of amino acids and fatty acids. Samples of the liver were taken and stored at −80 °C after quick-freezing in liquid nitrogen for determining the expression of antioxidase activity and calcium iron channel proteins. The tibia was stored at 4 °C for the further determination of skeletal properties after removing the fat and muscle.
2.3. Nutrient Digestibility
Apparent total tract digestibility (ATTD) of the nutrients were calculated using the indicator method as follows:
where RC is the concentration of chromium in the ration (%), RN is the concentration of nutrients in the ration (%), FN is the concentration of nutrients in the feces (%), FC is the concentration of chromium in the feces (%), and DN is the concentration of nutrients in the diet (%).
ATTD (%) = 100 − (RC × FN)/(FC × RN) × 100
2.4. Chemical Analysis
The diets and dried fecal samples were crushed and passed through a 60-mesh sieve for analysis. Dry matter (DM; Method 934.01), ether extract (EE; Method 920.39), crude protein (CP; Method 990.03), and chromium (Method 990.08) in the diets and feces were analyzed in duplicate [13].
2.5. Inflammatory Cytokines and Antioxidant Capacity in the Liver
The levels of antioxidant parameters including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) were determined using assay kits following the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Concentrations of interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-12 (IL-12), and tumor necrosis factor (TNF-α) were determined using commercially available porcine ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6. Carcass Traits
After slaughter, the carcass of pigs was divided according to the conventional procedure, and the carcass weight was measured after removing the head, hooves, tail and internal organs while the kidneys and leaf fat were retained. The oblique length of the carcass was the length from the inner edge of the union of the first rib and sternum to the anterior edge of the pubic symphysis. The backfat thickness at the 10th rib of the left half of the carcass was measured with vernier calipers, while the length and width of the longissimus dorsi were determined. The slaughter rate and eye muscle area were calculated as follows:
Slaughter rate (%) = the carcass weight/the live weight before slaughter × 100
Eye muscle area (cm2) = length × width × 0.7
Meat quality including shear force, cooking loss, drip loss, pH45min, pH24h, lightness (L*), redness (a*) and yellowness (b*) were determined immediately after the slaughter. The pH of the longissimus dorsi was determined 45 min and 24 h after the slaughter using a portable acidity meter (OPTP-STAR, Meister, Germany). The drip loss was determined according to the method described in a previous study [14]. Briefly, samples of approximately 30 g were cut from the longissimus dorsi, and then were stored in covered plastic boxes over sieved plastic racks for 48 h at 2 to 4 °C. After storage, the samples were re-weighed and the difference in weight was used to determine drip loss. In addition, about 100 g of samples from the longissimus dorsi were packaged under vacuum and cooked in a water bath at 80 °C for 45 min. The difference in weight before and after cooking was used to determine cooking loss. Subsequently, the samples after determination of the cooking loss were blotted dry and cooled for 10 h. A hollow metal probe was used to cut 10 subsamples from each block of meat along the length of the fiber direction, and the subsamples were used to determine the shear force by using an Instron machine (Stable Micro Systems Ltd., Surrey, UK).
2.7. Contents of Amino Acids (AA) and Fatty Acids (FA) in the Longissimus Dorsi
The longissimus dorsi was cut into 2 mm of thin slices to weight by using an aluminum box, and then freeze-dried (Tofflon Freezing Drying Systems, Shanghai, China). Lyophilized meat was subsequently crushed into powder to analyze the intramuscular fat concentration by using the Soxhlet petroleum ether extraction (XT15 Extractor, Ankom Technology Corp., Macedon, NY, USA) as described by Zhang et al. [15]. Concentrations of fatty acids were determined using classical gas chromatography (6890 Series, Agilent Technologies, Wilmington, DE, USA) as described in a previous report [16]. Moreover, methionine and cysteine were determined as methionine sulphone and cysteic acid using an amino acid analyzer (Hitachi L-8900, Tokyo, Japan) after cold performic acid oxidation overnight and hydrolyzing with 7.5 mol/L HCl at 110 °C for 24 h. Tryptophan was determined using high performance liquid chromatography (Agilent 1200 Series, Santa Clara, CA, USA) after LiOH hydrolysis for 22 h at 110 °C.
2.8. Skeletal Characteristics
Bone density was determined according to Archimedes’ principle as follows:
where A is the weight after leaving the water surface, B is the weight when fully immersed in distilled water, and p is the density of the distilled water.
Bone density = [A/(A − B)] × p
The bone mechanical properties were determined using a three-point bending test with an MTS-810 universal tensile tester (MTS Systems Corporation, Eden Prairie, MN, USA) [17]. The details on the analysis procedures of the bone mechanical properties followed those of a previous study [18].
2.9. Quantification PCR Analysis
Total ribonucleic acid (RNA) was extracted from the liver by the RN01-TRIpure Reagent (Aidlab Biotechnologies Co., Ltd., Beijing, China) according to the manufacturer’s protocol. The extracted RNA was quantified using NanoDrop 2000 (Thermo Fisher Scientific, MA, USA), and then diluted to the same concentration in each sample. The complementary deoxyribonucleic acid (cDNA) was produced using a reverse transcription kit (Takara, Kusatsu, Shiga, Japan). Quantitative polymerase chain reaction (PCR) was performed on a Riche light cycler 96 Real-Time PCR System (Roche, CA, USA). The PCR reaction procedure was: 5 min pre-denaturation at 95 °C, 20 s at 95 °C, 30 s at 60 °C, 40 cycles, and a melting curve of 65–90 °C. The primer sequences of each gene are shown in Supplementary Table S1. The relative expression of target genes to that of a housekeeping gene (GAPDH) was calculated using the 2−ΔΔCt method.
2.10. Statistical Analysis
Normality was verified and outliers were identified using the UNIVARIATE procedure of SAS 9.4 (SAS Institute, Cary, NC, USA), and a general linear model (GLM) of SAS was used to analyze the observations with the pen (replicate) as the experimental unit. An observation was considered an outlier if the value was more than three standard deviations away from the grand mean, but no outliers were observed in the study. The different dietary treatments were considered fixed effects, and the experimental animal status and feeding management (such as weaning age, humidity and temperature of the pens) were random effects. Means were separated using the LSMEANS statement and adjusted using Tukey’s multiple comparison tests. Polynomial contrast was conducted to determine the linear and quadratic effects of inclusion doses. A significant difference was considered when p < 0.05 and a tendency when 0.05 < p < 0.10.
3. Results
3.1. Growth Performance
There were no significant differences in the body weight (BW), ADG, ADFI and G: F of the pigs among different dietary groups (Table 2). However, the ADG in days 28–56 and days 1–84 showed a quadratic response as dietary 25(OH)D3 levels increased from 0 to 75 μg/kg (p < 0.05) with a maximum at 50 ug/kg. There was a trend that pig BW on day 56 and day 84 was maximized at 50 ug/kg and responded quadratically as inclusion levels of 25(OH)D3 increased (p < 0.10).

Table 2.
Effects of 25(OH)VD3 inclusion doses on growth performance of growing-finishing pigs.
3.2. Nutrient Digestibility
There were no significant differences in the digestibility of DM, EE, CP, calcium and phosphorus in pigs among different dietary groups (Table 3). However, the digestibility of calcium and phosphorus showed a quadratic response with a numerical maximum at 50 μg/kg to dietary 25(OH)D3 levels from 0 to 75 μg/kg (p < 0.05).

Table 3.
Effects of 25(OH)VD3 inclusion doses on nutrient digestibility of growing-finishing pigs.
3.3. Carcass Traits and Pork Quality
There were no significant differences in carcass traits (Table 4) and the composition of amino acids (Table 5) in the longissimus dorsi of the pigs between the 0 μg/kg and 50 μg/kg 25(OH)D3 groups. The dietary inclusion of 50 μg/kg 25(OH)VD3 decreased the concentrations of saturated and monounsaturated fatty acids in the longissimus dorsi of pigs, but increased the proportion of polyunsaturated fatty acids (p < 0.05; Table 6). In addition, providing 50 μg/kg 25(OH)VD3 increased a ratio of ∑ n-6 polyunsaturated fatty acid (PUFA) to ∑ n-3 PUFA and decreased the ratio of polyunsaturated fatty acids to saturated fatty acids (p < 0.05).

Table 4.
Effects of the dietary addition of 0 and 50 µg/kg 25(OH)VD3 on the carcass traits and pork quality of growing- finishing pigs.

Table 5.
Effects of dietary addition of 0 and 50 µg/kg 25(OH)VD3 on amino acid composition in the longissimus dorsi of growing-finishing pigs.

Table 6.
Effects of the dietary addition of 0 and 50 µg/kg 25(OH)VD3 on fatty acid composition of pork longissimus dorsi in growing-finishing pigs.
3.4. Skeletal Characteristics
There were no significant differences in phosphorus and bone mineral contents, bone mineral density and destruction deflection between the 0 μg/kg and 50 μg/kg 25(OH)D3 groups (Table 7). There was a trend that 50 μg/kg 25(OH)VD3 increased calcium concentration of the tibia (p < 0.10). The group of 50 μg/kg 25(OH)D3 significantly increased the break strength and stiffness in the tibia of pigs compared with the 0 μg/kg 25(OH)D3 group (p < 0.05).

Table 7.
Effects of the dietary addition of 0 and 50 µg/kg 25(OH)VD3 on the tibia from growing-finishing pigs.
3.5. Inflammatory Cytokines
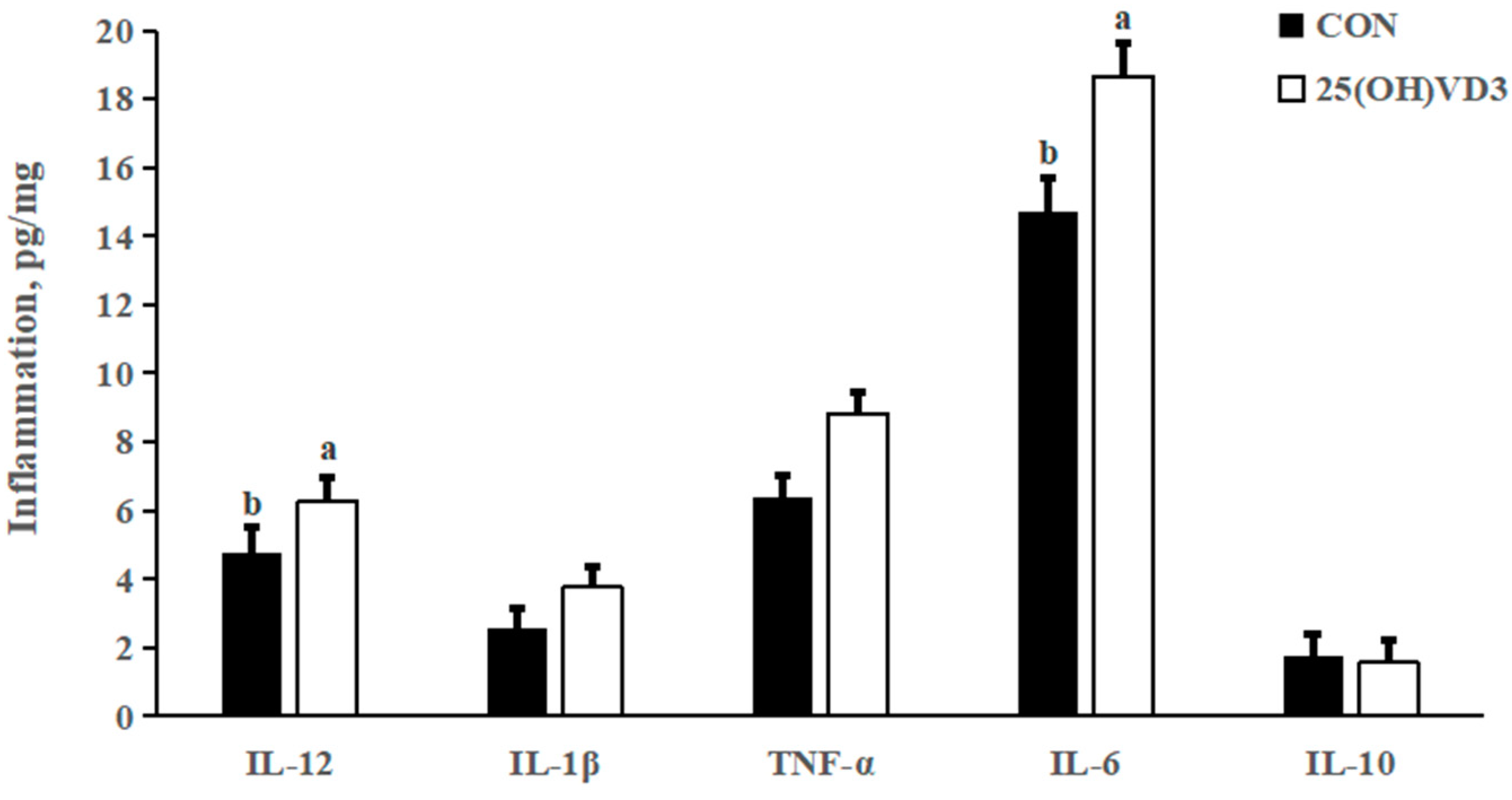
There were no significant differences in the concentrations of IL- 1β, TNF-α and IL-10 in the liver between the 0 μg/kg and 50 μg/kg 25(OH)D3 groups (Figure 1). However, provision of 50 μg/kg 25(OH)VD3 increased the concentrations of IL-12 and IL-6 in the liver of pigs compared with the 0 μg/kg 25(OH)VD3 group (p < 0.05).
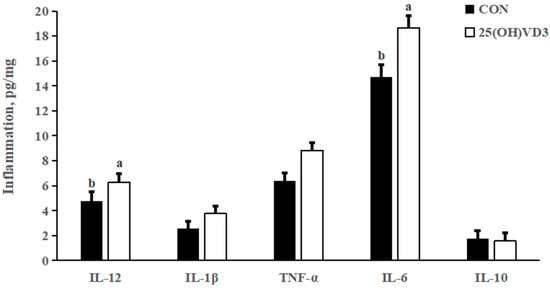
Figure 1.
Effects of 50 µg/kg 25(OH)VD3 on inflammatory cytokines concentration in the liver of growing-finishing pigs. Note: Superscript letters within the bar chart indicate significant differences (p < 0.05), n = 6. 25(OH)VD3, 25-hydroxyvitamin D3.
3.6. Antioxidase Activity
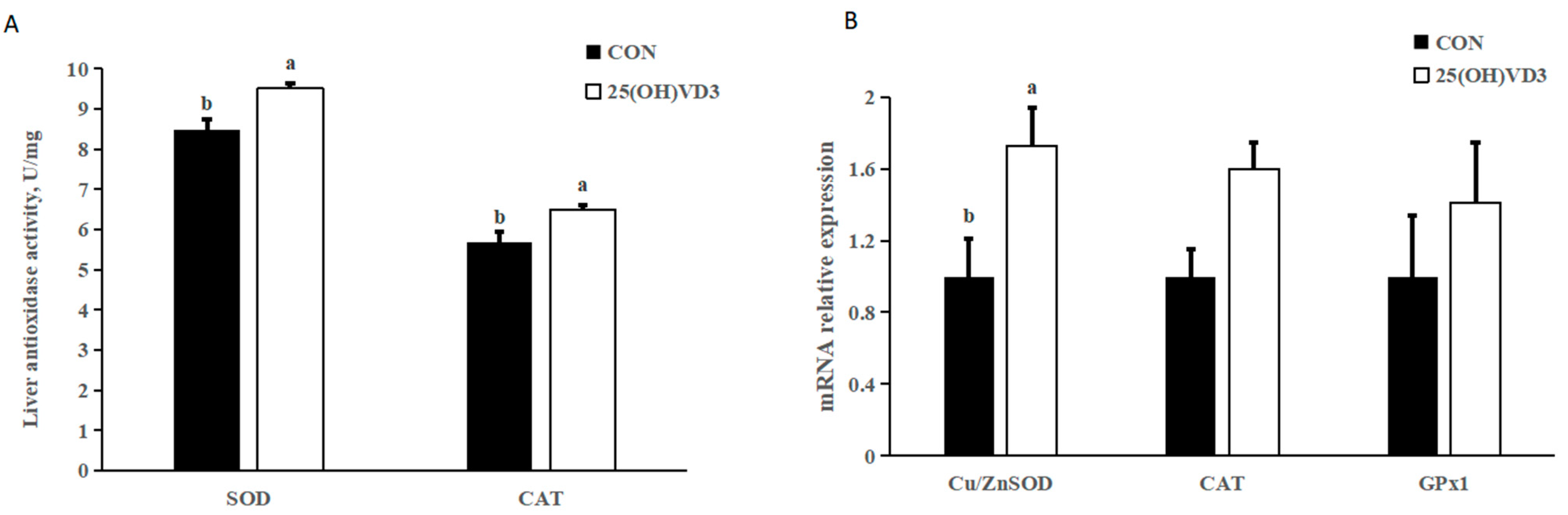
Providing 50 μg/kg 25(OH)VD3 increased the concentrations of SOD and CAT and the mRNA expression of Cu/Zn SOD in the liver of pigs compared with the 0 μg/kg 25(OH)D3 group (p < 0.05; Figure 2). There were no differences in the activity of the mRNA expression of CAT and GPx1 between the 0 μg/kg and 50 μg/kg 25(OH)D3 groups.
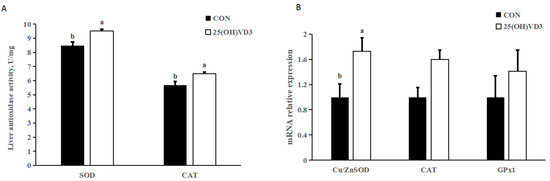
Figure 2.
Effects of 50 µg/kg 25(OH)VD3 on antioxidase activity in the liver of growing-finishing pigs. (A) Concentration of liver antioxidase activity. (B) mRNA expression of antioxidase genes. Note: Superscript letters within the bar chart indicate significant differences (p < 0.05), n = 6. 25(OH)VD3, 25-hydroxyvitamin D3.
3.7. mRNA Expression of Calcium Ion Channel Proteins
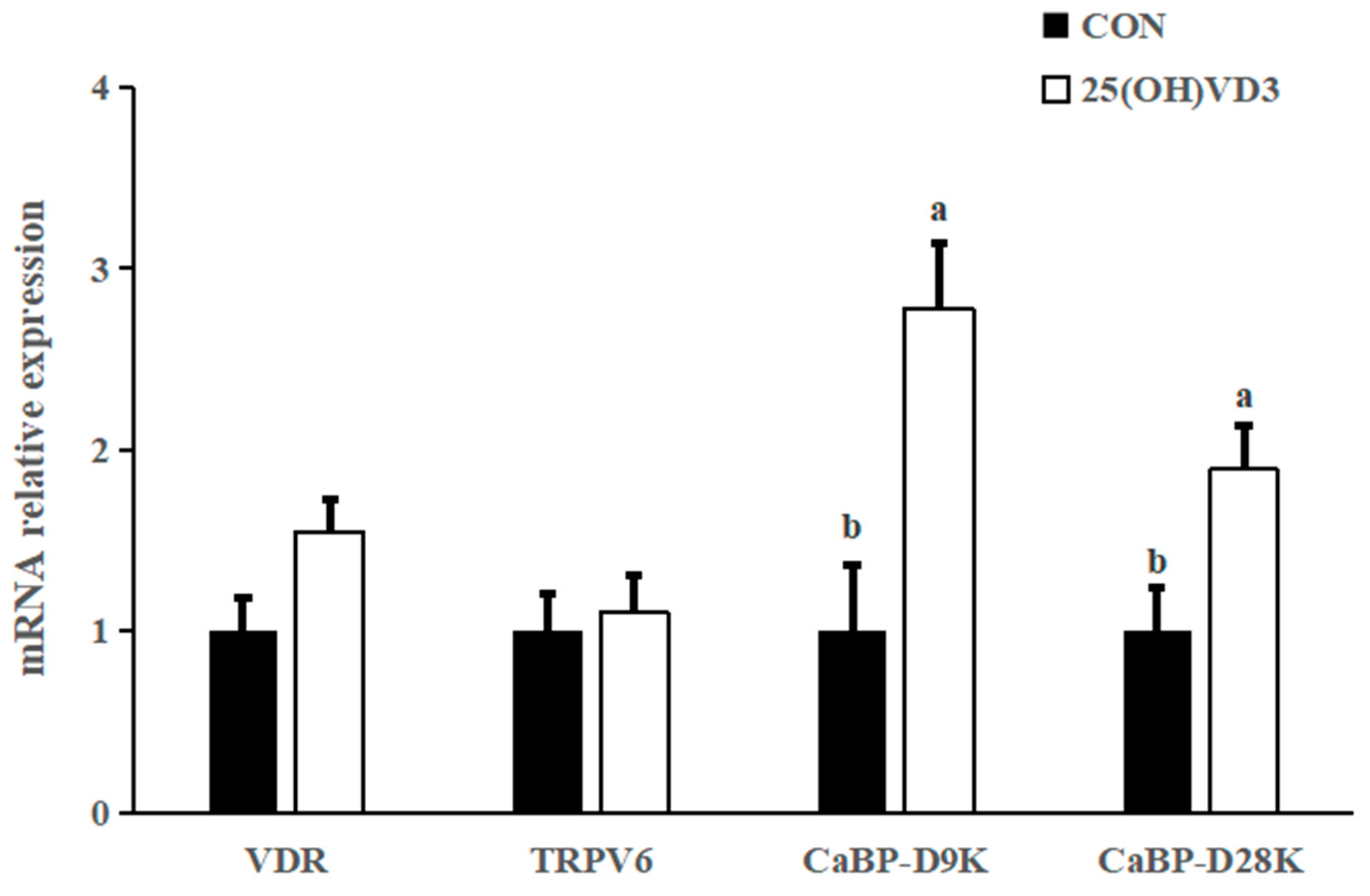
The dietary addition of 50 μg/kg 25(OH)VD3 tended to increase the mRNA expression of vitamin D receptor (VDR) (p < 0.10), calcium-binding protein D9k (CaBP-D9k) and calcium-binding protein D28k (CaBP-D28K) in the liver of pigs compared with the 0 μg/kg 25(OH)VD3 group (p < 0.05; Figure 3). There was no difference in the mRNA expression of TRPV6 between the 0 μg/kg and 50 μg/kg 25(OH)VD3 groups.
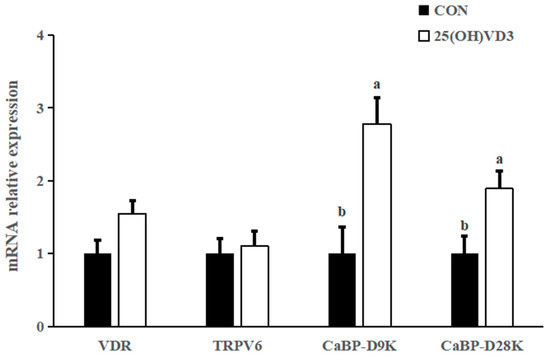
Figure 3.
Effects of 50 µg/kg 25(OH)VD3 on the mRNA expression of calcium ion channel proteins in the liver of finishing pigs. Note: Superscript letters within the bar chart indicate significant differences (p < 0.05), n = 6. 25(OH)VD3, 25-hydroxyvitamin D3.
4. Discussion
4.1. Effects of Different Inclusion Levels of 25(OH)VD3 on Growth Performance and Nutrient Digestibility
In the present study, the ADG of pigs in days 28–56 and days 1–84 and pig body weight on day 56 and day 84 showed a quadratic change as dietary 25(OH)VD3 levels increased from 0 to 75 μg/kg. The pigs had the greatest growth performance when the inclusion level of 25(OH)VD3 was 50 μg/kg in addition to the 25 ug/kg already in the diet. The above results were consistent with those in a previous study that showed a quadratic response of dietary 25(OH)VD3 levels on the ADG and body weight of weanling piglets, and an inclusion level of 50 μg/kg 25(OH)VD3 was recommended [8]. The results of a greatest growth performance in the 50 μg/kg 25(OH)VD3 for weanling piglets and growing-finishing pigs are consistent, indicating that responses of 25(OH)VD3 at an inclusion level of 50 μg/kg 25(OH)VD3 on pigs’ performance has no correlation to the growth phase of pigs from 8 kg to 120 kg, but further study should be conducted to confirm the above conclusion. In addition, the dietary supplementation of 25(OH)VD3 with different inclusion doses affected the quadratic digestibility of calcium and phosphorus in growing-finishing pigs, and the pigs in the group of 50 μg/kg 25(OH)VD3 showed the greatest digestibility of calcium and phosphorus in our study. The above result agreed with those in many previous studies, in which the dietary supplementation of 25(OH)VD3 increased calcium and phosphorus digestibility in sows and growing pigs [19]. Many previous studies have reported that providing an additional 50 μg/kg 25(OH)VD3 increased the digestibility of calcium and phosphorus, but did not affect the growth performance of growing-finishing pigs [20,21]. The reasons for the increased digestibility of calcium and phosphorus are associated with the improved activity of phytase and the upregulated expression of calcium ion channel proteins [21,22]. In addition, a previous study reported that dietary total phosphorus levels have marginal effects on the ileal digestibility of phosphorus in growing pigs [23]. Therefore, it is a useful strategy for supplementing the 25(OH)VD3 to increase the utilization of dietary phosphorus.
4.2. Effects of 50 μG/KG 25(OH)VD3 on Carcass Traits and Pork Quality
There were no significant differences in the carcass traits in the longissimus dorsi of pigs between the 0 μg/kg and 50 μg/kg 25(OH)VD3 groups, although the dietary supplementation of 50 μg/kg 25(OH)D3 increased the antioxidase activity in the liver, which was consistent with a previous report [24]. Those results indicated that 25(OH)VD3 has no influences on carcass traits, although 25(OH)VD3 can improve growth performance. A previous study reported that an increased activity of antioxidases improved the pork quality of finishing pigs [25]. In addition, samples from the pigs in the group of 50 μg/kg 25(OH)VD3 had decreased concentrations of saturated and monounsaturated fatty acids in the longissimus dorsi of the pigs, but an increased proportion of polyunsaturated fatty acids in the present study. However, a previous study reported that 50 μg/kg 25(OH)VD3 to the sows had no effect on the concentration of fatty acids in the sow’s milk and in the tibial and femoral cortical bones of new-born piglets [26]. The dietary addition of 50 μg/kg 25(OH)VD3 increased a ratio of ∑n-6 PUFA to ∑n-3 PUFA and decreased a ratio of polyunsaturated fatty acids to saturated fatty acids, which indicated that 25(OH)VD3 promoted the synthesis of polyunsaturated fatty acids in the growing-finishing pigs. The increased synthesis of polyunsaturated fatty acids induced by 25(OH)VD3 may be associated with the upregulating expression of calcium ion channel proteins, but the potential mechanism should be further clarified.
4.3. Effects of 50 μG/KG 25(OH)VD3 on Immune Function
In our previous study, a dietary supplementation of 50 μg/kg 25(OH)VD3 increased the concentrations of IL-1β in the jejunum and colon of weanling piglets. In addition, Zhang et al. [27] reported that 50 μg/kg 25(OH)VD3 added in the diet increased serum concentrations of IgM and IgG in weanling pigs. The above results indicate that 25(OH)VD3 improves the immune responses of pigs in resisting infection by pathogens, which is consistent with the results of the present study that dietary supplementation of 50 μg/kg 25(OH)VD3 increased concentrations of IL-12 and IL-6 in the liver of growing-finishing pigs. However, 25(OH)VD3 could decrease the concentrations of TNF-α, IL-2 and IL-6 through suppressing the activation of T cells [28]. Varying responses of 25(OH)VD3 on the concentrations of inflammatory cytokines should be associated with the healthy status of pigs.
4.4. Effects of 50 μG/KG 25(OH)VD3 on Antioxidant Capacity
The pigs that were fed an additional 50 μg/kg 25(OH)VD3 had increased concentrations of SOD and CAT and mRNA expression of Cu/Zn SOD in the liver compared with the pigs from the 0 μg/kg 25(OH)VD3 group, which indicates that 25(OH)VD3 can improve antioxidant capacity in growing-finishing pigs. The present results agree with those from many previous studies in which the dietary supplementation of 50 μg/kg 25(OH)VD3 increased the activity of T-AOC and GSH-Px in the serum of weanling piglets. The improved antioxidant capacity may result in an increase in the a* value of the carcass trait by reducing the oxidation of pork after the slaughter [29]. However, although 50 μg/kg 25(OH)VD3 increased the antioxidant capacity of the pigs, no significant improvements on the carcass trait were observed in our study.
4.5. Effects of 50 μG/KG 25(OH)VD3 on Bone Characteristics and Expression of Calcium Ion Channel Protein
Vitamin D3 can induce osteoblast proliferation through the VDR signaling pathway and promote bone formation and mineralization [30]. However, high-dose vitamin D3 supplementation will promote bone decomposition and absorption and inhibit bone mineralization [31]. In our study, there was a trend that 50 μg/kg 25(OH)VD3 increased the calcium concentration of the tibia. Furthermore, the addition of 50 μg/kg 25(OH)VD3 increased the bone breaking strength and stiffness in the tibia of pigs compared with the 0 μg/kg 25(OH)VD3 group. A previous study showed that dietary supplementation of 50 μg/kg 25(OH)VD3 fed to sows from late gestation to weaning increased calcium content, bone density and breaking strength in the tibias and femurs of sows [5]. Dietary supplementation of 25 μg/kg 25(OH)VD3 increased bone mineral content and breaking strength in the femur of weanling piglets in a previous study [8].
In addition, 50 μg/kg 25(OH)VD3 increased the mRNA expression of VDR, CaBP-D9K and CaBP-D28K in the tibia of pigs compared with the 0 μg/kg 25(OH)VD3 group. The above publication reported 50 μg/kg 25(OH)VD3 upregulated mRNA expression of duodenal VDR, transient receptor potential vanilloid 6 (TRPV6) and CaBP-D9k in sows, as well as ileal VDR and claudin-2, colonic VDR and CaBP-D9k in new-born piglets. Overall, a diet supplementing 50 μg/kg 25(OH)VD3 can improve bone characteristics and calcium deposition through upregulating the mRNA expression of calcium ion channel proteins.
5. Conclusions
The growth performance of growing-finishing pigs showed a quadratic change as inclusion levels of 25(OH)VD3 increased from 0 to 75 μg/kg, and the diet with an inclusion level 50 μg/kg 25(OH)VD3 showed a greater growth performance than 0, 25 and 75 μg/kg. A diet supplementing 50 μg/kg 25(OH)VD3 increased the concentrations of polyunsaturated fatty acids, but did not affect the amino acid concentrations in the longissimus dorsi of pigs. In addition, a diet supplementing 50 μg/kg 25(OH)D3 improved bone characteristics and calcium deposition through upregulating the mRNA expression of calcium ion channel proteins, resulting in an increased digestibility of calcium, as well as an improvement in liver antioxidant capacity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13010086/s1, Table S1: The primer sequences of antioxidase and calcium ion channel protein genes.
Author Contributions
Conceptualization, Z.Z. and G.Z.; investigation and methodology, J.C., B.Q. and X.Q.; formal analysis, Z.Z.; resources, G.Z.; data curation, Z.Z. and G.Z.; writing—original draft preparation, Z.Z. and G.Z.; writing—review and editing, J.Z.; supervision, J.Z.; project administration, J.Z.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (2021YDF1300201) and National Natural Science Foundation of China (32102560).
Institutional Review Board Statement
The study was approved by the Care and Use of Experimental Animal Committee of the China Agricultural University (AW20602202-1-2).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The Roles of Vitamin D in Skeletal Muscle: Form, Function, and Metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.-J.; Bendahan, D.; Bernard, M.; Martin, J.-C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef]
- Yasutake, Y.; Nishioka, T.; Imoto, N.; Tamura, T. A Single Mutation at the Ferredoxin Binding Site of P450 Vdh Enables Efficient Biocatalytic Production of 25-Hydroxyvitamin D3. Chembiochem 2013, 14, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Piao, X. Use of 25-hydroxyvitamin D3 in diets for sows: A review. Anim. Nutr. 2021, 7, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Specker, B.L.; Tsang, R.C.; Hollis, B.W. Effect of Race and Diet on Human-Milk Vitamin D and 25-Hydroxyvitamin D. Arch. Pediatr. Adolesc. Med. 1985, 139, 1134–1137. [Google Scholar] [CrossRef]
- Zhai, H.; Adeola, O.; Liu, J. Phosphorus nutrition of growing pigs. Anim. Nutr. 2022, 9, 127–137. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, Y.; Xu, Y.; Zhang, Z.; Wu, Y.; Cao, J.; Qiu, B.; Qin, X.; Han, D.; Piao, X.; et al. Dietary supplementation of 25-hydroxyvitamin D3 improves growth performance, antioxidant capacity and immune function in weaned piglets. Antioxidants 2022, 11, 1750. [Google Scholar] [CrossRef]
- Ovesen, L.; Brot, C.; Jakobsen, J. Food contents and biological activity of 25-hydroxyvitamin D: A vitamin D metabolite to be reckoned with? Ann. Nutr. Metab. 2003, 47, 107–113. [Google Scholar] [CrossRef]
- DeGroote, J.; Michiels, J.; Claeys, E.; Ovyn, A.; De Smet, S. Changes in the pig small intestinal mucosal glutathione kinetics after weaning. J. Anim. Sci. 2012, 90, 359–361. [Google Scholar] [CrossRef]
- Ma, X.; Shang, Q.; Hu, J.; Liu, H.; Brøkner, C.; Piao, X. Effects of replacing soybean meal, soy protein concentrate, fermented soybean meal or fish meal with enzyme-treated soybean meal on growth performance, nutrient digestibility, antioxidant capacity, immunity and intestinal morphology in weaned pigs. Livest. Sci. 2019, 225, 39–46. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Wiegand, B.R.; Parrish, F.J.; Morrical, D.G.; Huff-Lonerga, E. Feeding high levels of vitamin D3 does not improve tenderness of callipyge lamb loin chops. J. Anim. Sci. 2001, 79, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Straadt, I.K.; Rasmussen, M.; Andersen, H.J.; Bertram, H.C. Aging-induced changes in microstructure and water distribution in fresh and cooked pork in relation to water-holding capacity and cooking loss-a combined confocal laser scanning microscopy (CLSM) and low-field nuclear magnetic resonance relaxation study. Meat Sci. 2007, 75, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, L.; Qiao, S.; Mao, X.; Zeng, X. Effects of dietary leucine supplementation in low crude protein diets on performance, nitrogen balance, whole-body protein turnover, carcass characteristics and meat quality of finishing pigs. Anim. Sci. J. 2016, 87, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Keenan, M.J.; Hegsted, M.; Jones, K.L.; Delany, J.P.; Kime, J.C.; Melancon, L.E.; Tulley, R.T.; Hong, K.D. Comparison of Bone Density Measurement Techniques: DXA and Archimedes’ Principle. J. Bone Miner. Res. 1997, 12, 1903–1907. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Li, M.; Shang, Q.; Liu, S.; Piao, X. Maternal 25-hydroxycholecalciferol during lactation improves intestinal calcium absorption and bone properties in sow-suckling piglet pairs. J. Bone Miner. Metab. 2019, 37, 1083–1094. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, X.; Xiao, H.; Hou, L.; Wang, X.; Huang, Y.; Lin, Y.; Zheng, C.; Wang, L.; Jiang, Z. Effects of phytase and 25-hydroxyvitamin D3 supplementation on growth performance and bone development in weaned piglets in Ca-and p-deficient dietary. J. Sci. Food Agric. 2022, 102, 940–948. [Google Scholar] [CrossRef]
- Duffy, S.K.; Kelly, A.K.; Rajauria, G.; Clarke, L.C.; Gath, V.; Monahan, F.J.; O’Doherty, J.V. The effect of 25-hydroxyvitamin D3 and phytase inclusion on pig performance, bone parameters and pork quality in finisher pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1296–1305. [Google Scholar] [CrossRef]
- Regassa, A.; Adhikari, R.; Nyachoti, C.M.; Kim, W.K. Effects of 25-(OH)D3 on fecal Ca and P excretion, bone mineralization, Ca and P transporter mRNA expression and performance in growing female pigs. J. Environ. Sci. Health Part B 2015, 50, 293–299. [Google Scholar] [CrossRef]
- Proszkowiec-Weglarz, M.; Angel, R. Calcium and phosphorus metabolism in broilers: Effect of homeostatic mechanism on calcium and phosphorus digestibility. J. Appl. Poult. Res. 2013, 22, 609–627. [Google Scholar] [CrossRef]
- Liu, J.B.; Xue, P.C.; Cao, S.C.; Liu, J.; Chen, L.; Zhang, H.F. Effects of dietary phosphorus concentration and body weight on post-ileal phosphorus digestion in pigs. Anim. Feed Sci. Technol. 2018, 242, 86–94. [Google Scholar] [CrossRef]
- Mateo, R.D.; Wu, G.; Moon, H.K.; Carroll, J.A.; Kim, S.W. Effects of dietary arginine supplementation on the performance of lactating primiparous sows and nursing piglets. J. Anim. Sci. 2008, 86, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Cai, X.; Xiong, H.; Zhang, H.F. Effects of feeding frequency on meat quality traits and Longissimus muscle proteome in finishing pigs. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1175–1184. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, M.; Piao, X. Effects of 25- hydroxyvitamin D3 on growth performance, serum parameters, fecal microbiota, and metabolites in weaned piglets fed diets with low calcium and phosphorus. J. Sci. Food Agric. 2022, 102, 597–606. [Google Scholar] [CrossRef]
- Sandoval, J.L.; Ventura, D.E.; Fiallos, O.B.; Anderson, B.L.; Sparks, J.C.; Starkey, J.D.; Starkey, C.W. Efficacy and safety of a novel source of dietary 25-hydroxycholecalciferol in growing pigs. J. Anim. Sci. 2022, 100, skac260. [Google Scholar] [CrossRef]
- Vidyarani, M.; Selvaraj, P.; Jawahar, M.S.; Narayanan, P.R. 1, 25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine 2007, 40, 128–134. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.; Jiang, Z.; Zheng, C.; Zhou, G.; Yu, D.; Cao, T.; Wang, J.; Chen, F. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids 2008, 38, 95–102. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.-C.; Jurutka, P.W. Molecular Mechanisms of Vitamin D Action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. High dose 1,25(OH)2D3 inhibits osteoblast mineralization in vitro. Int. J. Mol. Med. 2012, 29, 934–938. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).